Back to Journals » OncoTargets and Therapy » Volume 15
Progression-Free Survival of a Patient with Advanced Hepatocellular Carcinoma Treated with Adoptive Cell Therapy Using Natural Killer Cells: A Case Report
Authors Hong G , Xie S , Guo Z, Zhang D , Ge S, Zhang S, Gao W
Received 2 November 2021
Accepted for publication 2 March 2022
Published 15 March 2022 Volume 2022:15 Pages 255—266
DOI https://doi.org/10.2147/OTT.S344707
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Guodai Hong,1 Silun Xie,2 Zikuan Guo,3 Diping Zhang,2 Sihai Ge,2 Suqin Zhang,1 Wenbin Gao1
1Department of Oncology, The Third Affiliated Hospital of Shenzhen University (Shenzhen Luohu People’s Hospital), Shenzhen, 518002, People’s Republic of China; 2Shenzhen Cell Biotechnology Co., Ltd, Shenzhen, 518054, People’s Republic of China; 3Beijing Clin- Biotechnology Co., Ltd, Beijing, 100070, People’s Republic of China
Correspondence: Wenbin Gao, Department of Oncology, The Third Affiliated Hospital of Shenzhen University (Shenzhen Luohu People’s Hospital), Luohu District, Shenzhen, 518002, People’s Republic of China, Tel +86 132 6677 8968, Email [email protected]
Abstract: Adoptive cell therapy (ACT) is a promising treatment that is considered safe and efficient. Natural killer (NK) cells play an important role in the innate immune system and destroy target cells such as tumor cells without prior sensitization. Here, we report a 59-year-old man with advanced diffuse hepatocellular carcinoma (HCC) who underwent 17 courses of NK cell treatment from March 2017 to July 2018. Although he presented with progressive disease, his hydrothorax and ascites decreased, and his state of mind, appetite and quality of life were markedly improved after treatment versus at admission. To date, his survival time is > 48 months. Here, we provide evidence that NK cell adoptive therapy has no adverse effects, enhances immune function, and improves the quality of life of patients with HCC.
Keywords: hepatocellular carcinoma, natural killer cell therapy, prolonged survival
Introduction
Hepatocellular carcinoma (HCC) is a commonly diagnosed cancer worldwide.1 In China, HCC ranks fourth and third in incidence and mortality, respectively, among malignancies. Furthermore, HCC is the third and sixth most common cancer and the third and fourth leading cause of cancer-related death in men and women, respectively.2 Most patients with HCC are diagnosed in the intermediate or terminal stage; advanced diffuse HCC is the terminal stage and has an extremely poor prognosis and median survival of <3 months.3
The current main treatments for HCC are surgery (liver resection or liver transplantation), radiotherapy, transcatheter arterial chemoembolization (TACE), and targeted therapy (eg, sorafenib).4 Adoptive cell therapy (ACT) is a promising approach for treating HCC in clinical applications.5,6 As innate cells are the first line of immune defense, natural killer (NK) cells can lyse target cells or tumor cells without prior sensitization. In humans, NK cells are always defined as CD3−CD16+CD56+. NK cell function is mediated by the balance of killer activating receptors (KARs) and killer inhibitory receptors (KIRs) on the cells. Under normal circumstances, KARs can bind ligands such as major histocompatibility class I (MHC I) expressed on normal cells, which prevents NK cell activation. When cells are infected or transformed into tumor cells, KIR expression is reduced or lost, and KARs play a dominant role in KIR and KAR interactions. Then, NK cells can directly recognize and eliminate the target cells.7
Although NK cells have strong cytotoxic effects against tumor cells, they account for only 10~15% of peripheral blood mononuclear cells (PBMCs). To increase the number of NK cells and enhance their cytotoxicity, efficient approaches have been adopted for large-scale ex vivo NK cell expansion from PBMCs under good manufacturing practice (GMP) conditions.8,9 Clinical trials have evaluated the effects of ACT with NK cells on solid tumors such as melanoma, lymphoma, ovarian cancer, and breast cancer.10–12 Based on these successful cultivation efforts, we have established a new method for NK cell expansion to meet clinical application needs. Here, we report a case of a 59-year-old man with advanced diffuse HCC. He received 17 courses of NK cell infusion in succession without any other treatment. We evaluated the curative effect at the end of the treatment according to the clinical data.
Patients and Methods
Patient
A 59-year-old man was diagnosed with advanced diffuse HCC, T3N1M1 (ie, stage IV disease according to the tumor-node-metastasis (TNM) staging system) in June 2016. He had a history of chronic hepatitis B virus (HBV) infection but no history of diseases such as tuberculosis, hepatitis, schistosomiasis, chronic bronchitis, coronary heart disease, and kidney disease; major trauma; food and drug allergies; or transfusion reactions. He was treated with TACE under general anesthesia (practical medication: oxaliplatin, 50 mg; pirarubicin, 30 mg; iodized oil, 30 mL) in July 2016. He underwent peritoneal puncture operation and drug injection under general anesthesia (practical medication: Endostar, 18 mL; cisplatin, 75 mg; lobaplatin, 50 mg) in October, November, and December 2016. In March 2017, he underwent intraperitoneal perfusion (practical medication: Endostar, 6 mL). He completed 17 courses of NK cell therapy from March 2017 to July 2018. Each course of treatment was administered monthly and infused on two consecutive days. We obtained the patient’s written informed consent according to the Declaration of Helsinki.
NK Cell Preparation
First, 500 μL NK activator (Clin-Biotechnology Co., Ltd., Beijing, China) was aspirated by a Pasteur pipette and added to a T-175 culture flask (Corning Incorporated, NY, USA). Then, 20 mL phosphate-buffered saline (PBS) was added and mixed thoroughly so that the coating solution was dispersed at the bottom of the flask. The flask was stored at 4°C for >4 hours before use.
Next, approximately 50~80 mL peripheral blood was collected from the patient, transferred to a 50-mL centrifuge tube (Corning Incorporated), and centrifuged at 1800 rpm for 15 minutes. The plasma in the top layer was collected and heat-inactivated in a 56°C water bath. After 30 minutes, the tube was removed and allowed to cool to <37°C and then centrifuged at 3000 rpm for 6 minutes. The supernatant was collected and stored at 4°C until further use. The same volume of saline was added to the centrifuge tube containing blood cells. An equal volume of the suspension was transferred carefully to a 50 mL centrifuge tube containing 15 mL lymphocyte separation solution and centrifuged at 2000 rpm for 25 minutes (the speed slowed to its lowest). The middle monocyte layer was collected and washed twice with saline, and then the monocytes were counted.
Then, the coating solution in the T-175 culture flask was discarded, and the bottom of the flask was washed with 20 mL saline. NK cell culture medium (CTS TM AIM-V TM Serum-free Medium [Thermo Fisher Scientific, Waltham, MA, USA]; 5% plasma; 25 ng/mL recombinant human IL-15 [Beijing T&L Biotechnology Co., Ltd., Beijing, China]; 1000 U/mL recombinant human IL-2 [Shandong Jintai Biotechnology Co., Ltd., Jinan, China]) was prepared. The NK cell culture and expansion procedures were as follows: on day 1, NK cell culture medium and monocytes were added to the T-175 culture flask. The initial cell density was adjusted to 2 × 106/mL and then incubated at 37°C with 5% CO2. On days 4 and 6, an equal volume of NK cell culture medium was added to the culture flask. On day 8, the cell density was adjusted to 1.6×106 /mL, and the total culture medium was transferred to a 2-L cell culture bag (Gas-Permeable Cell Culture Bag, Corning Incorporated); the medium was replenished every 2 or 3 days according to the cell growth. On day 14, midterm sampling inspection was performed (the test included bacteria, fungi, mycoplasma, endotoxin, cell purity and cell viability). On days 17 and 18, the medium was collected into a 250-mL centrifuge tube (Corning Incorporated) and centrifuged at 1600 rpm for 6 minutes, and the supernatant was discarded. The precipitate was washed twice with saline. The NK cell infusion solution consisted of 100 mL saline dissolved with NK cells (cell density, 3–5 × 10 7 /mL) and 5% human albumin (Shenzhen Weiguang Biological Products Co. Ltd., Shenzhen, China). The total cell number was counted, and bacteria, fungi, mycoplasma, endotoxin, and cell purity were detected for a final time. We also detected the release of perforin (Alexa Fluor 647-Mouse Anti-Human, Perforin, Becton, Dickinson and Company) and granzyme B (FITC-Mouse Anti-Human, Granzyme B, Becton, Dickinson and Company) to demonstrate NK cell viability.
Therapeutic Procedure
The patient received 17 courses of NK cell therapy from March 2017 to July 2018. Each course consisted of two infusions and was administered monthly. The patient received no other therapies during the NK cell therapy period.
Inspection Approach
Bacteria and fungi were detected with nutrient agar plates and Sabouraud dextrose agar plates with antimicrobics (Huankai Microbial Sci & Tech Co., Ltd., Guangdong, Guangzhou, China), respectively. Endotoxin within the culture medium was examined with a Mycoplasma Isolation & Identification Tube (Zhuhai Encode Medical Engineering Co., Ltd., Guangdong, Zhuhai, China). A few induced NK cells were collected and washed once with PBS, and then fluorescein isothiocyanate (FITC)-mouse-anti-human CD3 and phycoerythrin (PE)-mouse anti-human CD56 were added. The cells were incubated in the dark for 15 minutes and washed once with PBS, and the cell purity was detected by an Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NY, USA). Cell viability was also detected with flow cytometry using FITC-7-amino-actinomycin D (7-AAD) staining.
Detection of Immune Function
Lymphocyte subsets were detected by flow cytometry (Accuri C6, BD Biosciences) before and after the NK cell infusions. Peripheral blood (7–8 mL) was collected to monitor immune function. The CD8+ T cells (T suppressor cells/T cytotoxic lymphocytes, reference range: 320–1250 cells/μL), CD4+ T cells (T helper cells, reference range: 450–1440 cells/μL), CD3−CD19+ cells (B lymphocytes, reference range: 90–560 cells/μL), and CD3−CD56+ cells (NK cells, reference range: 80–350 cells/μL) were stained with fluorescent antibodies (BD Biosciences) and counted.
Evaluation of Liver Function
Alanine aminotransferase (ALT, reference range: 9–50 U/L), aspartate aminotransferase (AST, reference range: 15–40 U/L), total bilirubin (TBil, reference range: 5–21 μmol/L), and albumin (ALB, reference range: 40–55 g/L) were measured with an automatic biochemical analyzer (AU5800, Beckman Coulter, Indianapolis, IN, USA) to assess liver function during NK cell treatment.
Detection of Tumor Markers
Tumor markers are indicators that reflect tumor occurrence and development. Alpha fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and CA15-3 levels are sensitive markers for the progression of liver cancer. Therefore, we detected these four markers during NK cell treatment via immunohistochemical staining.
Evaluation of Safety
The safety of the treatment was evaluated using the routine blood index. The leukocyte (reference range: 3.5–9.5 × 109/L), blood platelet (reference range: 125–350 × 109/L), neutrophil (reference range: 2–7 × 109/L), and lymphocyte (reference range: 0.8–4×109/L) counts were detected with an automatic hematology analyzer (DxH 600, Beckman Coulter).
Evaluation of Health-Related Quality of Life Issues
Health-related quality of life issues were assessed with The European Organization for Research and Treatment for Cancer Quality of Life Questionnaire (EORTC QLQ)-C30, version 3.0.13 It is a cross-cultural, cross-country core scale widely used for evaluating the quality of life of patients with cancer. The scale contains 30 items comprising five functional dimensions (physiological function, role function, emotional function, cognitive function, social function), five symptom dimensions (fatigue, nausea and vomiting, pain), six single items assessing common symptoms (loss of appetite, dyspnea, constipation, diarrhea, sleep disturbance), general health/quality of life dimensions and perceived financial impact. Higher scores for the functional and general health dimensions indicate better functional status and quality of life, while higher scores for the symptom dimensions indicate more symptoms or problems (worse quality of life). Related studies have shown that the EORTC QLQ-C30 has good reliability and validity for evaluating the quality of life of patients with cancer.14 The EORTC QLQ-C30 score is calculated on a scale of 1–4. Here, we followed the EORTC scoring manual.15 After linear transformation, the score for all scales ranged from 0–100. The scores for each scale are reported as the mean (SD) or as the median.
Evaluation of Therapeutic Efficiency
The treatment response was assessed after the NK cell infusions. Curative effect was evaluated as complete response, partial response, stable disease or progressive disease based on the Response Evaluation Criteria in Solid Tumors (RECIST).16
Results
Outcome of NK Cell Preparation
After 17–18 days of culture, we counted the total number of NK cells and evaluated their purity. Here, we used an in vitro culture cell number count assay and flow cytometry. The fold expansions in the whole count assay were 1307–1548 (we assumed that there were 5–10% NK cells in the peripheral blood; the initial number of peripheral blood monocytes was calculated as 50 million). CD3−CD56+ NK cells accounted for a low proportion before expansion and then increased to ≥80% on day 17 or 18 (Figure 1).
To assess crucial activation and inhibitory markers, we analyzed the activating receptors CD226 and CD314 (PE-Mouse Anti-Human, CD226; APC-Mouse Anti-Human, CD314; both, Becton, Dickinson and Company, NJ, Franklin Lakes) and the inhibitory receptor CD158a (FITC-Mouse Anti-Human, CD158a, Becton, Dickinson and Company). Figure 2 shows that CD226 expression was high after cell expansion, and CD158a expression was decreased, but not significantly. We also detected the release of perforin (Alexa Fluor 647-Mouse Anti-Human, Perforin, Becton, Dickinson and Company) and granzyme B (FITC-Mouse Anti-Human, Granzyme B, Becton, Dickinson and Company), which were expressed at high levels after cell culture (Figure 2).
NK Cell Therapy
The patient received NK cell infusions from March 2017 to July 2018. The patient received one treatment course per month. One course involved intravenous transfusion once daily on two consecutive days (Table 1).
 |
Table 1 The Number of Infused Cells and the Cell Purity and Cell Viability of the NK Cell Infusions |
Physical Indicators After Treatment
The change in the lymphocyte subsets suggested that immune function was improved or decreased. Through the 17-course treatment, the T suppressor cell/T cytotoxic lymphocyte (CD8+ T cell) count was greatly improved, while the T helper cell (CD4+ T cell) count was maintained. The NK cell (CD3−CD56+ cells) count increased, while the B lymphocyte (CD3−CD19+ cells) count was unchanged (Figure 3, Table 2).
 |
Table 2 Immune Cell Counts Tests Before and After 17 Course of Treatment with NK Cells |
 |
Figure 3 The variation in lymphocyte subsets during therapy. CD8+ T cell and NK cell counts were increased after treatment. CD4+ T cell and B cell counts were changed, but not significantly. |
The patient’s liver function did not deteriorate significantly. After the treatment, the ALT and AST levels increased slowly, and the TBil and ALB levels remained in the reference ranges (Figure 4, Table 3). At the same time, the levels of all tumor markers except AFP improved during the NK cell infusions (Figure 5, Table 4).
 |
Table 3 Liver Function Tests Before and After 17 Course of Treatment with NK Cells |
 |
Table 4 Tumor Markers Tests Before and After 17 Course of Treatment with NK Cells |
 |
Figure 4 Changes in liver function during therapy. We detected the ALT, AST, ALB, and TBil levels. ALT, AST, and ALB levels were increased but remained within the reference range. |
 |
Figure 5 Changes in tumor markers during therapy. CEA, CA15-3, and CA19-9 levels increased after treatment, and AFP levels increased slightly. |
There were no adverse reactions, such as fever, vomiting or convulsions, during the transfusion. Routine blood testing showed that the leukocyte, blood platelet, neutrophil and lymphocyte counts remained at normal levels (Figure 6, Table 5).
 |
Table 5 Blood Routine Tests Before and After 17 Course of Treatment with NK Cells |
 |
Figure 6 Routine blood tests during therapy. The leukocyte, blood platelet, neutrophil, and lymphocyte counts were stable and remained in the reference range. |
The patient’s quality of life was greatly improved. The EORTC QLQ-C30 showed that the functional dimension and health dimension scores increased from ≤20 to approximately 80. The symptom dimension scores decreased from 60 to ≤10 (Figure 7).
 |
Figure 7 The EORTC QLQ-C30 score during therapy. The function and health dimension scores increased gradually. The symptom dimension score was slightly decreased after therapy. |
Curative Effect
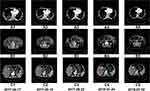
We performed computed tomography (CT) before and after NK cell treatment for imaging-based diagnosis to assess the clinical efficiency. Hydrothorax and ascites were greatly decreased after six treatment courses. The patient has maintained this status since the end of therapy. Moreover, multiple tumors in the right liver lobe decreased in size after 17 courses of NK cell therapy (Figure 8).
Discussion
The risk factors for liver cancer include nonalcoholic fatty liver disease, hepatocirrhosis, HBV, HCV, type II diabetes, and excess food and water, smoking, and alcohol consumption.17 In China, HBV and HCV are considered major contributors to liver cancer.18 Apart from traditional radiotherapy, we have applied chemotherapy (sorafenib, 5-fluorouracil) and immunotherapy (monoclonal antibodies, immune checkpoint inhibitors) for hepatoma treatment.19 However, the 5-year survival rate for patients with TNM stage III disease after resection is 16%.20
ACT is a novel method involving the selective activation and expansion of immune cells obtained from the patient’s own peripheral blood or tumor issues to enhance immune function. A meta-analysis of postoperative adoptive immunotherapy for HCC showed that ACT significantly reduced the risk of 1- and 3-year recurrence but had no obvious effects on the 3-year survival rate.21 In recent years, numerous clinical trials have shown that ACT with NK cells achieves better tolerance and reliable safety in acute myeloid leukemia, non-Hodgkin lymphoma, gastric or colorectal cancer, multiple myeloma, and other solid tumors than other approaches.22–27 Regarding HCC, Lin et al reported that cryoablation combined with adoptive transfer of NK cells prolonged the median progression-free survival of patients with advanced HCC.28 Moreover, a case report showed that allogenic NK cell immunotherapy had better efficacy against HCC after liver transplantation.29 Furthermore, NK cells have strong cytotoxicity against HCC cells via ex vivo expansion with an autologous feeder layer and anti-CD3 antibody.30 On the other hand, an in vitro NK cell expansion system based on K562 feeder cells expressing membrane-bound IL-15, IL-21, or the costimulatory molecule 4–1BB ligand (4–1BBL) enhanced proliferation and cytotoxicity without telomere shortening.31 Generally, genetically engineered feeder cells should be irradiated before application.32,33 K562 cells expressing membrane-bound IL-21 exhibit high NK cell activation with intensive growth patterns and high expression of activating receptors and cytokines.31,34–36 Fujisaki et al reported that irradiated K562 cell membrane-bound IL-15 and 4–1BBL cocultured with PBMCs produced an enhanced cytotoxic effect on acute myeloid leukemia cells in a murine model.37 A clinical trial indicated that membrane-bound IL-21 expanded the number of NK cells for infusions and improved the leukemia relapse rate after haploidentical transplantation (median survival, 14.7 months).38
In addition to immune checkpoint inhibitors, the chimeric antigen receptor T (CAR-T) technique is emerging as a potential approach. As CD19 is a specific target in acute B-lymphoblastic leukemia, AFP, glypican-3 (GPC-3), NK group 2 member D ligand (NKG2DL), and New York esophageal squamous cell carcinoma 1 (NY-ESO-1) are potential nonspecific targets in unresectable advanced HCC.39 A clinical trial has shown that GPC-3 CAR-T cell therapy for GPC-3-positive patients with advanced HCC achieved a better response than traditional approaches, but toxicity remains a major obstacle in practical application.40
In the present research, we used a commercial NK cell expansion kit to culture a sufficient number of NK cells at high purity for clinical application. After a large number of highly active NK cells are injected into a patient, the activation of NK cells depends on the strength of the stimulatory signals of activating receptors and inhibitory receptors. When NK cells recognize cancer cells and activate them, they release perforin and granzymes to initiate cancer cell apoptosis and cytotoxic killing mechanisms.41 Activated NK cells also release cytokines such as IFNγ and TNFα, which not only synergistically kill cancer cells but also activate other immune cells and enhance immune defense capabilities.42
In the early stages of NK cell therapy in this patient, multiple liver cancer lesions were still slowly growing (Figure 8C1–C4), as indicated by the increase in AFP (Figure 5), and the hepatocellular carcinoma lesion size reached a maximum at 1 year after treatment. After multiple cycles of NK cell therapy, the general condition of the patient improved, his quality of life improved, and his liver function was normal. This suggests that the treatment with NK cells leads to an antitumor immune response, but this process may be very slow, as it took a long time to generate enough of an immune response to promote the regression of cancer tissue. As treatment continued, the antitumor response induced by NK cells eventually led to the gradual shrinkage and partial disappearance of multiple liver cancer lesions (Figure 8C5), and AFP decreased significantly. After 1 year and 4 months of NK cell infusion therapy, the patient’s disease gradually stabilized; his physical indicators remained at normal levels, tumor burden was significantly reduced, and his quality of life was significantly improved.
Ethics Approval and Consent to Participate
This study was approved by The Third Affiliated Hospital of Shenzhen University (IRB ID: 20160817, 2018LHYYZLNK-006-01). The patient provided written informed consent according to the Declaration of Helsinki.
Consent for Publication
The patient provided written informed consent for the case details to be published.
Acknowledgments
The authors would like to thank all members of the study team, the patient and his family.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Parikh ND, Marshall VD, Singal AG, et al. Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: an analysis of the SEER-Medicare database. Hepatology. 2017;65(1):122–133. doi:10.1002/hep.28881
4. Zhu ZX, Huang JW, Liao MH, Zeng Y. Treatment strategy for hepatocellular carcinoma in China: radiofrequency ablation versus liver resection. Jpn J Clin Oncol. 2016;46(12):1075–1080. doi:10.1093/jjco/hyw134
5. Cao J, Kong FH, Liu X, Wang XB. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2019;25(27):3649–3663. doi:10.3748/wjg.v25.i27.3649
6. Comoli P, Chabannon C, Koehl U, et al. Development of adaptive immune effector therapies in solid tumors. Ann Oncol. 2019;30(11):1740–1750. doi:10.1093/annonc/mdz285
7. Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi:10.1126/science.1198687
8. Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26(2):161–172. doi:10.1016/j.smim.2014.02.002
9. Lim O, Lee Y, Chung H, et al. GMP-compliant, large-scale expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells in vitro and in vivo. PLoS One. 2013;8(1):e53611. doi:10.1371/journal.pone.0053611
10. Yang Y, Lim O, Kim TM, et al. Phase I study of random healthy donor-derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumors. Cancer Immunol Res. 2016;4(3):215–224. doi:10.1158/2326-6066.CIR-15-0118
11. Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17(19):6287–6297. doi:10.1158/1078-0432.CCR-11-1347
12. Geller MA, Cooley S, Judson PL, et al. A Phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98–107. doi:10.3109/14653249.2010.515582
13. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in Oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi:10.1093/jnci/85.5.365
14. Schut AW, Timbergen MJM, Lidington E, et al. The evaluation of health-related quality of life issues experienced by patients with desmoid-type fibromatosis (the QUALIFIED study)-a protocol for an international cohort study. Cancers. 2021;13(13):3068. doi:10.3390/cancers13133068
15. Witkowski D. EORTC QLQ-C30 Scoring Manual. University of Copenhagen; 2021.
16. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
17. Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2362–2368. doi:10.1158/1055-9965.EPI-11-0643
18. Liu Z, Mao X, Jiang Y, et al. Changing trends in the disease burden of primary liver cancer caused by specific etiologies in China. Cancer Med. 2019;8(12):5787–5799. doi:10.1002/cam4.2477
19. Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314. doi:10.1016/j.bbcan.2019.188314
20. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
21. Xie F, Zhang X, Li H, et al. Adoptive immunotherapy in postoperative hepatocellular carcinoma: a systemic review. PLoS One. 2012;7(8):e42879. doi:10.1371/journal.pone.0042879
22. Nguyen R, Wu H, Pounds S, et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J Immunother Cancer. 2019;7(1):81. doi:10.1186/s40425-019-0564-6
23. Cooley S, He F, Bachanova V, et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019;3(13):1970–1980. doi:10.1182/bloodadvances.2018028332
24. Bachanova V, Sarhan D, DeFor TE, et al. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother. 2018;67(3):483–494. doi:10.1007/s00262-017-2100-1
25. Ishikawa T, Okayama T, Sakamoto N, et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int J Cancer. 2018;142(12):2599–2609. doi:10.1002/ijc.31285
26. Shah N, Li L, McCarty J, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol. 2017;177(3):457–466. doi:10.1111/bjh.14570
27. Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15(12):1563–1570. doi:10.1016/j.jcyt.2013.06.017
28. Lin M, Liang S, Wang X, et al. Cryoablation combined with allogenic natural killer cell immunotherapy improves the curative effect in patients with advanced hepatocellular cancer. Oncotarget. 2017;8(47):81967–81977. doi:10.18632/oncotarget.17804
29. Xie S, Wu Z, Zhou L, et al. Iodine-125 seed implantation and allogenic natural killer cell immunotherapy for hepatocellular carcinoma after liver transplantation: a case report. Onco Targets Ther. 2018;11:7345–7352. doi:10.2147/OTT.S166962
30. Hosseinzadeh F, Ai J, Ebrahimi-Barough S, et al. Natural killer cell expansion with autologous feeder layer and Anti-CD3 antibody for immune cell therapy of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2019;20(12):3797–3803. doi:10.31557/APJCP.2019.20.12.3797
31. Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7(1):e30264. doi:10.1371/journal.pone.0030264
32. Somanchi SS, Lee DA. Ex vivo expansion of human NK cells using K562 engineered to express membrane bound IL21. Methods Mol Biol. 2016;1441:175–193.
33. Baek H-J, Kim J-S, Yoon M, et al. Ex vivo expansion of natural killer cells using cryopreserved irradiated feeder cells. Anticancer Res. 2013;33(5):2011–2019.
34. Ojo EO, Sharma AA, Liu R, et al. Membrane bound IL-21 based NK cell feeder cells drive robust expansion and metabolic activation of NK cells. Sci Rep. 2019;9(1):14916. doi:10.1038/s41598-019-51287-6
35. Wang X, Lee DA, Wang Y, et al. Membrane-bound interleukin-21 and CD137 ligand induce functional human natural killer cells from peripheral blood mononuclear cells through STAT-3 activation. Clin Exp Immunol. 2013;172(1):104–112. doi:10.1111/cei.12034
36. Streltsova MA, Erokhina SA, Kanevskiy LM, et al. Recurrent stimulation of natural killer cell clones with K562 expressing membrane-bound interleukin-21 affects their phenotype, interferon-gamma production, and lifespan. Int J Mol Sci. 2019;20(2):443. doi:10.3390/ijms20020443
37. Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. doi:10.1158/0008-5472.CAN-08-3712
38. Ciurea SO, Schafer JR, Bassett R, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130(16):1857–1868. doi:10.1182/blood-2017-05-785659
39. Rochigneux P, Chanez B, De Rauglaudre B, Mitry E, Chabannon C, Gilabert M. Adoptive cell therapy in hepatocellular carcinoma: biological rationale and first results in early phase clinical trials. Cancers. 2021;13(2):271. doi:10.3390/cancers13020271
40. Shi D, Shi Y, Kaseb AO, et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin Cancer Res. 2020;26(15):3979–3989. doi:10.1158/1078-0432.CCR-19-3259
41. Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15(6):388–400. doi:10.1038/nri3839
42. Kashii Y, Giorda R, Herberman RB, et al. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J Immunol. 1999;163(10):5358–5366.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.