Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Progress in the Development of Stem Cell-Derived Cell-Free Therapies for Skin Aging
Authors Chou Y , Alfarafisa NM , Ikezawa M, Khairani AF
Received 8 August 2023
Accepted for publication 7 November 2023
Published 22 November 2023 Volume 2023:16 Pages 3383—3406
DOI https://doi.org/10.2147/CCID.S434439
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Anne-Claire Fougerousse
Yoan Chou,1 Nayla Majeda Alfarafisa,2 Maiko Ikezawa,3 Astrid Feinisa Khairani1,2
1Graduate School of Master Program in Anti Aging and Aesthetic Medicine, Faculty of Medicine, Universitas Padjadjaran, Jatinangor, West Java, Indonesia; 2Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jatinangor, West Java, Indonesia; 3Department of Anatomy and Cell Biology, Graduate School of Medicine, Gunma University, Gunma, Japan
Correspondence: Astrid Feinisa Khairani, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jalan Raya Bandung – Sumedang Km 21, Jatinangor, West Java, 45363, Indonesia, Tel +62-22-7795594, Email [email protected]
Introduction: The skin is a vital organ as the body’s largest barrier, but its function declines with aging. Therefore, research into effective regeneration treatments must continue to advance. Stem cell transplantation, a cell-based therapy, has become a popular skin-aging treatment, although it comes with drawbacks like host immune reactions. Stem cell-derived cell-free therapies have emerged as an alternative, backed by promising preclinical findings. Stem cell secretomes and extracellular vesicles (EVs) are the key components in cell-free therapy from stem cells. However, comprehensive reviews on the mechanisms of such treatments for skin aging are still limited.
Purpose: This review discusses stem cell-derived cell-free therapy’s potential mechanisms of action related to skin aging prevention by identifying specific molecular targets suitable for the interventions.
Methods: A search identified 27 relevant in vitro studies on stem cell-derived cell-free therapy interventions in skin aging model cells without restricting publication years using PubMed, Scopus, and Google Scholar.
Results: Stem cell-derived cell-free therapy can prevent skin aging through various mechanisms, such as (1) involvement of multiple regenerative pathways [NFkb, AP-1, MAPK, P-AKT, NRF2, SIRT-1]; (2) oxidative stress regulation [by reducing oxidants (HO-1, NQO1) and enhancing antioxidants (SOD1, CAT, GP, FRAP)]; (3) preventing ECM degradation [by increasing elastin, collagen, HA, TIMP, and reducing MMP]; (4) regulating cell activity [by reducing cell senescence (SA-β-gal), apoptosis, and cell cycle arrest (P53, P12, P16); and enhancing autophagy, cell migration, and cell proliferation (Ki67)] (5) Regulating the inflammatory pathway [by reducing IL-6, IL-1, TNF-⍺, and increasing TGF-β]. Several clinical trials have also revealed improvements in wrinkles, elasticity, hydration, pores, and pigmentation.
Conclusion: Stem cell-derived cell-free therapy is a potential novel treatment for skin aging by cell rejuvenation through various molecular mechanisms.
Keywords: cell-free therapy, extracellular vesicles, secretome, skin aging, stem cell
Graphical Abstract:
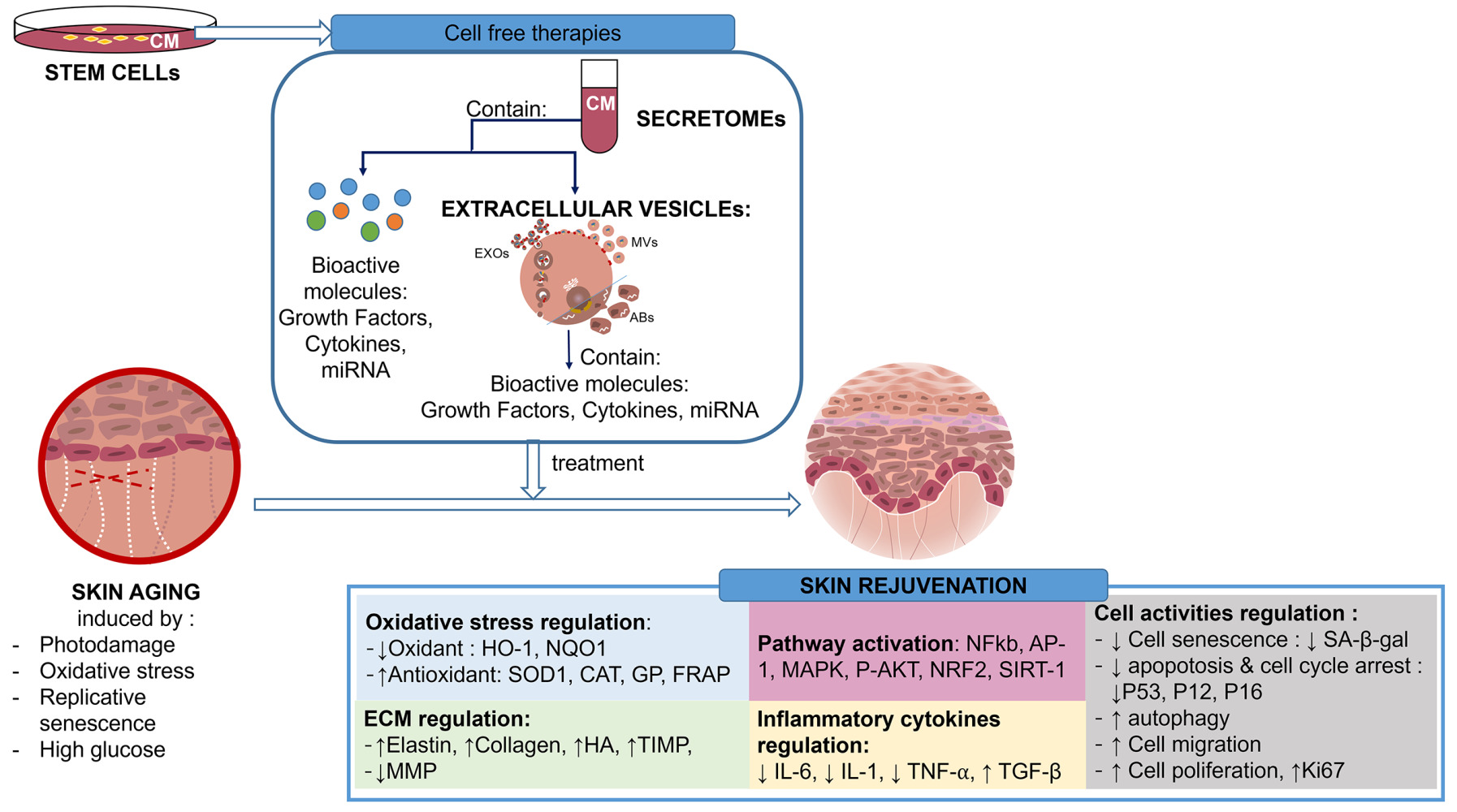
Introduction
Mesenchymal stem cells (MSCs) are multipotent cells that exist in almost all tissues, including bone marrow, adipose tissue, human umbilical cord blood, muscle, and synovium.1 MSCs have been widely researched and used in regenerative medicine to reconstruct various tissues, including those related to the musculoskeletal system, nervous system, myocardium, liver, cornea, trachea, and skin.1 Although numerous preclinical and clinical studies have confirmed the safety of MSC-related therapies, there is still concern that using MSCs in clinical settings may carry some risks. The main risks include fibrosis, inflammation, and tumorigenicity.1 Cell retention and low cell survival after transplantation are also observed in MSC therapy.2
Stem cell-derived cell-free therapy, referred to as cell-free therapy, uses the secreted products of cells to stimulate the regulation of many biological processes, including leading endogenous and progenitor cells to sites of injury, mediating apoptosis, proliferation, migration, and angiogenesis.3 Unlike conventional stem cell-based therapies, cell-free therapies in regenerative medicine provide significant benefits, including reduced immune rejection, tumorigenicity, and pathogen transmission.3
Some materials often used in cell-free therapies are secretomes and extracellular vesicles (EVs).4,5 There is a previous review by Damayanti et al that discussed the advantages and biological activity of mesenchymal stem cell secretomes on the skin for applications such as wound healing, photoprotection, promotion of hair growth, psoriasis treatment, and other antimicrobial applications.2 Other studies have also proven that cell-free therapy can be used as a skin-aging therapy.6
Skin is a substantial body organ and a protective barrier against the external environment. As we age, every human organ’s performance gradually deteriorates, including the skin. Age-related structural changes in the skin include thinning, loss of elasticity, dryness, wrinkles, and changes in pigmentation.7 The skin dermis loses thickness as well, which explains why the skin has the appearance of being paper-thin.8,9 On the cellular level, skin aging can cause flattening of the dermo-epidermal junction, decrease melanocyte production, and lower immunological response due to a significant decline in Langerhans cells.10 The complicated biological process of skin aging is controlled by several variables, including genetics and environmental factors such as UV radiation from sun exposure or polluted air.11
Cell-free therapy protects the skin from aging by increasing cell regeneration and reducing the damaging effects of the sun’s free radicals and ultraviolet (UV) rays on the skin.6 Various substances or ingredients that slow skin aging include antioxidants, herbs, botulinum toxin, hyaluronic acid, and others. However, using those substances has some limitations, such as a lack of product standardization, effectiveness for long-term use, side effects, and others.12–14 Research is still needed to find an ideal novel therapy for skin aging, such as cell-free therapy.
This review will focus on in vitro studies and explore how the molecular mechanism of cell-free therapy, both secretomes and EVs, works on skin aging. We also discuss the potential of cell-free therapy in clinical settings to bridge the gap from laboratory research to clinical applications, which may bolster the idea of cell-free therapy as a novel therapeutic option for delaying the skin aging process. Understanding cell-free therapies is critical for developing safe and effective regenerative medicine treatments.
Methods
Our narrative review’s primary focus was on in vitro studies that explored interventions related to skin aging, guided by the PICO framework: population (skin cells within an aging model), intervention (secretomes or extracellular vesicles derived from stem cells), comparison (control group), and outcome (effects related to aging prevention or rejuvenation). We included original research conducted in the English language within the context of aging models involving skin cells (fibroblasts, keratinocytes, and melanocytes) without imposing any restrictions on publication years. We excluded articles related to reviews, wound healing research, and interventions with conditioned medium therapies from non-stem cell sources.
We conducted a search across PubMed, Scopus, and Google Scholar, employing keywords such as “secretome stem cells for skin aging”, “extracellular vesicle stem cells for skin aging”, “exosomes stem cells for skin aging”, “microvesicles for skin aging”, and “stem cell conditioned medium for skin aging”. Initially, this search yielded 373 articles, which were subsequently reduced to 204 after eliminating duplicate entries. Through abstract screening, we identified 101 eligible articles. They were all carefully reviewed and evaluated. This process led to the selection of 27 relevant in vitro studies (Figure 1), which are summarized in Tables 1 and 2
 |
Figure 1 Literature search process. |
 |
Table 1 Secretome Therapy for Skin Aging |
 |
Table 2 Extracellular Vesicles Therapy for Skin Aging |
Skin Aging
A complex combination of extrinsic and intrinsic factors causes skin aging.42 Whether intrinsic or extrinsic, aging impacts collagen and elastin in the dermis, both qualitatively and quantitatively.9 Coarse wrinkles, a rough texture, pallor, uneven pigmentation, and a loss of skin elasticity are clinical signs of extrinsically aging skin.7 This aging process can occasionally deviate from chronological age. Meanwhile, intrinsic skin aging can appear as a loss of elasticity and uniform pigmentation associated with age.42 Skin aging generally results in morphological changes that cause aesthetic dissatisfaction, structural changes for the skin as the body’s largest mechanical barrier, and physiological changes such as decreased Vitamin D metabolism, immune regulation, reduced absorption of percutaneous drugs, UV protection, and others.8,11,42
Extrinsic aging is a superposition of intrinsic aging, which means that extrinsic aging occurs concurrently with intrinsic aging and is an aging mechanism that aggravates existing inherent aging processes.8 Intrinsic skin aging is a natural process influenced by genetics, race, metabolism, and hormones.11 The inherent aging process advances gradually. The process underpinning intrinsic skin aging is the same as the mechanism underlying cell aging. These processes include long-term oxidative stress buildup, telomere shortening, cellular senescence, and disruption of protein homeostasis, which results in the accumulation of damaged proteins and impaired cell function.8,11 Oxidative stress raises hypoxia-inducible factors (HIFs) and NFκB levels. HIFs then influence the expression of genes that regulate cellular metabolism.8 Both HIFs and NFκB promote the expression of proinflammatory cytokines such as interleukin (IL)-1 and IL-6, vascular endothelial growth factor (VEGF), and tumor necrosis factor (TNF)-α.8 Skin atrophy, disruption of the dermal ECM, and flattening of the dermal-epidermal junction (DEJ) characterized elderly skin.11
UV rays, particularly medium-wavelength ultraviolet radiation (UVB, 280–320nm) and long-wavelength ultraviolet radiation (UVA, 320–400 nm), are the main cause of extrinsic skin aging, also called photoaging.8 UV rays cause sunburn, erythema, hyperpigmentation, and photocarcinogenesis.8 UVB rays are restricted to the skin’s superficial epidermal layer and cause direct damage, resulting in cell senescence, apoptosis, or carcinogenesis.8,11 UVB radiation primarily affects keratinocytes and promotes Langerhans cell depletion.11 It also causes DNA mutations and stimulates immunosuppression by releasing cytokines such as TNF- and IL-10.11 UVA rays penetrate the dermis deeper and produce reactive oxygen species (ROS), which cause biological changes in DNA, membrane lipids, mitochondria, and proteins.8,11 In the dermis, UVA induces microtears of collagen fibers, aggravating hyperpigmentation caused by UVB rays, and can act as a photo-sensitizer (Figure 2).7,10,42,43
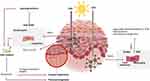 |
Figure 2 Photoaging of the skin is mainly caused by UV radiation. UVB rays are restricted to the skin’s superficial epidermal layer and cause direct damage. UVA rays penetrate deeper into the dermis.7,10,42,43 |
Free radicals induced by UV radiation set off a chain of molecular reactions and release enzymes that break down connective tissue, giving the skin a rough texture.8,11 ROS plays an essential role in mediating cell damage and apoptosis.6,32 UV-induced skin photoaging is correlated with activation protein 1 (AP-1) and nuclear factor kappa B (NF-κB) activation.11,15 It reduces collagen expression while increasing the expression of matrix metalloproteinases (MMPs), particularly MMP-1, MMP-3, and MMP-9, resulting in increased degradation of matrix proteins and the formation of wrinkles.8,11,44 The primary role of collagen types 1 and 3 in the skin is to support the tensile strength of the dermis and minimize wrinkles.16 Photoaging, instead of intrinsic aging, demonstrated a considerable drop in type 1 collagen rather than type 3 collagen.9
Another molecular mechanism in extrinsic aging is the presence of membrane and nuclear signaling.8 The synthesis of CYR61 contributes to skin aging since it causes an increase in MMP-1 and TGF-b receptors, eventually disrupting collagen transcription. ROS production stimulates the transcription of tropoelastin, a component of mature elastic fibers.8 Fibulins 2 and 5, as well as fibrillin-1, components of the dermal elastic fiber’s microfibrillar fraction, are also increased. That mechanism results in the disruption of extracellular membrane structures, such as elastic fibers and basement membranes.8 Mitochondrial dysfunction and neutrophil infiltration also play a role in skin aging (Figure 3).8,11
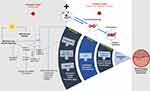 |
Figure 3 Mechanisms of extrinsic and intrinsic skin aging. Extrinsic aging occurs with intrinsic aging and is an aging mechanism that exacerbates existing aging processes. Aging is associated with AP-1 and NF-kB activation, which reduce collagen expression while increasing MMPs. CYR61 production accelerates skin aging by disrupting collagen transcription. The decline of cell function is also affected by oxidative stress, telomere shortening, senescence, and protein disruption.8,11 |
Skin Aging In vitro Research Model
In general, there are several types of cell senescence, such as epigenetically induced senescence, oxidative stress-induced senescence, chemotherapy-induced senescence, mitochondrial dysfunction-associated senescence, DNA damage-induced senescence, oncogene-induced senescence, and replicative senescence.45,46 Researchers attempt to simulate this process in vitro using various induction or treatment methods, such as (1) ultraviolet radiation as a photoaging model,15,17–21 (2) administration of H2O2 or tbOH as an oxidative stress-induced senescence model,22,23 (3) passaging as a replicative senescence model that describes natural aging,23 and (4) high glucose medium as an aging model due to hyperglycemia condition.46 We observed that the photoaging model is one of the most common methods used in in vitro studies of skin aging.
In vitro research on skin aging usually uses cell lines from fibroblasts (HDF, HFF, NDHF, CCD-986Sk, 3T3-A31) or keratinocytes (HaCaT) to simulate the skin (Tables 1 and 2). The cells can be cultured in 2D and 3D (two- and three-dimensional) models.47 Fibroblasts, the most abundant cell type in the dermis, are used to evaluate the features of skin aging in the dermis.48 Keratinocytes are used to evaluate epidermal homeostasis.48 Keratinocytes act as a barrier against environmental harm by regulating oxidative stress, glucose metabolism, and inflammatory mediators.32 Along with keratinocytes, the epidermis is the location of a structured network of melanocytes, which produce pigments that protect skin against ultraviolet radiation.49 Although uncommon, melanocyte cells are used in skin aging research to study the effects of hyperpigmentation caused by skin aging.24,50 Initially, 2D culture methods were frequently utilized to research mammalian cells.47 In vitro studies have recently begun extensively using the 3D culture method because it can provide an overview of cell interactions more similar to those in the in vivo environment.47,48
There are some manifestations of cell senescence, such as Increased lysosomal content as marked by the activity of the senescence-associated beta-galactosidase (SA-β-gal), escalation of DNA damage, overexpression of the cell cycle regulators and cyclin-dependent kinase inhibitors (p16 and p21), overexpression of SASP (senescence-associated secretory phenotypes), morphology changes, and apoptosis resistance.33,45,46,51,52
Biological Features of MSC-Free Therapy
Cell-free therapy has several advantages compared to cell-based therapy, including low tumorigenicity, the ability to treat diseases with minimal side effects, emergency suitability, ease of storage, and low immunogenicity.3,6,53 Secretomes have several advantages over stem cells because medium can be manufactured and transported more easily, so it is appropriate for emergencies because it can be prepared in large quantities to save cost and time while still being available for treatment when needed.3 Furthermore, because it is cell-free, it has a low risk of getting recipient rejection reaction after the treatment (low immunogenicity).3,6
Secretomes contain various soluble factors consisting of cytokines and growth factors such as (1) FGF7, IL-6, IL-11, TGF, and PDGF that are important in the wound healing process; (2) PDGF, VEGF, HGF, and M-CSF that are important for hair growth; (3) laminins and TGFβ1 that contribute for epithelization; (4) FGF-4, FGF-6, and FGF-7/KGF which are known to induce fibroblast and keratinocyte proliferation; (5) EGF, TGFα, PDGF-AA, PDGF-AB, M-CSF, and PLGF which are known to promote epithelial cell proliferation and migration; (6) ang-1, VEGF, PLGF, IGF-1 and HGF that are important for angiogenesis; (7) IGFBP that are known for inducing cells chemotaxis and proliferation; (8) TIMP-1 and TIMP-2, which are known to inhibit ECM degradation. Due to its essential bioactive ingredients, secretomes may become a potential cell-free therapy for skin aging.25,26
Secretomes can be isolated from various types of stem cells, such as bone marrow, adipose-derived, epidermal progenitor, dermal progenitor, and extra-embryonic perinatal (placenta, fetal membrane).54 Stem cells secrete secretomes into the medium, which is also called conditioned medium (CM).3 Soluble factors and vesicles secreted by stem cells can act directly by mediating intracellular pathways in injured cells or indirectly by inducing the secretion of functionally active products from nearby tissues (Table 3).3 These soluble factors contain many bioactive molecules that can enter the dermis through the micro-pores, activating fibroblasts, stimulating collagen production and remodeling, and promoting skin regeneration. Therefore, secretomes can be used as a cell-free therapy for skin aging.6
 |
Table 3 The Comparison Between Secretomes and Extracellular Vesicles |
EVs are particles that are naturally released from the cell (Table 3). They cannot replicate, as they lack a functional nucleus.55 All cells, including MSCs, can secrete various types of EVs. EVs can be found in almost every biological fluid, including plasma, serum, saliva, amniotic fluid, breast milk, urine, and even human skin.58,59 EVs are thought to play a role in regulating skin homeostasis during aging.59 EVs can also be isolated and characterized from conditioned media.55 EVs are collected from conditioned media via the ultracentrifugation method.55 EVs also acted as cell-free therapies via bioactive molecules.55
EVs play an important role in cell-to-cell communication because of their ability to transport nucleic acids (including mRNAs and miRNAs), proteins, and lipids between cells.57 Researchers usually categorize EVs into different types based on size, biogenesis, and functions: exosomes, microvesicles, apoptotic bodies, ectosomes, and oncosomes.34,58 Microvesicles are more commonly used to describe medium-to-large EVs (150 to 1000 nm).56 Exosomes (Exo) are small EVs with a 30–150 nm size range. It is important to note that reports somewhat vary on vesicle size classification (Table 3).56
Exosomes, also known as small extracellular vesicles (sEV), are one of the vital secretory products of MSCs with a diameter that ranges from 30–150 nm.4,56 According to another study, exosomes have a diameter of less than 100 nm with a density of 1.10–1.18 g/mL.60 When observed through an electron microscope, exosomes have a typical “disk-like” structure and flat spherical shape.35,36 EVs develop from larger intracellular vesicles known as multivesicular bodies (MVBs). MVBs are intraluminal vesicles (ILVs) formed by endosomal membrane internal budding.37 Endosomes initially fuse with endocytic vesicles to exocytose their contents and then undergo a series of changes before becoming late endosomes or MVBs. The cargo encapsulated within MVBs is sorted during maturation.60 MVBs migrate to the cell’s perimeter and fuse with the plasma membrane. Exosomes are then exocytosed into the extracellular space.61 These small vesicles contain specific proteins, lipids, and nucleic acids that can be transmitted and act as signaling molecules to change the function of other cells.62 They are also high in protein markers (tetraspanins, TSG101, and Hsp70).43 Exosomes are advantageous in their biological function as cell-free therapy due to their small size, excellent stability, low cytotoxicity and immunogenicity, and target specificity (Table 3).35
Ectosomes/ microvesicles are formed by plasma membrane protrusions that eventually detach and are shed in the extracellular space, with diameters ranging from 50 to 500 nm.59,61 Apoptotic bodies are a byproduct of apoptosis and contain biomaterial from the dying cell. Their sizes range from 50 to 5000 nm, or 1000–5000 nm, and a density between 1.16 and 1.28 g/mL (Table 3).4,37 Apoptotic bodies are made up of partially degraded cellular components. These EVs are essential in cellular homeostasis because they can remove apoptotic cells and provide immunomodulation (Figure 4).4,56
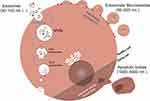 |
Figure 4 Extracellular vesicle (EVs) classification and biogenesis. EVs usually categorize EVs into different types based on size, biogenesis, and functions, namely exosomes, ectosomes/microvesicles, and apoptotic bodies. Exosomes originate from MVBs and are released into the extracellular space through exocytosis. Ectosomes/ microvesicles form through plasma membrane protrusions and detach to enter the extracellular environment. Apoptotic bodies, resulting from cell death, contain biomaterial from the dying cell.4,56 |
EVs have the potential to treat a wide range of skin conditions, including infection, inflammatory skin disease, scarring, alopecia, pigmentation, skin rejuvenation, and skin cancer.63 MSC-derived extracellular vesicles/exosomes exhibit tissue repair effects by modifying gene expression and protein production in recipient cells. They also activate regeneration-related pathways such as Wnt/ß-catenin, AKT, ERK, and STAT3.7 EVs are pretty safe to use as cell-free therapy. This is supported by the EV toxicity test in the study by Ha et al on HDF, 3T3-A31, and RAW 264.7 cells.64 They also proved that exosomes (nano-sized EVs) increased procollagen type 1 and elastin in HDF. Other results showed that exosomes decreased inflammatory cytokines (IL-6, IL-27, and IFN-B) in RAW 265.7 cells and did not induce phototoxicity in 3T3-A31.64 Exosomes also show no skin sensitization, no acute oral toxicity, and no effect on skin irritation in the in vivo model.64
Among the numerous forms of EVs, exosomes are the most prominent.65 MSC-derived exosomes are enriched with critical bioactive molecules that influence cell function, including peptides, enzymes, cytokines, chemokines, growth factors, messenger RNAs (mRNAs), micro RNAs (miRNAs), and long noncoding RNAs (lncRNAs) molecules.66,67 miRNAs perform essential gene-regulatory roles in cell senescence, primarily by inhibiting target mRNA translation, which may be related in the anti-photoaging action of EVs. Non-coding RNA includes tRNA, lncRNA, microRNA, and circRNA.35
Secretome Therapy for Skin Aging
Previous studies have shown the secretomes’ therapeutic effect on skin aging through various mechanisms, such as increasing collagen synthesis through decreasing collagen degradation factors (such as MMPs);15–22,27 increasing MMP inhibiting factor (TIMP);17,22 increasing elastin;17 increasing cell proliferation;21,22,27 decreasing apoptosis;15,19,27 increasing cell migration;16,25 increasing antioxidants;15,22,23 protecting cells from oxidative stress damage;15,22,23,28 and modulating various signaling transduction pathways such as MAPK, AP-1, NF-kB, WNT3A/ β –catenin15,18,23 (Table 1).
As mentioned above, secretomes show potential for skin rejuvenation due to their richness in various bioactive molecules.16,20,26,29 Shu Guo et al found platelet-derived growth factors AA (PDGF-AA) in ADSC-CM, which stimulates ECM protein synthesis and mediates cell proliferation and ECM remodeling.17 TGF-β1, another secretome growth factor, can stimulate collagen synthesis and alleviate collagen degradation. These findings are supported by Xu Yang et al, who used DA-CM in their study.20 Kim et al found UCB-MSC-CM contained a high level of GDF-11 that affects cell proliferation, migration, and ECM production.16
Secretomes can intervene in skin cell aging through various mechanisms. Pathways WNT3A/ β-catenin, NF-κB, and MAPK/AP-1 play a role in aging, and secretomes have been shown to regulate these pathways.15,18,23 Wnt/β-catenin is critical in mediating procollagen type 1 production and skin damage via TGF- β.18 MAPK phosphorylation activates the NF-kB, followed by the transcription factor AP-1 complex.15 Both the AP-1 and NF-kB pathways have been shown to influence the homeostasis of MMPs and heme oxygenase-1 (HO-1) during skin aging.15 Secretomes improve cell damage due to ROS by increasing key antioxidant defense system members such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase.15,22–24
MMPs can degrade almost all ECM components and play an essential role in skin aging. MMP activity is downregulated by secretome therapy.15,19,20,22 Furthermore, secretomes increased the level of TIMP.17,22 UV radiation and oxidative stress exposure contribute to skin aging by activating MMP-1, resulting in collagen degradation and loss of skin elasticity, which can be inhibited by secretomes therapy as marked by an increase in the levels of collagen, elastin, and hyaluronic acid.19,26
Secretomes have also been shown to regulate p16, p21, and p53 levels.30 p16 is a tumor suppressor gene and a senescence marker.45 This gene’s expression has been shown to increase with age.45 When the cell cycle regulator p16 is expressed, cells are arrested in the G1 phase, proliferation stops, and senescence appears.19 Secretomes therapy drastically reduced p16 expression.19,31 The tumor suppressor p53 regulates cell cycle arrest, apoptosis, and cellular senescence.22 The expression of p21 is controlled by p53.45 The secretomes therapy reduced the expression of p53 and p21.22,30,31
Secretomes therapy has been shown to reduce cell senescence populations, as indicated by decreased SA-β-gal expression.20,22 SA-β-gal is an enzyme generated by lysosomes from senescent cells and is often used as a biomarker of aging.45 Secretomes also increased cell proliferation.17,19,21–23 Ki67, a proliferation marker, increased following secretomes therapy.22
Extracellular Vesicle Therapy for Skin Aging
Extracellular vesicles (EVs) produced from mesenchymal stem cells (MSC-EVs) have the potential to be used as “cell-free” therapies since they can improve organ function, promote healing, and reduce inflammation.56 Similar to secretomes, EVs can promote skin rejuvenation by increasing cell proliferation and migration and improving ECM components.33,34,37,38 Previous studies have shown that EVs protect the skin from aging by reducing intracellular ROS production and promoting antioxidant enzyme expression (Table 2).33–36,39–41
Several researchers are attempting to create EVs in better form utilizing various ways to deliver better therapeutic effects, such as EVs loaded with circular RNA (circ_0011129),35 using chitosan (CS) hydrogel,38 or primed with TGFβ.34 The therapeutic effect of EVs depends on the microenvironment, which is proven in research by Vu et al.34 The study discovered that exosomes from TGFβ-stimulated UCMSCs had a greater ability to encourage cell migration. Moreover, total protein elastin levels are associated with TGFβ-stimulated UCMSCs.34 In addition to exosomes, the study discovered that the other EV subpopulations, microvesicles, and apoptotic bodies also have regeneration effects.34 Various growth factors are present in each of the three EV subpopulations.34 The regenerative effects of EVs can have different results depending on the type (EXOs, ABs, or MVs).34 Probably, this is because the dominant component of the growth factor content found in each type of EV is different. For example, the researcher found that ABs have a dominant composition of FGF-2. MVs and EXOs have a dominant composition of HGF.34 Even so, the use of apoptotic bodies as skin aging therapy needs to be explored further.
EV treatment can improve dermal ECM function and thus restore cellular function by increased expression of ECM-related genes such as COL I, COL III, Elastin, HAS II, and HAS III;34 and decreased expression of MMPs;35,37,38,41 (Table 2). Wu Pei et al research shows that EVs may reduce skin redness, scaling, and inflammatory cell infiltration. Their study used exosomes from human cord mesenchymal stem cells (hucMSC).36
Previous studies found that EVs from MSCs have anti-aging effects, most likely due to antioxidant activity. The protective effect of oxidative stress by EVs is due to increased GPX-1 gene expression,41 SIRT1 expression,36 antioxidant enzyme expression (SOD-1 and CAT),40 mTOR/Ps6 inhibition,39 and NRF2 pathway regulation.32 NRF2 plays a crucial role in controlling the expression of antioxidant enzymes after skin damage. SOD, glutathione peroxidase (GSH-PX), and CAT are produced because of a cascade effect caused by the NRF2 pathway in skin injury.32 EVs protected cells from ROS damage under stress conditions by adaptive activation of NRF2.32
Bridging the Gap from Bench to Bed
Cell-free therapy has demonstrated favorable outcomes for intrinsic aging, as shown by studies that show cell-free therapy has positive effects in replicative senescence skin aging models.37,38 One of the primary contributors to premature aging is UV radiation (photoaging). In vitro studies shown in Tables 1 and 2 have consistently indicated that cell-free therapy protects against UV radiation,15,17–20,24,25,27–29,37,40,41 thereby reducing the clinical symptoms of premature aging.
ROS plays a significant role in causing cellular damage.6,8,32 Cell-free therapy has antioxidant properties that prevent cellular damage. Furthermore, it promotes cell proliferation and reduces apoptosis, thereby supporting the normal homeostasis of skin cells. As a result, the skin can retain its normal physiological function.22,26,32,35
Several clinical trials have recently been reported supporting cell-free therapy for skin aging. Clinical trials have shown cell-free therapy can enhance skin texture by improving wrinkles, elasticity, and hydration.68 Wrinkles, elasticity, and hydration are related to the balance of the ECM component. In vitro studies in Tables 1 and 2 show that cell-free therapy can prevent ECM degradation by increasing elastin, collagen, HA, and TIMP and reducing MMP.15–22,27,35,37,38,41 A study by Kim et al revealed that the topical application of USC-CM increased dermal density and reduced skin wrinkles in the CM group, involving 22 participants aged 18–55.16 These results are consistent with their in vitro findings, which demonstrated enhanced collagen type 1 and 3, elastin gene expression, and reduced MMP-1 expression in HDF cells.16 Another study by Sohn et al found that applying a 5% CM formula topically improved skin texture in females aged 29–69 with mild to moderate wrinkles.23 Their study also found that cell-free therapy reduced fibroblast aging by activating MAPK signaling that supports type I collagen production and enhanced antioxidant levels.23
Clinical trials also have demonstrated the efficacy of cell-free therapy pigmentation improvement.68 Pigmentation is closely linked to melanin production.8 In vitro research by Park et al revealed that cell-free therapy exhibits anti-melanogenic properties by reducing MITF, tyrosinase, TRP-1, and TRP-2 levels.24
Another clinical trials have demonstrated that cell-free therapy can improve skin texture by improving pore size.68 This improvement is likely associated with sebum production and sebaceous gland activity, closely linked to hormonal factors and IGF-1.68 In an in vitro study, Balasubramanian et al revealed that cell-free treatment contains various components promoting skin regeneration, including IGF-1, which is essential in migration, proliferation, anti-apoptosis, and angiogenesis.26 However, further research is required to explore the correlation of IGF-1 with clinical improvements in pore size during the aging process.
Studies conducted by Kim et al and Jihee et al have demonstrated that topical application of cell-free therapy over 3–4 weeks resulted in improvements in skin aging signs and erythema, with no reports of severe adverse effects.16,69 The improvement in erythema may be associated with the regulation of inflammatory cytokines. An in vitro study conducted by Wu Pei et al demonstrated that cell-free therapy can downregulate the expression of the inflammatory cytokine TNF-α, which may be linked to the improvement of erythema.36
Aside from topical application,16,23,69 Topical cell-free therapy may be combined with other skin-resurfacing technologies, such as micro-needling and laser treatments, to achieve the desired skin rejuvenation benefits with considerable impact. The increased efficacy is due to the capacity of micro-needling and laser treatments to improve cell-free therapy penetration into the skin, allowing it to optimize its effects.68 Knowing that skin aging can result in psychological stress,10,23 this combination of topical cell-free therapy and skin resurfacing modalities is probably preferable because of the significant outcomes.
A systematic review study by Ting Jung Lin et al, based on clinical trials published from 2014 to 2020, reported that the combination of micro-needling or laser therapy with topical cell-free therapy significantly improved skin aging symptoms, including reduced wrinkles, pigmentation, and pores.68 Furthermore, recent studies published from 2020–2023 also provide similar clinical evidence for using CM-stem cells in skin aging (Table 4).70–72 These studies indicate that the clinical use of cell-free therapy can be considered relatively safe.68,71 Mild side effects, such as redness, edema, petechiae, and pain, may occur, although they are most likely a result of the resurfacing procedures, which may be expected.70,72
 |
Table 4 The Clinical Evidence of Cell-Free Therapy for Skin Aging |
The transdermal distribution of USC-CM by micro-needling, carried out five times at two-week intervals, is safe and produces better results in terms of skin brightness, texture, and patient satisfaction, according to research by Liang et al.71 Another research by Yusharyahya et al indicates improvements in wrinkles when ADSC-CM is administered twice at four-week intervals in patients undergoing micro-needling or fractional laser treatments.70 These studies collectively suggest that the short-term use of cell-free therapy (4 weeks) might already have shown effects, while long-term use (10 weeks) is considered relatively safe.
Although there is currently not much research on EV products for skin aging reasons, clinical data supporting the use of EVs in skin aging therapy is already available. According to research by Park et al, administration of ADSC-Exo solution via micro-needling resulted in improvements in skin wrinkles, elasticity, hydration, and pigmentation.72 These results are consistent with the histological evaluation, which shows improvements in extracellular matrix (ECM) components. In this trial, topical exosome application resulted in temporary erythema, edema, and petechiae that resolved in a week, but no serious adverse effects were observed.72
Conclusion
Our review shows that cell-free therapy has benefits for skin aging. Secretome and EVs (especially exosomes) are cell-free therapies secreted from mesenchymal stem cells. The comparison of characteristics between secretomes and EVs is presented in Table 3. Secretomes and EVs contain many bioactive substances mediating cell-to-cell communication, such as cytokines and growth factors. These bioactive substances play a role in preventing skin aging through various mechanisms, such as (1) involvement of multiple regenerative pathways [NFkb, AP-1, MAPK, P-AKT, NRF2, SIRT-1]; (2) oxidative stress regulation [by reducing oxidants (HO-1, NQO1) and enhancing antioxidants (SOD1, CAT, GP, FRAP)]; (3) preventing ECM degradation [by increasing elastin, collagen, HA, TIMP, and reducing MMP]; (4) regulating cell activity [by reducing cell senescence (SA-β-gal), apoptosis, and cell cycle arrest (P53, P12, P16); and enhancing autophagy, cell migration, and cell proliferation (Ki67)] (5) regulating the inflammatory pathway [by reducing IL-6, IL-1, TNF-⍺, and increasing TGF-β]. Several clinical trials have also supported the in vitro research by showing positive results regarding skin aging, such as improvements in wrinkles, elasticity, hydration, pores, and pigmentation.
Nevertheless, it is difficult to conclude which cell-free therapy is effective in treating skin aging due to various stem cell sources and research methods. There is a need for further research regarding the effectiveness of comparisons between various cell-free therapies, especially between secretomes and EVs. From a technical perspective, there are several challenges, such as acquiring an ideal source of stem cells, establishing optimal culture conditions,73 and variations in research methodologies across different studies.
Cell-free therapy has exhibited many encouraging findings in its endeavor to counteract the effects of skin aging through various molecular mechanisms. This form of therapy holds promise as a novel intervention for preventing skin aging. Nonetheless, comprehensive research is paramount to attaining more trustworthy and dependable results.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886. doi:10.3390/cells8080886
2. Damayanti RH, Rusdiana T, Wathoni N. Mesenchymal stem cell secretome for dermatology application: a review. Clin Cosmet Investig Dermatol. 2021;14:1401–1412. doi:10.2147/CCID.S331044
3. Xia J, Minamino S, Kuwabara K, Arai S. Stem cell secretome as a new booster for regenerative medicine. Biosci Trends. 2019;13(4):299–307. doi:10.5582/bst.2019.01226
4. Tan MI, Alfarafisa NM, Septiani P, et al. Potential cell-based and cell-free therapy for patients with COVID-19. Cells. 2022;11(15):1–20. doi:10.3390/cells11152319
5. D’arrigo D, Roffi A, Cucchiarini M, Moretti M, Candrian C, Filardo G. Secretome and extracellular vesicles as new biological therapies for knee osteoarthritis: a systematic review. J Clin Med. 2019;8(11):1867. doi:10.3390/jcm8111867
6. Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11(1):312. doi:10.1186/s13287-020-01831-3
7. Shimizu Y, Ntege EH, Sunami H. Current regenerative medicine-based approaches for skin regeneration: a review of literature and a report on clinical applications in Japan. Regen Ther. 2022;21:73–80. doi:10.1016/j.reth.2022.05.008
8. McGraw-Hill Education. Fitzpatrick’s Dermatology.
9. El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):398–405. doi:10.1034/j.1600-0625.2002.110502.x
10. Farage M, Miller KW, Maibach HI. Textbook of Aging Skin. Springer; 2017; doi:10.1007/978-3-662-47398-6
11. Bonté F, Girard D, Archambault JC, Desmoulière A. Skin Changes During Ageing. Biochem Cell Biol Age. 2019;91:249–280. doi:10.1007/978-981-13-3681-2_10
12. Fernandes A, Rodrigues PM, Pintado M, Tavaria FK. A systematic review of natural products for skin applications: targeting inflammation, wound healing, and photo-aging. Phytomedicine Int J Phytother Phytopharm. 2023;115:154824. doi:10.1016/j.phymed.2023.154824
13. Sadowska-Bartosz I, Bartosz G. Effect of antioxidants on the fibroblast replicative lifespan in vitro. Oxid Med Cell Longev. 2020;2020:1–15. doi:10.1155/2020/6423783
14. Philipp-Dormston WG, Bergfeld D, Sommer BM, et al. Consensus statement on prevention and management of adverse effects following rejuvenation procedures with hyaluronic acid-based fillers. J Eur Acad Dermatol Venereol JEADV. 2017;31(7):1088–1095. doi:10.1111/jdv.14295
15. Li L, Ngo HTT, Hwang E, et al. Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. Int J Mol Sci. 2020;21(1). doi:10.3390/ijms21010049
16. Kim YJ, Seo DH, Lee SH, et al. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem Biophys Rep. 2018;16(September):96–102. doi:10.1016/j.bbrep.2018.10.007
17. Guo S, Wang T, Zhang S, et al. Adipose-derived stem cell-conditioned medium protects fibroblasts at different senescent degrees from UVB irradiation damages. Mol Cell Biochem. 2020;463(1–2):67–78. doi:10.1007/s11010-019-03630-8
18. Xu X, Wang H, Zhang Y, et al. Adipose-derived stem cells cooperate with fractional carbon dioxide laser in antagonizing photoaging: a potential role of Wnt and β-catenin signaling. Cell Biosci. 2014;4(1):1–11. doi:10.1186/2045-3701-4-24
19. Song SY, Jung JE, Jeon YR, Tark KC, Lew DH. Determination of adipose-derived stem cell application on photo-aged fibroblasts, based on paracrine function. Cytotherapy. 2011;13(3):378–384. doi:10.3109/14653249.2010.530650
20. Xu Y, Zhang JA, Xu Y, et al. Antiphotoaging effect of conditioned medium of dedifferentiated adipocytes on skin in vivo and in vitro: a mechanistic study. Stem Cells Dev. 2015;24(9):1096–1111. doi:10.1089/scd.2014.0321
21. Kwon TR, Oh CT, Choi EJ, et al. Conditioned medium from human bone marrow-derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice. Photodermatol Photoimmunol Photomed. 2016;32(3):120–128. doi:10.1111/phpp.12224
22. Jung JY, Shim JH, Choi H, Lee TR, Shin DW. Human dermal stem/progenitor cell-derived conditioned medium improves senescent human dermal fibroblasts. Int J Mol Sci. 2015;16(8):19027–19039. doi:10.3390/ijms160819027
23. Sohn SJ, Yu JM, Lee EY, et al. Anti-aging properties of conditioned media of epidermal progenitor cells derived from mesenchymal stem cells. Dermatol Ther. 2018;8(2):229–244. doi:10.1007/s13555-018-0229-2
24. Park YM, Lee MJ, Jeon SH, Hrůzová D. In vitro effects of conditioned medium from bioreactor cultured human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) on skin-derived cell lines. Regen Ther. 2021;18:281–291. doi:10.1016/j.reth.2021.08.003
25. Seetharam RN. Human mesenchymal stromal cells-derived conditioned medium based formulation for advanced skin care: in vitro and in vivo evaluation. J Stem Cells Res Dev Ther. 2019;5:1–8. doi:10.24966/srdt-2060/100012
26. Balasubramanian S, Thej C, Walvekar A, et al. Evaluation of the secretome profile and functional characteristics of human bone marrow mesenchymal stromal cells-derived conditioned medium suggest potential for skin rejuvenation. J Cosmet Dermatol Sci Appl. 2017;07(01):99–117. doi:10.4236/jcdsa.2017.71010
27. Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009;53(2):96–102. doi:10.1016/j.jdermsci.2008.08.007
28. Wang X, Wang Q, Yin P, et al. Secretome of human umbilical cord mesenchymal stem cell maintains skin homeostasis by regulating multiple skin physiological function. Cell Tissue Res. 2023;391(1):111–125. doi:10.1007/s00441-022-03697-8
29. Son WC, Yun JW, Kim BH. Adipose-derived mesenchymal stem cells reduce MMP-1 expression in UV-irradiated human dermal fibroblasts: therapeutic potential in skin wrinkling. Biosci Biotechnol Biochem. 2015;79(6):919–925. doi:10.1080/09168451.2015.1008972
30. Li M, Zhao Y, Hao H, et al. Umbilical cord–derived mesenchymal stromal cell–conditioned medium exerts in vitro antiaging effects in human fibroblasts. Cytotherapy. 2017;19(3):371–383. doi:10.1016/j.jcyt.2016.12.001
31. Pan C, Lang H, Zhang T, et al. Conditioned medium derived from human amniotic stem cells delays H2O2‑induced premature senescence in human dermal fibroblasts. Int J Mol Med. 2019;44(5):1629–1640. doi:10.3892/ijmm.2019.4346
32. Wang T, Jian Z, Baskys A, et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials. 2020;257(June):120264. doi:10.1016/j.biomaterials.2020.120264
33. Guo JA, Yu PJ, Yang DQ, Chen W. The antisenescence effect of exosomes from human adipose-derived stem cells on skin fibroblasts. BioMed Res Int. 2022;2022:12–14. doi:10.1155/2022/1034316
34. Vu DM, Nguyen VT, Nguyen TH, et al. Effects of extracellular vesicles secreted by TGFβ-Stimulated umbilical cord mesenchymal stem cells on skin fibroblasts by promoting fibroblast migration and ECM protein production. Biomedicines. 2022;10(8):1–12. doi:10.3390/biomedicines10081810
35. Zhang Y, Zhang M, Yao A, et al. Circ_0011129 encapsulated by the small extracellular vesicles derived from human stem cells ameliorate skin photoaging. Int J Mol Sci. 2022;23(23). doi:10.3390/ijms232315390
36. Wu P, Zhang B, Han X, et al. HucMSC exosome-delivered 14-3-3ζ alleviates ultraviolet radiation- induced photodamage via SIRT1 pathway modulation. Aging. 2021;13(8):11542–11563. doi:10.18632/aging.202851
37. Oh M, Lee J, Kim YJ, Rhee WJ, Park JH. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int J Mol Sci. 2018;19(6):1–18. doi:10.3390/ijms19061715
38. Zhao X, Liu Y, Jia P, et al. Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts. Stem Cell Res Ther. 2021;12(1):1–15. doi:10.1186/s13287-021-02262-4
39. Shi HZ, Zeng JC, Shi SH, Giannakopoulos H, Zhang QZ, Le AD. Extracellular vesicles of GMSCs alleviate aging-related cell senescence. J Dent Res. 2021;100(3):283–292. doi:10.1177/0022034520962463
40. Xu P, Xin Y, Zhang Z, et al. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther. 2020;11(1):1–14. doi:10.1186/s13287-020-01777-6
41. Deng M, Yu Z, Li D, et al. Human umbilical cord mesenchymal stem cell-derived and dermal fibroblast-derived extracellular vesicles protect dermal fibroblasts from ultraviolet radiation-induced photoaging: in vitro. Photochem Photobiol Sci. 2020;19(3):406–414. doi:10.1039/c9pp00421a
42. Toutfaire M, Bauwens E, Debacq-Chainiaux F. The impact of cellular senescence in skin ageing: a notion of mosaic and therapeutic strategies. Biochem Pharmacol. 2017;142:1–12. doi:10.1016/j.bcp.2017.04.011
43. Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20(3):291–301. doi:10.1016/j.jcyt.2017.11.002
44. Zhan JYX, Wang XF, Liu YH, et al. Andrographolide sodium bisulfate prevents UV-induced skin photoaging through inhibiting oxidative stress and inflammation. Mediators Inflamm. 2016;2016:1–12. doi:10.1155/2016/3271451
45. Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28(6):436–453. doi:10.1016/j.tcb.2018.02.001
46. Hernandez-Segura A, Brandenburg S, Demaria M. Induction and validation of cellular senescence in primary human cells. J Vis Exp. 2018;2018(136):1–10. doi:10.3791/57782
47. Smithmyer ME, Cassel SE, Kloxin AM. Bridging 2D and 3D culture: probing impact of extracellular environment on fibroblast activation in layered hydrogels. AIChE J Am Inst Chem Eng. 2019;65(12). doi:10.1002/aic.16837
48. Weinmüllner R, Zbiral B, Becirovic A, et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. Npj Aging Mech Dis. 2020;6(1). doi:10.1038/s41514-020-0042-x
49. Gruber F, Kremslehner C, Eckhart L, Tschachler E. Cell aging and cellular senescence in skin aging — recent advances in fibroblast and keratinocyte biology. Exp Gerontol. 2020;130:110780. doi:10.1016/j.exger.2019.110780
50. Bellei B, Migliano E, Tedesco M, et al. Adipose tissue-derived extracellular fraction characterization: biological and clinical considerations in regenerative medicine. Stem Cell Res Ther. 2018;9(1):1–18. doi:10.1186/s13287-018-0956-4
51. Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571:183–192. doi:10.1038/s41586-019-1365-2
52. Wlaschek M, Maity P, Makrantonaki E, Scharffetter-Kochanek K. Connective Tissue and Fibroblast Senescence in Skin Aging. J Invest Dermatol. 2021;141(4):985–992. doi:10.1016/j.jid.2020.11.010
53. Tang Tao T, Lv Li L, Lan Yao H, Liu Cheng B. Extracellular vesicles: opportunities and challenges for the treatment of renal diseases. Front Physiol. 2019;10(March):1–12. doi:10.3389/fphys.2019.00226
54. Foo JB, Looi QH, Chong PP, et al. Comparing the therapeutic potential of stem cells and their secretory products in regenerative medicine. Stem Cells Int. 2021;2021. doi:10.1155/2021/2616807
55. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1). doi:10.1080/20013078.2018.1535750
56. Tieu A, Lalu MM, Slobodian M, et al. An analysis of mesenchymal stem cell- derived extracellular vesicles for preclinical use. ACS nano. 2020;14:9728–9743. doi:10.1021/acsnano.0c01363
57. Jarrige M, Frank E, Herardot E, et al. The future of regenerative medicine: cell therapy using pluripotent stem cells and acellular therapies based on extracellular vesicles. Cells. 2021;10(2):1–29. doi:10.3390/cells10020240
58. Gurunathan S, Kang MH, Qasim M, Khan K, Kim JH. Biogenesis, membrane trafficking, functions, and next generation nanotherapeutics medicine of extracellular vesicles. Int J Nanomedicine. 2021;16:3357–3383. doi:10.2147/IJN.S310357
59. Terlecki-Zaniewicz L, Pils V, Bobbili MR, et al. Extracellular vesicles in human skin: cross-talk from senescent fibroblasts to keratinocytes by miRNAs. J Invest Dermatol. 2019;139(12):2425–2436.e5. doi:10.1016/j.jid.2019.05.015
60. Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J. Exosomes: new molecular targets of diseases. Acta Pharmacol Sin. 2018;39(4):501–513. doi:10.1038/aps.2017.162
61. da Ferreira AF, Gomes DA. Stem cell extracellular vesicles in skin repair. Bioengineering. 2019;6(1):1–18. doi:10.3390/bioengineering6010004
62. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
63. Li Y, Xiao Q, Tang J, Xiong L, Li L. Extracellular vesicles: emerging therapeutics in cutaneous lesions. Int J Nanomedicine. 2021;16(June):6183–6202. doi:10.2147/IJN.S322356
64. Ha DH, Kim SD, Lee J, et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul Toxicol Pharmacol. 2020;115:104686. doi:10.1016/j.yrtph.2020.104686
65. Ma ZJ, Yang JJ, Lu YB, Liu ZY, Wang XX. Mesenchymal stem cell-derived exosomes: toward cell-free therapeutic strategies in regenerative medicine. World J Stem Cells. 2020;12(8):814–840. doi:10.4252/WJSC.V12.I8.814
66. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8(12):1605. doi:10.3390/cells8121605
67. Lee H, Cha H, Park JH. Derivation of cell-engineered nanovesicles from human induced pluripotent stem cells and their protective effect on the senescence of dermal fibroblasts. Int J Mol Sci. 2020;21(1). doi:10.3390/ijms21010343
68. Lin TJ, Huang YL, Kang YN, Chen C. Effectiveness of topical conditioned medium of stem cells in facial skin nonsurgical resurfacing modalities for antiaging: systematic review and meta-analysis of randomized controlled trials. Aesthetic Plast Surg. 2023;47(2):799–807. doi:10.1007/s00266-022-03168-z
69. Kim J, Kim B, Kim S, Lee YI, Kim J, Lee JH. The effect of human umbilical cord blood–derived mesenchymal stem cell media containing serum on recovery after laser treatment: a double-blinded, randomized, split-face controlled study. J Cosmet Dermatol. 2020;19(3):651–656. doi:10.1111/jocd.13063
70. Yusharyahya SN, Ven Japranata V, Sitohang IBS, et al. A comparative study on adipose-derived mesenchymal stem cells secretome delivery using microneedling and fractional CO2 laser for facial skin rejuvenation. Clin Cosmet Investig Dermatol. 2023;16:387–395. doi:10.2147/CCID.S401839
71. Liang X, Li J, Yan Y, et al. Efficacy of microneedling combined with local application of human umbilical cord-derived mesenchymal stem cells conditioned media in skin brightness and rejuvenation: a randomized controlled split-face study. Front Med. 2022:9. doi:10.3389/fmed.2022.837332
72. Park G, Kwon HH, Seok J, et al. Efficacy of combined treatment with human adipose tissue stem cell‐derived exosome‐containing solution and microneedling for facial skin aging: a 12‐week prospective, randomized, split‐face study. J Cosmet Dermatol. 2023:15872. doi:10.1111/jocd.15872
73. Tan KX, Chang T, Lin X. Secretomes as an emerging class of bioactive ingredients for enhanced cosmeceutical applications. Exp Dermatol. 2022;31(5):674–688. doi:10.1111/exd.14570
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
