Back to Journals » Research and Reports in Urology » Volume 15
Progressing Towards Same-Day Discharges After Robotic-Assisted Radical Prostatectomy; Safe and Cost Effective to Discharge Without Routine Blood Tests
Authors Chislett B, Omran G, Harvey M, Bolton D, Lawrentschuk N
Received 8 August 2023
Accepted for publication 28 September 2023
Published 9 October 2023 Volume 2023:15 Pages 471—477
DOI https://doi.org/10.2147/RRU.S429819
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Bodie Chislett,1,2 Ghadir Omran,1,2 Michael Harvey,1,2 Damien Bolton,1 Nathan Lawrentschuk3
1Department of Urology, Austin Health, Heidelberg, Melbourne, VIC, Australia; 2Young Urology Researchers Organisation (YURO), Melbourne, VIC, Australia; 3Department of Urology, Royal Melbourne Hospital, Park Ville, Melbourne, VIC, Australia
Correspondence: Bodie Chislett, Tel +61409171823, Email [email protected]
Introduction: Changing population demographics and the recent SARS-CoV-2 pandemic have forever changed healthcare, with increasing demands on straining systems. The economic cost is yet to be fully realised, with growing concerns around the current system’s ability to accommodate the ageing comorbid population. Consequently, a paradigm shift has taken place in healthcare systems, prioritizing cost accountability. In the absence of established guidelines or robust literature, the use of laboratory tests postoperatively is often guided solely by clinician preference. This study presents a retrospective analysis that investigates the utility and cost implications of routine postoperative investigation following robotic-assisted radical prostatectomies. The findings aim to emphasise the importance of evidence-based practices and cost-effective approaches in postoperative care.
Materials/Methods: A retrospective analysis was performed on all robotic-assisted radical prostatectomies (RARP) identified from a single institution between 29th June 2017 to 28th June 2019. This interval was chosen in an attempt to avoid bias or confounding variables associated with the SRS-CoV-2 pandemic. A single clinician conducted a comprehensive medical record review using unit record numbers corresponding to identified procedural codes. Demographics and variables were recorded, including postoperative test results, hospital length of stay and 30-day readmission rates. Patients were assigned to either ‘Routine Postoperative tests’ (RPOT) or ‘No Routine Postoperative tests’ (No RPOT) and a comparative analysis was performed. Using the Australian National Pharmaceutical Benefits Scheme (PBS) pricing guide, total expenditure was calculated.
Results: A total of 319 patients were included in the study with an average of 2.5 tests per patient within the first 24 hours. Routine postoperative tests had no bearing on outcomes, with comparable readmission rates between cohorts, and a significantly shorter length of stay in the “No routine postoperative tests” group when compared to the “Routine Postoperative Tests”. A total of 1028 tests were performed within the first 48 hours following surgery with expenditure on routine testing totalling $20,516 based on the Australian PBS pricing schedule. Abnormal results were returned on 96% of patients. In the RPOT group, 18 out of the 20 common interventions occurred from 302 RARP. Among the patients in the RPOT group, eight individuals underwent blood transfusions. However, none of these patients met the hospital-specific criteria for transfusion, which require a hemoglobin level below 70 or symptomatic presentation with a hemoglobin level below 80.
Conclusion: The data suggests routine postoperative laboratory has no bearing on re-admission rates, with patients experiencing significantly shorter hospital stays. Furthermore, our results indicate inefficient use of routine postoperative laboratory, with few clinical interventions, frequent abnormal results, and significant accumulative expenses.
Keywords: robotic-assisted radical prostatectomy, minimally invasive, cost-effective, postoperative care, postoperative tests, same-day discharge
Introduction
Changing population demographics will increase global healthcare resource demand in the coming decades. The economic cost of this is yet to be fully realised, with growing concerns around the current system’s ability to accommodate the ageing comorbid population. This concern has only been heightened during the recent COVID-19 pandemic, with global healthcare systems crippled. Consequently, a shift has occurred in healthcare system paradigms, with a renewed emphasis on ensuring cost accountability.
The thoughtless use of routine blood tests is one area requiring further examination, often utilised without consideration or provocation. Despite their small nominal fees, routine bloods have high accumulative costs. In a postoperative setting, routine laboratory tests, or routine blood tests, assess biochemical health, identify complications and aid clinical decision-making. However, routine postoperative tests (RPOT) are often ordered inappropriately at the descretion of clinicians, failing to impact clinical decision-making.1 Historically, RPOT are collected the day following surgery, consisting of a full blood count (FBC) and serum urea, electrolytes and creatinine (UEC) at a minimum. However, as there is a lack of evidence or guidelines to standardise clinical practice, clinical discretion dictates the current use of routine postoperative tests (RPOT) and, therefore, test use and frequency may vary between clinicians.
The necessity and utilisation of RPOT theoretically should be declining, corresponding to the advancements in minimally invasive surgery and enhanced recovery protocols. The introduction of the robotic-assisted radical prostatectomy (RARP) is an example of modern techniques and the advantages they provide, having been shown to reduce intraoperative blood loss, length of stay (LOS) and overall postoperative complication rates.2,3 To date, one previous publications have assessed the utility of RPOT following RARP, but failed to address the safety of abandoning RPOT, following a RARP.4 A comprehensive literature review identified eight other publications evaluating the use of RPOT following minimally invasive arthroplasties, general surgeries, and gynaecological surgeries.1,5–11 All papers observed that investigating low-risk procedures is either over utilised, superfluous, or should be discouraged. Discarding RPOT after a RARP may have broader implications. The same-day discharge (SDD) of patients following RARP has been growing in popularity, an outpatient process that inherently omits RPOT. As more institutions are adopting this pathway, there is great importance in ensuring that waiving RPOT is safe.
The use of routine postoperative investigations needs further exploration, given the lack of supporting literature and potential cost to healthcare services. Herein, we aim to assess the safety of forgoing RPOT in uncomplicated routine RARP by comparing 30-day readmission rates and hospital length of stay (LOS) between the two cohorts. We also hope to draw inference on the utility and cost of RPOT through measuring clinical interventions initiated upon laboratory results and an accumulative cost of individual tests respectfully.
Materials and Methods
A retrospective review was conducted for patients who underwent a robotic-assisted radical prostatectomy at a single Australian institution. Cases were identified using a hospital-specific procedural code (9,263,300) for both primary or salvage RARP. Inclusion criteria were set as patients that underwent RARP identified during two years from 29th June 2017 to 28th June 2019, with the former coinciding with the introduction of procedural codes. This period was chosen to avoid confounders created by the SARS-CoV-19 pandemic. A single clinician conducted a comprehensive medical record review using unit record numbers corresponding to identified procedural codes. Demographics and variables were recorded, including postoperative test results, hospital length of stay and 30-day readmission rates.
Patients were assigned to either the routine postoperative tests (RPOT) or no routine postoperative tests (No RPOT) groups. RPOT were defined as laboratory test results received on file in the 24 hours following surgery derived from whole blood or serum analysis, such as full blood examinations (FBE). Fluid analysis from other sources, such as drain tube fluid, and tests historically ordered in response to either signs or symptoms, such as troponin, were not considered routine and therefore excluded. Groups were compared for re-admission rates and hospital length of stay. Patients with documented evidence as to the indication for postoperative laboratory testing or LOS greater than 4 days were excluded from the analysis.
Total laboratory test use was tallied for the 24-hour and 48-hour periods following surgery. Using the “Pharmaceutical Benefits Scheme” pricing code, the cumulative cost of RPOT was estimated. The frequency of clinical interventions predicated on laboratory test results was assessed using a combination of medical record review and additional hospital-specific procedural codes. Interventions were predetermined prior by a group of senior clinical staff. Procedural codes were used to identify the following interventions: packed red blood cells (1,370,602), platelets (1,370,603) and hyperkalaemia fluid bolus (E875). Medical records were reviewed for antibiotics, oral potassium, or other electrolyte supplementation with no existing procedural codes. Antibiotic use before initial laboratory results were excluded from postoperative interventions, deemed prophylactic instead of responsive medicine. Postoperative laboratory tests were also reviewed for abnormal values based on the hospital’s normal range (Figure 1).
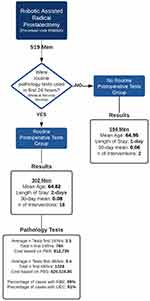 |
Figure 1 Flow diagram of methodology and results summary. |
This retrospective quality assessment publication strictly adheres to the Declaration of Helsinki’s ethical guidelines. The research protocol and data collection procedures received approval from the appropriate ethical review board. As the study is retrospective, obtaining individual patient informed consent was not possible, nor necessary as deemed by the review board. However, patient data was handled confidentially, and identifiers were removed to ensure anonymity. Data security measures were strictly implemented, with restricted access for authorized personnel, and electronic data stored securely.
Results
A total of 519 cases were identified, all conducted by 19 surgeons between 29th June 2017 to 28th June 2019. Revision procedures were included in the study, though none were identified as radical procedures. A total of 23 patients were excluded from the analysis, having either a documented complication or a long stay (greater than 4 days). The majority of these cases were Clavien-Dindo 2–3, with 3 cases having Clavien-Dindo classification 4. Postoperative care was managed in conjunction with consultant-led perioperative medical teams. The “RPOT” group had 302 patients, while 194 were assigned to the “no-postoperative investigation” group. The mean age of both groups was 64 years, with both cohorts having an average of Charlson Comorbidity Index of 2. All men in the No RPOT group were considered uncomplicated, having no documentation of postoperative complications or any laboratory blood test results on file.
A proportions test was conducted to test whether the re-admission rates differ between the investigated and non-investigated patients (Figure 2). With a p-value > 0.05, we cannot reject the null hypothesis that there is no difference between the two groups in terms of 30-day readmission rates. The two groups have similar re-admission rates (Table 1).
 |
Table 1 30-Day Readmission Rate |
 |
Figure 2 Proportions test for re-admission rates between RPOT and No RPOT groups. |
Patient length of stay was analysed using a time-to-event approach, with discharge from the hospital being the “event”. The diagram below is a Kaplan Meier (KM) analysis chart representing the length of stay in the hospital for the “RPOT” and “No RPOT” groups (Figure 3). We used a KM curve to determine each group’s trajectory to determine if an appreciable difference in length of stay between the two groups existed. With a p-value < 0.05 we can reject the null hypothesis that both groups’ length of stay in hospital is the same. The results suggest the “No RPOT” group stayed in hospital less time than the “RPOT” group, with a median length of stay of 1 and 2 days respectively.
 |
Figure 3 Length of stay comparison between RPOT and No RPOT. |
With one intervention assigned per episode, 20 interventions were recorded (3.8%). The majority of interventions occurred in the RPOT group, with 18 out of 20 at a rate of 1 in 17 cases. Eight patients (2.6%) in the “RPOT” group underwent blood transfusions, with none meeting the hospital-specific transfusion criteria of haemoglobin less than 70 (mg/dL) or symptomatic with haemoglobin less than 80 (mg/dL). A review of operative notes revealed no patients were documented to have blood loss greater than 1000mL, which would be an indication for postoperative investigation. No patients underwent blood transfusions in the “No RPOT” group, and no patient in the study at any time underwent a platelet transfusion. One episode of potassium supplementation was recorded in the “RPOT” group. Fourteen patients (4.6%) were administered antibiotics in the days following surgery, with 12 of them occurring following the return of abnormal blood results.
A cumulative tally recorded for all postoperative investigations showed a total of 760 tests were ordered within the first 24 hours, with an average of 2.5 per patient. Cost analysis was performed using the “Pharmaceutical Benefits Scheme” pricing schedule. The overall cost for routine investigations following all procedures totalled $12,739, equating to $42 per patient. This number jumped to $68 per patient over the first 48 hours. A total of 290 cases (96%) returned abnormal results in one or more parameters. Full blood examinations were the most frequently ordered test, with 99% of RPOT cases receiving at least one. Urea, electrolytes, and creatinine was the second most frequently ordered test (91% of RPOT cases).
Discussion
Continued surgical advancements have transformed once-invasive procedures into minimally invasive. Such developments ought to correspond with shorter hospital admissions, fewer complications, and faster recoveries. It follows that clinicians should have less need for routine blood tests following such surgeries, theoretically translating into fewer tests ordered, though without evidence to support this hypothesis, it remains speculative. To affect change in imbued clinical practices, evidence must be provided to support the notion that RPOT is unnecessary in many circumstances postoperatively.
With a comparable 30-day readmission rate, we observe that routine postoperative laboratory tests have no bearing on patient outcomes following RARP. While overall complication rates were not assessed, previous publications estimate it to occur in approximately 5% of cases.2,12 Furthermore, it appears that deliberately omitting RPOT following RARP is an established practice by some clinicians, with no patient within the No RPOT group having a postoperative test during their admission. With comparable 30-day re-admission rates, overall low complication rates, and a clinically established practice for safely discharging patients without RPOT following RARP, we suggest forgoing routine laboratory use in healthy men following an uncomplicated procedure. Patients should be selected by experienced clinicians, as is indicative of our methodology. While these suggestions may already reflect some clinicians’ and institutions’ established practice, we believe this is the first published data to assess the safety of waiving blood tests following RARP.
Expectantly, we observed a shorter median length of stay in the “No RPOT” group compared with the “RPOT” Group, with 1 and 2 days, respectively. These results are consistent with previous works, including Xia et al, who reported LOS following RARP to be 1–2 days in most institutions.12 Multiple contributing factors dictate inpatient length of stay following surgery, with the use of postoperative investigations unlikely to impact significantly. Rather, a noncausal relationship between LOS and postoperative tests should be expected, as both are indirect indicators of a patient’s health, including comorbidities. Regardless, interesting inferences can be made from inpatient LOS. Primarily, the correlation between No RPOT and LOS suggests clinicians are adept at identifying patients suitable for accelerated discharge, a key element in SDD pathways many are implementing.
SDD RARP is garnering popularity, with more institutions adopting the outpatient framework.13–16 There are a number of barriers to performing SDD for RARP, with the primary concern being reliable safety netting and analgesia. With ensured analgesia and established evidence showing clinical observation is unwarranted in many cases, SDD pathways would gain greater support. Correct identification of suitable cases is paramount to SDD RARP success, as not all cases are appropriate. Our results show clinicians are skilled at identifying suitable patients for accelerated pathways, and patients may be safely discharged without postoperative tests, an integral part of clinical observation. Our findings, therefore, support the sustainability and safety of SDD RARP, though they fall short of suggesting clinical observation can be safely abandoned following RARP.
In addition to not affecting outcomes for RARP, we believe RPOT are unnecessary given the small number of clinical interventions in comparison to the abnormal results, with 96% of test panels returning one or more negative results. This suggests very few abnormal RPOT lead to actionable intervention. Our findings are consistent with previous investigations into RPOT following joint arthroplasty and female pelvic surgery identified during our literature review, which contends RPOT are unnecessary and should be discouraged.10–16 While low singular fees apply to each test, our data highlights how accumulative totals of laboratory tests have a significant financial impact on healthcare expenditure. Significant savings could be made on overall expenditure ($39 per patient within the first 24hrs). Additionally, in the setting of RARP, many biochemical markers assessed on FBE and UEC are unnecessary, with alternative tests such as haemoglobin underutilised that incur significantly fewer costs. While curbing clinician practice habits may be difficult, with potentially significant financial windfalls, this is an area that hospital administrations should explore.
While our data is insightful to the utility of postoperative laboratory tests, there are several inherent limitations and biases. No adjustments were made for confounding variables, including comorbidities, when assessing the patient length of stay and re-admission rates. Further, due to inconsistencies in documentation, adjustments for other potential confounders were not made. However, at a minimum, the data suggests RPOT can be safely forgone in otherwise healthy patients (those without comorbidities) following uncomplicated procedures. Further limitations arose during the data collection process, with written documentation often eligible, limited, or lacking clinical rationale as to the role of routine laboratory. Poor documentation likely leads to an underestimation of complications. Further, as clinicians were not blinded during the medical record review, this study is vulnerable to bias.
Conclusion
With continued advances in minimally invasive surgery and enhanced postoperative recovery, foreseeable surgeries traditionally requiring close patient observation and routine investigations can be foregone. We have added to the growing body of literature suggesting that postoperative laboratory testing following routine surgery is unwarranted and can be safely abandoned, the first paper to assess after robotic-assisted radical prostatectomies. Potentially large windfalls can be experienced by a modern system needing cost efficiency. However, strong evidence will be needed to adjust the historical clinical practice of ordering routine laboratory tests following surgery.
Disclosure
Professor Nathan Lawrentschuk is an instructor at Device Technologies, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. X-D W, Xiao P-C, Zhu Z-L, Liu J-C, Y-J L, Huang W. The necessity of routine postoperative laboratory tests in enhanced recovery after surgery for primary Hip and knee arthroplasty: a retrospective cohort study protocol. Medicine. 2019;98(18):e15513. doi:10.1097/MD.0000000000015513
2. Ilic D, Evans SM, Allan CA, Jung JH, Murphy D, Frydenberg M. Laparoscopic and robot-assisted vs open radical prostatectomy for the treatment of localized prostate cancer: a Cochrane systematic review. BJU Int. 2018;121(6):845–853. doi:10.1111/bju.14062
3. Cao L, Yang Z, Qi L, Chen M. Robot-assisted and laparoscopic vs open radical prostatectomy in clinically localized prostate cancer: perioperative, functional, and oncological outcomes: a Systematic review and meta-analysis. Medicine. 2019;98(22):e15770. doi:10.1097/MD.0000000000015770
4. Mottet N, Bellmunt J, Briers E, et al. Guidelines on Prostate Cancer. Europ Assoc Urol. 2017;53:1–137.
5. Lin J-M, Cao Z-Y, Peng A-F, et al. Are routine postoperative laboratory tests really necessary after lumbar spinal surgery? World Neurosurg. 2019;124:e748–e754. doi:10.1016/j.wneu.2018.12.214
6. Paynter JW, Raley JA, Kyrkos JG, et al. Routine postoperative laboratory tests are unnecessary after primary reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2020;29(8):1656–1664. doi:10.1016/j.jse.2019.12.023
7. Gilde AK, Downes KL, Leverett S, Miranda MA. Routine postoperative complete blood counts are not necessary after most primary total hip and knee arthroplasties. J Arthroplasty. 2021;36(4):1257–1261. doi:10.1016/j.arth.2020.10.047
8. Shaner JL, Karim AR, Casper DS, Ball CJ, Padegimas EM, Lonner JH. Routine postoperative laboratory tests are unnecessary after partial knee arthroplasty. J Arthroplasty. 2016;31(12):2764–2767. doi:10.1016/j.arth.2016.05.052
9. Howell EP, Kildow BJ, Karas V, et al. Clinical impact of routine complete blood counts following total knee arthroplasty. J Arthroplasty. 2019;34(7):S168–72. doi:10.1016/j.arth.2019.03.016
10. Sears S, Mangel J, Adedayo P, Mims J, Sundaresh S, Sheyn D. Utility of preoperative laboratory evaluation in low-risk patients undergoing hysterectomy for benign indications. Eur J Obstet Gynecol Reprod Biol. 2020;248:144–149. doi:10.1016/j.ejogrb.2020.03.041
11. Murphy AM, Tunitsky-Bitton E, Krlin RM, Barber MD, Goldman HB. Utility of postoperative laboratory studies after female pelvic reconstructive surgery. Am J Obstet Gynecol. 2013;209(4):
12. Xia L, Taylor BL, Pulido JE, Mucksavage P, Lee DI, Guzzo TJ. Predischarge predictors of readmissions and postdischarge complications in robot-assisted radical prostatectomy. J Endourol. 2017;31(9):864–871. doi:10.1089/end.2017.0293
13. Ploussard G, Dumonceau O, Thomas L, et al. Multi-institutional assessment of routine same day discharge surgery for robot-assisted radical prostatectomy. J Urol. 2020;204(5):956–961. doi:10.1097/JU.0000000000001129
14. Wolboldt M, Saltzman B, Tenbrink P, Shahrour K, Jain S. Same-day discharge for patients undergoing robot-assisted laparoscopic radical prostatectomy is safe and feasible: results of a pilot study. J Endourol. 2016;30(12):1296–1300. doi:10.1089/end.2016.0552
15. Abaza R, Martinez O, Ferroni MC, Bsatee A, Gerhard RS. Same day discharge after robotic radical prostatectomy. J Urol. 2019;202(5):959–963. doi:10.1097/JU.0000000000000353
16. Reddy S, Noel J, Moschovas M, et al. Same day discharge protocol for robotic assisted radical prostatectomy: the experience of a high-volume referral center. J Endourol. 2022;36:934–940. doi:10.1089/end.2021.0730
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
