Back to Journals » International Journal of Nanomedicine » Volume 18
Progress in Pluronic F127 Derivatives for Application in Wound Healing and Repair
Authors Li S, Yang C, Li J, Zhang C, Zhu L, Song Y, Guo Y, Wang R, Gan D, Shi J, Ma P, Gao F, Su H
Received 24 April 2023
Accepted for publication 10 July 2023
Published 7 August 2023 Volume 2023:18 Pages 4485—4505
DOI https://doi.org/10.2147/IJN.S418534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Shanshan Li,1,* Cheng Yang,1,* Junqiang Li,1,* Chao Zhang,1 Liaoliao Zhu,1 Yang Song,1 Yongdong Guo,1 Ronglin Wang,1 Dongxue Gan,1 Jingjie Shi,1 Peixiang Ma,1 Fei Gao,2 Haichuan Su1
1Department of Oncology, The Second Affiliated Hospital, Air Force Medical University, Xi’an City, People’s Republic of China; 2Center for Peptide Functional Materials and Innovative Drugs, Institute of Translational Medicine, Shanghai University, ShangHai City, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haichuan Su, Department of Oncology, The Second Affiliated Hospital, Air Force Medical University, No. 569 Xinsi Road, Baqiao District, Xi’an City, People’s Republic of China, Email [email protected] Fei Gao, Center for Peptide Functional Materials and Innovative Drugs, Institute of Translational Medicine, Shanghai University, No. 99 ShangDa Road, BaoShan District, ShangHai City, People’s Republic of China, Email [email protected]
Abstract: Pluronic F127 hydrogel biomaterial has garnered considerable attention in wound healing and repair due to its remarkable properties including temperature sensitivity, injectability, biodegradability, and maintain a moist wound environment. This comprehensive review provides an in-depth exploration of the recent advancements in Pluronic F127-derived hydrogels, such as F127-CHO, F127-NH2, and F127-DA, focusing on their applications in the treatment of various types of wounds, ranging from burns and acute wounds to infected wounds, diabetic wounds, cutaneous tumor wounds, and uterine scars. Furthermore, the review meticulously examines the intricate interaction mechanisms employed by these hydrogels within the wound microenvironment. By elucidating the underlying mechanisms, discussing the strengths and weaknesses of Pluronic F127, analyzing the current state of wound healing development, and expanding on the trend of targeting mitochondria and cells with F127 as a nanomaterial. The review enhances our understanding of the therapeutic effects of these hydrogels aims to foster the development of effective and safe wound-healing modalities. The valuable insights provided this review have the potential to inspire novel ideas for clinical treatment and facilitate the advancement of innovative wound management approaches.
Keywords: Pluronic F127, F127-CHO, F127-NH2, F127-DA, wound healing and repair
Introduction
Every year, hundreds of millions of people experience wounds of different etiologies.1–4 Once a wound is formed, it is difficult to heal if the person is afflicted by an underlying disease such as diabetes, and can easily lead to other complications. Some wounds may not heal for months or years, which not only has a serious impact on patients’ health, but also increases their financial burden. Therefore, the development of biomaterials that promote wound healing has received increasing research attention. Hydrogels are excellent materials for treating wounds.They have a network structure similar to that of extracellular matrices, can absorb wound exudate, and can provide a wet environment to the wound.5 These systems are also able to deliver drugs6 and cells to promote tissue repair.7 Researchers have developed various functional hydrogels have been developed according to the requirements of different tissues.8,9
Pluronic F127 (F127) is an FDA-approved hydrogel10 with the composition polyoxyethylene-polyoxypropylene-polyoxyethylene (PEO-PPO-PEO), where PEO is hydrophilic and PPO is lipophilic. When this copolymer is heated to physiological temperature (ie, ~37 °C), the hydrophobic PPO block dehydrates and crosslinks with the hydrophilic PEO block to form spherical microgels. These microgels then crosslink with one another to form porous three-dimensional reticular hydrogels, which revert to the solution phase at 4 °C11–14 (Scheme 1). As previously reported, Pluronic F127 is temperature-sensitive,15 injectable,16 biodegradable,17 non-toxic,10 and biocompatible.18 This article reviews the research and applications of F127 and its derivatives in wound dressing, acute and chronic wound closure, seamless wound closure, deep wound closure, wound infection healing, and wound repair.
 |
Scheme 1 Changes in the morphology of F127 at different temperatures. Adapted from Klouda L, Mikos AG, 68(1), Thermoresponsive hydrogels in biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics, 34–45, Copyright (2008), with permission from Elsevier.19 |
Wound healing is a dynamic and complex tissue repair and regeneration process consisting of a hemostatic, inflammatory, proliferative, and remodeling phase. In the hemostatic phase, the body activates the clotting system after an injury, activating platelets and releasing growth factors, which in turn begins the healing process. In the inflammatory phase, neutrophils reach the wound site to kill local bacteria within minutes after injury while releasing pro-inflammatory factors; in the later stages of injury, macrophages emerge to engulf bacteria, dead neutrophils, and damaged tissues to play a reparative role and release transforming growth factors, cytokines, and chemokines to inhibit further inflammation. In the proliferative phase, macrophages, fibroblasts, endothelial cells, and keratinocytes work together to cause the body to produce new tissue and blood vessels within 1–2 days after injury, completing the re-epithelialization of the wound and the formation of granulation tissue. In the final proliferation stage, the wound edges slowly shrink and come closer together. In the remodeling phase, tissue begins to remodel with the formation of a mature scar during the proliferative phase. During this phase, the body simultaneously produces and breaks down collagen, maintaining a balance between the need for tensile strength and the remodeling of new tissue to form new skin or scar tissue with functionality.20,21 Wounds are classified as acute and chronic based on the rate of healing.22 In this review, acute wounds23,24 and chronic wounds(diabetic wounds)25,26 are discussed. Acute wounds generally require hemostasis and infection prevention, while diabetic wounds have a high glycemic microenvironment with persistent and enhanced periods of inflammation (hypoxia), and prolonged open wounds, resulting in extreme susceptibility to infection, which in turn also delays healing.27,28 Burns are one of the most common types of wounds and can be classified into four categories according to the depth of the wound: Wounding only affecting the superficial epidermis (the germ layer is alive), wounds affecting the epidermal germ layer and dermal papillae, wounds affecting the deep dermis, and wounds affecting the entire skin layer.29 This review covers superficial epidermal burns,30,31 deep burns,32 and deep partial-thickness burn.33
The F127 hydrogel was first synthesized and applied to treat burns by Schmolka in 1972.30 Later, F127 was combined with bactericidal agents (eg, silver nitrate and silver lactate) for the treatment of thermal burns.31 It was also found that the addition of silver nanoparticles (AgNPs) led to complete inhibition of the growth of Staphylococcus aureus and Pseudomonas aeruginosa. The gel formulation exhibited an anti-biofilm activity and > 95% survival of human fibroblasts. The commercially available 1% silver sulfadiazine cream was highly cytotoxic to human fibroblasts, with 18% fibroblast survival after 4 h of application, suggesting that the gel may be an alternative to 1% silver sulfadiazine cream for wound treatment.34 F127 gel stimulates the expression of the vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGF-β1), which promote healing.35
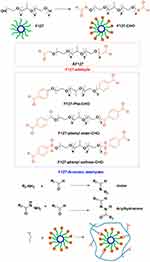
F127 hydrogels usually have poor mechanical strength, adhesion and self-healing ability. These issues can be addressed by the formation of various derivatives, such as aldehyde-terminated Pluronic F127 (F127-CHO), alkoxyamine-terminated Pluronic F127 (F127-NH2), and Pluronic F127 diacrylate (F127-DA) (Figure 1).
 |
Figure 1 F127 derivatives for wound healing and repair. |
Aldehyde-Terminated Pluronic F127 Hydrogels
In 2011, the Mei group obtained the filtrate F127-CHO by dissolving F127 in dry dichloromethane and adding Dess-Martin periodinane, stirring overnight at room temperature, followed by cold ether treatment of the product and filtering of the precipitate (Scheme 2).36 Subsequently, the investigators performed similar reactions using acetaldehyde, 4-hydroxybenzaldehyde, 4-carboxybenzaldehyde and methanesulfonyl chloride/4-hydroxybenzaldehyde to introduce hydrophilic aldehyde groups into the structure to obtain F127-aldehyde (AF127)37 and F127-aromatic aldehyde hydrogels (F127-Phe-CHO,33,38 F127-phenyl ester-CHO,39 and F127-phenyl sulfone-CHO)25 (Scheme 2).
The investigators found that the aldehyde-terminated F127 hydrogels exhibited enhanced mechanical strengths and superior self-healing and tissue adhesion properties compared to the original F127 hydrogels. Their successful application in wound repair and regeneration was attributed to two main mechanisms: Firstly, the formation of a C=N bond can promote tissue healing and repair. More specifically, the aldehyde-terminated F127 reacts readily with an amine group25 to form an imine or with a hydrazide40 to form an acylhydrazone (Scheme 2). Imines and acylhydrazones exhibit greater mechanical strength in hydrogels, while imine structures tend to be more potent in wound healing than acylhydrazide-containing structures, and acylhydrazones exhibit good self-healing ability in slightly acidic environments (ie, pH 4.0–6.0).41,42
Second, F127-CHO self-assembles in water into nano-cavities whose inner cavities can be used to load additional substances that promote wound healing and tissue repair. More specifically, the aldehyde-containing F127 hydrogel contains a spherical cavity owing to the interaction between the aldehyde group, hydrophilic PEO, and the lipophilic PPO. This cavity can be used to load substances such as curcumin,23 7.8-dihydroxy flavones,43 bromelain/EGF,32 insulin,25 and ceria-based nanocomposites,44 among others.
Formation of Hydrogels with Hydrazide-Based Polymers at the Aldehyde End of Pluronic F127
As mentioned above, aldehyde-terminated F127 hydrogels react with hydrazide groups to form acylhydrazones, which can be used for sports wounds, wound infections, deep burns, and uterine scars. Currently, the known aldehyde-terminated F127 hydrogels include AF127,40 F127-Phe-CHO,33 and F127-phenyl ester-CHO.45 In addition, aromatic acylhydrazones are more stable than aliphatic acylhydrazones due to conjugation effect,46 thereby accounting for the fact that the F127-aromatic aldehydes are mechanically stronger than F127-aldehydes. Their corresponding hydrazide-based polymers can be divided into two main categories: adipic dihydrazide derivatives (adipic dihydrazide,47 adipic dihydrazide-modified hyaluronic acid,39 adipic dihydrazide-modified γ-polyglutamic acid)38 and the three-armed PEO hydrazides48 (Scheme 3).
 |
Scheme 3 Reaction ofF127-CHO with hydrazine-based polymers to form Schiff bases. |
Adipic Dihydrazide Derivatives
Adipic dihydrazide is commonly employed as a cross-linking agent for form aldehyde-containing compounds. Yang et al synthesized injectable, self-healing, thermosensitive hydrogels of dynamically cross-linked acylhydrazones based on F127-Phe-CHO using adipic dihydrazide.47 Subsequently, Chen et al reported the use of adipic dihydrazide modified hyaluronic acid (HAAD) instead of adipic dihydrazide, and dissolved lyophilized HAAD with F127-Phe-CHO in PBS to form an injectable thermosensitive hydrogel. The mechanical strength of this hydrogel (modulus of elasticity G′between 1000.0 ~ 10,000.0 Pa) is similar to that of the natural skin tissue, with an elongation at break of 2400.0% and a transient recovery rate of 85.2% after 3 compressions, in addition to good biocompatibility, tissue adhesion, and fluid absorption properties, which effectively promoted the repair of deep burn wounds.33 Based on the above hydrogel, Gu et al replaced F127-Phe-CHO with acetaldehyde-terminated F127 (AF127) (Scheme 4), and formed hydrogels by micelles of AF127, HAAD, and dopamine-functionalized oxidized hyaluronic acid (OHA-Dop) dissolved in PBS. Owing to the unconjugated nature of the resultant aliphatic hydrazide, this hydrogel exhibitedless-favorable mechanical properties.40 The hydrogel is supplemented with OHA-Dop was then examined as dopamine readily forms π-π stacking interactions and hydrogen bonds with the amine, imidazole, and thiol groups on biological substrates, thereby leading to strong adhesion.37 The hydrogel exhibited an adhesion force of 31 kPa. AF127 micelles, dynamically cross-linked and immobilized on HA-ADH hydrogel network by C=N bonding, showed much higher mechanical strength, rapid self-healing, and enhanced shear thinning behavior compared to single network HA-ADH hydrogels. Compared to commercial Mepitel, this hydrogel has excellent wound healing properties (greater wound shrinkage, more collagen deposition, less scarring, simultaneous production of skin appendages, granulation tissue, and blood vessels) and is a new approach to wound healing.40
 |
Scheme 4 Injectable, self-healing and strongly-adhesive hydrogel dressings in wound healing. Adapted with permission from Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing, ACS Appl. Mater. Inter. 2020;12(52):57782–57797. Copyright (2020) American Chemical Society.40 |
Zhou et al resuspended Lactobacillus rhamnosus in HA-ADH solution and dissolved F127-phenyl ester-CHO and fucoidan in PBS solution, after which the two solutions were mixed to prepare different ratios of hydrogels (Scheme 5). Among them, Lactobacillus rhamnosus antagonizes Candida albicans, inhibiting infection by Pseudomonas aeruginosa, promoting the healing of skin wounds, and reducing scar formation. Compared with F127-Phe-CHO, F127-phenyl ester-CHO contains a greater number of ester groups, leading to the formation of additional hydrogen bonds and a relatively enhanced mechanical strength. The hydrogel has comparable antibacterial and healing rates compared to commercially available Prontosan gels, but is superior to Prontosan gels in terms of collagen deposition. In conclusion, the hydrogel is a potential alternative for the treatment of superbug-based infections and wounds.39 In another study, Deng loaded umbilical cord mesenchymal stem cells (UCMSCs) and asiaticoside microspheres (AMs) onto F127-phenyl ester-CHO and AHA hydrogels for uterine scar repair.45 It was found that asiaticoside inhibits scar proliferation and promotes the healing of initially inflamed wounds. The UCMSCs repaired the damaged endometrial epithelial cells. Angiogenesis experiments pointed to an increase in this hydrogel’s vascular connections and vascular length (184.3±9.8 and 1.24±0.04, respectively). The system slowed the release of AMs for up to 7 days, reduced endometrial fibrosis, promoted endometrial cell proliferation, facilitated glandular regeneration, and restored the uterus in rats, thus demonstrating its potential clinical application in uterine scar repair.45
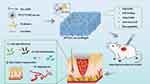 |
Scheme 5 Preparation of an injectable, self-healing [email protected] hydrogel via Schiff base for superbug-infected wounds. Reprinted with permission from Injectable and Self-Healing Probiotics-Loaded Hydrogel for Promoting Superbacteria-Infected Wound Healing, ACS Appl. Mater. Inter. 2022;14(18):20538–20550. Copyright (2022) American Chemical Society.39 |
Cai et al formed two-dimensional PDA nanosheets loaded with NO donor N,N′-disubstituted-butyl-N,N′-dinitroso-1,4-phenylenediamine (BNN6) to form PDA- BNN6 using DNA as a template, in the presence of tris(hydroxymethyl)aminomethane. The hydrazine group of γ-PGA-ADH with the aldehyde group of F127-Phe-CHO loaded PDA- BNN6, the hydrogel was obtained in less than 55s by the Schiff base reaction and the micellar action of F127-Phe-CHO38 (Scheme 6). NO is of particular interest because of its close association with wound healing, including the control of skin blood flow, skin defense and tissue repair systems.15 In addition, PDA NSs have recently been reported as a new material for use in photothermal therapy (PTT). The photothermal conversion performance of PDA NS in this system (56.1%) was better under 808 nm NIR, while NO was released on demand. In the antibacterial experiments, the hydrogel achieved 98.9% and 99.7% bactericidal efficiency against E. coli and S. aureus in vitro. The storage modulus (G′) and loss modulus (G″) of the hydrogel at 37 °C are higher than those at 25 °C, indicating that F127-CHO not only enhances the mechanical properties, but also promotes the formation of the hydrogel with the increase of temperature. In summary, the combination of photothermal effect and NO gas was synergistically antibacterial, thereby providing a new approach for the preparation of wound dressings for infected skin.38 Hydrogels synthesized from F127-CHO and adipic dihydrazide derivatives are one of the key materials for use in wound repair and skin regeneration.
 |
Scheme 6 (a) Preparation of PDA-BNN6 nanosheets. (b) Raw materials and preparation of hydrogels. (c) Application of hydrogels in wound infection sites. Used with permission of [Royal Society of Chemistry], from [Polydopamine Nanosheets Doped Injectable Hydrogel with Nitric Oxide Release and Photothermal Effects for Bacterial Ablation and Wound Healing. Liu G, Wang L, He Yet al 10, 23, 2021]; permission conveyed through Copyright Clearance Center, Inc.38 |
Three-Armed PEO Acylhydrazides
Chen et al reported a dynamically cross-linked acylhydrazone composed of three-armed PEO acylhydrazine and F127-Phe-CHO. The three arm PEO acyl hydrazide contains three hydrazide groups, which increases the probability of forming acylhydrazone with F127-Phe-CHO. The acylhydrazone bond is a reversible chemical bond under acidic and neutral conditions, and the bond has self-healing properties, good mechanical properties and adaptability. In addition, F127-Phe-CHO forms self-assembled micelles and Schiff bases, which have dual effects and give the hydrogels excellent in mechanical properties, including tensile properties (stretching to 117 times the initial length), high toughness (tensile toughness of 14.1 MJ m−3), and good self-healing ability (self-healing strength within 24 h is 85% of the initial strength).48 The hydrogel has the potential to be used as a sports wound dressing.
Formation of Hydrogels with Amine-Modified Compounds at the Aldehyde-Capped End of Pluronic F127
The aldehyde-terminated F127 can form hydrogels with amines via Schiff bases and is commonly used for full-thickness skin wounds, joint wounds, diabetic wounds, burn wounds and skin tumors. The most commonly used amines for this purpose can be divided into three categories: quaternized chitosan (QCS),23 ε-polylysine derivatives (ε-polylysine-coated MnO2 NSs25 and polyvinyl alcohol modified with sulfhydryl and amine groups),32 and polyethylenimine-coated cerium oxide nanorods44 (Scheme 7).
 |
Scheme 7 Reaction of F127-CHO with various amino-modified compounds to form Schiff bases. |
Quaternized Chitosan
The parent ring structure of QCS contains many amines and hence can form dynamic imines upon reaction with aldehydes.49 In addition, QCS is more soluble than chitosan under physiological conditions, and has been demonstrated to possess antifungal50 and antioxidant properties.51 Through the combination of QCS and curcumin-loaded F127-Phe-CHO, Guo et al formed an antimicrobial hydrogel system with self-healing properties and good mechanical properties. The content of F127-Phe-CHO allows controlled release of curcumin. The physical cross-linking of F127-Phe-CHO micelles and the dynamic Schiff base interaction with QCS make the hydrogel self-healing. The weight of the hydrogel can reach 100 g when the adhesive force is tested with fresh pig skin, which may be due to the close contact between the aldehyde group of the hydrogel and the amino group on the surface of the tissue to form a Schiff base. This hydrogel system accelerated wound healing, increased the thickness of granulation tissue, and promoted the distribution of collagen and VEGF can be used as a joint wound dressing.23
ε-PL Derivatives
ε-Polylysine (ε-PL) is known to exhibit antimicrobial properties.52 Zhang et al prepared hydrogels from ε-PL-coated manganese dioxide NSs (EM) and insulin-loaded F127-phenyl sulfone-CHO micelles. Note that the MnO2 NSs catalyze the decomposition of endogenous H2O2 into O2 to reduce the oxidative stress in cells. Thus, through the synergistic combination of the positively charged ε-PL and MnO2 NSs, the resulting hydrogel exhibited extraordinary antibacterial properties (The authors used a diabetic trauma model with MRSA infection to test the antibacterial effect and wound healing ability of the hydrogel and observed that it was the most effective bactericide on day 3 with approximately 100% bactericidal effect on day 14) against multidrug-resistant (MDR) bacteria. In addition, it was demonstrated that this hydrogel is pH and redox responsive, and that it can control the release of insulin, thereby regulating blood glucose levels and accelerating the healing (wound healing rate on day 7 was 78.2%) of diabetic wounds infected by MDR bacteria. This hydrogel therefore provides a new strategy for wound healing in diabetic patients.25
Yang et al employed ε-PL for Schiff base formation with F127-Phe-CHO, wherein F127-Phe-CHO was cross-linked with ε-PL and sulfhydryl-modified polyvinyl alcohol (PVA-SH/ε-PL). It was loaded with bromelain and the epidermal growth factor stepwise to obtain hydrogels for wound cleansing and healing.32 In this system, pineapple proteins were commonly employed in burn debridement53 as the sulfhydryl groups in PVA-SH prevent their oxidation. By using the swelling test to evaluate its liquid absorption performance and moisturizing property, the hydrogel had the largest swelling ratio at 50 min. It then gradually decreased, with the liquid absorption performance up to 59.62%, moisturizing rate up to 88%, and biodegradation rate over 40% after 72 h. These results indicate that the hydrogel has good liquid absorption, biodegradation, and moisturizing rate. This hydrogel is therefore a potentially injectable wound dressing for the treatment of deep burn wounds.32
Later, Lei et al generated F127-OTs by the room temperature reaction of F127 with pyridine and toluenesulfonyl chloride (TsCl), later added 4-hydroxybenzaldehyde to obtain F127-Phe-CHO. ε-PL can also react with F127-OTs to obtain FEPL, and F127-Phe-CHO forms FCE with FEPL, which is loaded with monodisperse polydopamine functionalized biological by Schiff base activated glass nanoparticles (BGN@PDA) to kill melanoma, antibacterial, and heal wounds under the action of NIR Laser and heat. Among them, BGN@PDA can stimulate skin repair under photothermal conditions. The hydrogel effectively kills tumor cells (>90%) and inhibits tumor growth (94% inhibition at day 18) in a subcutaneous skin tumor model. Higher wound healing rates with this hydrogel compared to commercial 3M dressings. This nanocomposite hydrogel promotes wound healing in skin tumors via PTT54 (Scheme 8).
 |
Scheme 8 (A) Chemical synthesis process of hydrogel; (B) Schematic diagram of BNG@PDA; (C) Application of hydrogel in tumor wound healing. Reprinted with permission from Injectable Self-Healing Antibacterial Bioactive Polypeptide-Based Hybrid Nanosystems for Efficiently Treating Multidrug Resistant Infection, Skin-Tumor Therapy, and Enhancing Wound Healing. Adv. Funct. Mater. 2019;29(22):1806883. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.54 |
Polyethyleneimine-Coated Cerium Oxide Nanorods
Common hydrogel dressings are prone to bioflocculation, which leads to bacterial infection and generates large amounts of reactive oxygen species (ROS) that hinder wound repair and skin regeneration.55 To address this issue, Lei et al designed a novel nanocomposite hydrogel material. Firstly, PEI/PVP@ CeO2 nanorods were obtained by adding polyethyleneimine (PEI) and polyvinylpyridone (PVP) to CeO2 aqueous solution after the reaction. Next, F127 was reacted with TsCl to form F127-TsCl, followed by the addition of 4-hydroxybenzaldehyde to obtain F127-CHO. Finally, PEI/PVP@ CeO2 was linked with F127-CHO using Schiff base to form nanocomposite hydrogels (FVEC hydrogels)44 (Scheme 9). Cerium oxide adheres strongly to tissues and exhibits a high ROS scavenging activity, thereby protecting cells from oxidative stress.56 A rat model evaluated skin wound healing, and FVEC hydrogel completely closed the wound in mice on day 14, and the wound was covered by new smooth skin, which was superior to the existing treatment 3M Tegaderm film healing efficiency. The healing effect was further evaluated by histological examination (H&E staining), which concluded that hair follicles and adipocytes could be generated at day 14. By implanting the hydrogel subcutaneously on the back of mice and subsequently examining the mice histologically, it was found that the hydrogel degraded significantly at 3 days and was essentially completely degraded after 7 days due to hydrolysis of Schiff base in the in vivo environment. This amphiphilic F127 micelles being broken down in vivo. Combined with the thermal sensitivity, injectability, biocompatibility, and biodegradability of F127-CHO hydrogels. This provided a new strategy for the healing and regeneration of full-thickness skin wounds (wounds that extend from the epidermis and dermis to subcutaneous tissue, along with fascia and muscle injuries).44
 |
Scheme 9 Schematic diagram of hydrogel formation by polyethyleneimine-coated CeO2 nanorods and F127-Phe-CHO for skin wound repair. Reprinted from Gong X, Luo M, Wang M, et al. Injectable self-healing ceria-based nanocomposite hydrogel with ROS-scavenging activity for skin wound repair, Regen. Biomater. 2022;9(1):rbab074. This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.44 |
Alkoxyamine-Terminated Pluronic F127 Hydrogels
Some researchers synthesized Schiff bases by another method (F127-NH2 and OHA), where F127-NH2 was synthesized as follows. The synthesis of F127-NH2 is as follows: PPh3 and N-hydroxyphthalimide were added to F127 dissolved in tetrahydrofuran, and DIAD was added in drops at 0°C. The reaction was carried out at room temperature for 24 h. Pluronic F127 bis-phthalimide derivatives were precipitated by quenching in petroleum ether, crystallized and purified, then dissolved in dichloromethane. After that, hydrazine monohydrate was added in drops at room temperature for 12 h. The filtrate was obtained by removing the precipitate as F127-NH2 (Scheme 10). At 37°C, the G′ value of F127-NH2/OHA hydrogel was approximately 3000pa, the G″ value was approximately 500 pa, and the bond strength of pigskin was 4.6 kPa, much higher than F127 hydrogel. In vitro studies also demonstrated its biocompatibility and its anti-adhesive effect on fibroblasts. Furthermore, its ability to adhere to tissues was moderate, and due to its self-fixation ability, this hydrogel was suitable for use as a physical barrier to prevent post operative adhesions.57
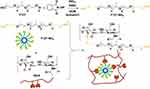 |
Scheme 10 Synthesis of F127-NH2 from F127, where F127 reacts with OHA to form an oxime hydrogel. |
Pluronic F127 Diacrylate Hydrogels
F127-DA hydrogels were first synthesized by Tirelli et al in 2002. The synthesis method is as follows: Under nitrogen atmosphere, F127 dissolved in toluene was extracted by reflux through a Soxhlet extractor. The extract was cooled slowly in a flask placed in an ice bath. After that, triethylamine, dichloromethane, and acryloyl chloride were added sequentially in the flask with a dropping funnel. Dichloromethane was added continuously and diluted, stirred for 12h, filtered, and precipitated to obtain a viscous oil F127-DA (Scheme 11). The F127-DA hydrogel self-assembled under aqueous conditions to form polymeric micelles with a vinyl-bearing surface for the solubilization and loading of hydrophobic drugs.58 F127-DA hydrogels are generally used in combination with compounds that possess alkenes, since these can be subjected to photopolymerization.59 Photocurable alkene radical polymerization can be carried out under UV or 405 nm light irradiation to form hydrogels with good biocompatibility, temperature sensitivity, and strong mechanical properties (high strength and toughness).60,61 In such systems, the alkenes-containing compounds can be divided into two categories: acrylate-modified polymers(poly(ethylene glycol)diacrylate (PEG-DA) and quaternized chitosan diacrylate (QCSDA)), and methacrylate-modified compounds (glycidyl methacrylate functionalized quaternized chitosan (QCSG), glycidyl methacrylate COS, sulfobetaine methacrylate (SBMA), and modified sodium alginate (MAlg)), as presented in Scheme 11.
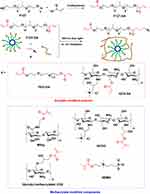 |
Scheme 11 Synthesis of F127-DA from F127 by the reaction of F127-DA with alkenes. |
Formation of Hydrogels with F127-DA and Acrylate-Based Polymers
Acrylate-based polymers are common light-curing hydrogel materials that have been widely studied in the fields of four-dimensional (4D) printing62 and spinal cord regeneration.63 The following subsections summarize the use of F127-DA with acrylate-based polymers (PEG-DA or QCSDA) photocuring for sutureless wound closure.
PEG-DA
PEG-DA is a common hydrogel that is known to gel rapidly using a photoinitiator. It possesses a high water content and a good elasticity, and therefore, has been widely used as a new scaffold in tissue engineering64 and regenerative medicine.65 Wang et al investigated the application of a hydrogel patch synthesized from PEG-DA, QCS and tannic acid (TA) for sutureless wound closure.66 The first application of TA was in 1928, when Davidson et al employed it to treat burns.67 Owing to the presence of multiple hydroxyl groups in its structure, TA can be used as a substitute for the hydrogel binder dopamine, and readily forms hydrogen bonds to improve the adhesion of the hydrogel. Meng et al used the non-swellable and highly mechanical F127-DA and PEG-DA as raw materials to form hydrogel patches in the presence of a photoinitiator under 395 nm irradiation, and demonstrated the potential of these patches to close and repair wounds without the requirement for sutures.61 Based on the above literature, Wang et al used PEG-DA, F127-DA, MAlg, and TA as raw materials to form new insoluble hydrogels using UV light, in which TA formed hydrogen bonds with PEG-DA, F127-DA, and MAlg, both PEG-DA and MAlg can be photocured with a double bond of F127-DA to improve the mechanical strength. Measurements from tensile and compressive tests revealed that the hydrogel containing 20% F127-DA had 7.7 times higher tensile stress, 3.7 times higher compressive stress, and an increase in Young’s modulus from 0.5 ± 0.1 kPa to 2.1 ± 0.2 kPa than the hydrogel without F127-DA. The bond strength of hydrogels with TA added was 102 times higher (0.41 | 0.05 kPa) than that without TA added. This hydrogel patch could be degraded and absorbed in vivo. The high fracture strength of sealed incisions compared to commercial adhesive pad seals makes them more suitable for wireless wound healing.68 In summary, the hydrogel synthesized by F127-DA and PEG-DA can generally be used for wireless suture wound closure.
QCSDA
The quaternary ammonium salt of chitosan has been demonstrated to possess antifungal and antioxidant properties. Therefore, to enhance the reactivity of QCS, the introduction of acrylate to a chitosan quaternary ammonium salt via a double bond linkage was examined. Pei et al used F127-DA, QCSDA, silk fibroin (SF), and TA to form hydrogels, wherein SF exhibited hemostatic function. Rheological and tensile tests tested the mechanical properties of the hydrogels. The tensile strain was 767% at 2% SF content, which showed good elastic properties. TA formed hydrogen bonds with other components, which increased the tensile stress and decreased the elongation; in contrast, the hydrogel with SF content of 2% soaked in TA for 16 h had the most maximum tensile stress (76.22 kPa) and good elongation (629%). In conclusion, the hydrogel had good mechanical properties. The adhesion of the hydrogel to pigskin was quantitatively determined by the bending and shearing method, and its adhesive strength was 11.41×0.99 and could adhere to metal, plastic, glass, and wet tissue paper. In addition, the described hydrogel exhibited low dilatability, toughness, antibacterial, antioxidant, and hemostatic functions, while also promoting tissue regeneration.24 This hydrogel holds promise for clinical wound healing.
Formation of Hydrogels Between F127-DA and Methacrylate-Based Polymers
Methacrylate-based polymers are commonly employed in contact lenses69 and 3D printing,70 and have recently been examined for the preparation of hydrogels for tissue repair. As summarized in the following subsections, F127-DA has been QCSG, combined with glycidyl methacrylated(COS) and SBMA to provide a range of new hydrogels for acute wounds, postoperative wounds, and diabetic wounds.
QCSG
QCSG can exert hemostatic effects through electrostatic adsorption of positively charged quaternary ammonium functional groups. Carbon nanotubes (CNTs) are conductive and responsive to NIR stimuli responsive ability and can improve the gel’s mechanical properties. Guo et al used CNT, QCSG and F127-DA as the substrate formula to form injectable antimicrobial conductive cryogels loaded with ibuprofen for wound dressing. The cryogel showed better hemostasis in a mouse tail amputation model and a liver injury model (CNT content of 4 mg/mL in the cryogel system showed better hemostasis than gauze and gelatin hemostatic sponges). The cryogel has excellent healing capabilities (high wound shrinkage, low inflammatory cells, and high angiogenesis) compared to Tegaderm™ film. In addition has strong mechanical strength, electrical conductivity, rapid blood-triggered shape recovery, sustained drug stability, hemocompatibility, slow release cytocompatibility and high blood uptake capacity, providing a potential new approach to the clinical application of F127.71
Glycidylmethacrylated COS
Yoo et al reported the preparation of a hydrogel by mixing photoluminescent glycidyl methacrylated COS and F127-DA loaded with recombinant human epidermal growth factor (rhEGF) for wound care in diabetic ulcers. This hydrogel acts as a thermoresponsive hydrogel hat serves as a wound adhesive. It was found that the incorporation of COS and rhEGGs significantly enhanced epidermal differentiation during wound healing. The release rate of rhEGF was dependent on the degradation rate of the hydrogel. This hydrogel could facilitate wound healing and promote keratinized cell differentiation in epidermal tissue, which is beneficial for diabetic wound care.26 Later, the authors replaced rhEGF with the basic fibroblast growth factor and heparin, and proposed that the resulting hydrogel could be used as a protein delivery systems and a tissue engineering scaffold.72
SBMA
SBMA is a promising amphiphilic material that has recently been used in antifouling and wound healing regeneration owing to its highly hydrophilic nature.73,74 Ashraf et al found that N,N′-methylene bis(acrylamide)(MBAAm) can be used as a cross-linking agent for SBMA.75 Based on this, Fu et al used MBAAm and F127-DA as cross-linking agents for SBMA to generate mechanically reactive hydrogel dressings for acute wounds. The hydrogel’s ultimate tensile strength, tensile strain and compressive stress were 112 kPa, 1420% and 1.41 MPa, respectively. The bonding strength of the hydrogel to pig skin was evaluated by the lap-shear method, and the adhesion force of the hydrogel to the pig skin tissue was 5.97 kPa. The drug release can be controlled under the application of mechanical force. In terms of protein adsorption, there was a 35% reduction over commercial chitinous dressings and an 8% reduction over commercial UrgoTul Ag dressings. In terms of adhesion of Staphylococcus aureus to the dressing, it was reduced by ~ 2.33 orders of magnitude compared to commercial UrgoClean. In conclusion, this hydrogel dressing can be used as an alternative to acute sports wound dressings.76
Conclusions
This review summarizes recent work on Pluronic F127 hydrogels and their derivatives (F127-CHO, F127-NH2 and F127-DA) for wound healing and repair. The F127 molecule contains hydrophobic (PPO) and hydrophilic (PEO) segments, and this hydrophobic property allows it to self-assemble into nanomicelles in water, commonly used for the solubilization and loading of hydrophobic drugs. In addition, F127 is thermosensitive, non-toxic, injectable, biocompatible, biodegradable. It is used as a drug carrier, wound dressing, surfactant, and 3D printing ink preparation. F127 was reacted with Dess-Martin periodinane, to obtain F127-CHO, which readily reacts with amine or hydrazine to form dynamic C=N bonds, enhancing mechanical properties, self-healing properties, and tissue adhesion properties. This hydrogel is mainly used for burns, bacterial/superbacterial infections, diabetic wounds, and uterine scars. F127 is obtained by reacting with PPh3, DIAD, and N2H2·H2O to obtain F127-NH2, which reacts with the aldehyde group of OHA to form a hydrogel with high modulus, stability, and self-fixation ability for preventing postoperative adhesions. The molecular chain of F127 The introduction of propylene groups forms F127-DA, which has light-curing ability, excellent mechanical properties, and exhibits high strength and toughness in diabetic wound care and severe wounds. When PEG-DA or TA is present in the system, it can be used for sutureless wound closure. In summary, F127 derivatives have important applications in wound repair and regeneration (Table 1).
 |
Table 1 Summary of the Application of F127 Derivatives in Wound Healing |
In general, F127 is usually readily cleared and degraded in vivo, thus limiting the distribution and therapeutic effect of F127 in vivo, in addition to having moderate mechanical strength, adhesion and self-healing ability. F127-CHO and F127-NH2 can form Schiff bases to improve these partial properties, but Schiff bases are dynamic and not mechanically strong enough to be used in high strength mechanics such as bones, teeth and 3D printing. F127-DA is irreversible once chemical bonds are formed with double bonded light curing, at which point it has excellent mechanical strength and toughness, but at the same time will lose self-healing properties. In addition, F127 and its derivatives are generally not used alone and need to be added to other materials to improve performance. These limits its application in biomedical fields.
Future Perspective
In order to enhance the mechanical strength, media adhesion and self-healing ability of F127 hydrogel during wound healing and repair, and to solve the problem of rapid removal and degradation in vivo, these problems can be solved in the future by chemical modification of F127 (position, number, type, and spatial site resistance, etc.), physical modification of cavity-loaded substances (temperature sensitizers, photosensitizers, pH responders, electrosensitizers, and enzymes, etc.), binding to other molecules (ligands, enzymes, drugs and proteins, cell membranes and mitochondria, etc.), and binding to nanomaterials (nanoparticles, nanowires, polymeric nanoparticles and nanofibers, etc.).
For wound healing, there is still a lot of room for development of F127 derivatives. For example, for wound dressings, there is a need to improve healing efficiency and time. For wireless suture adhesives, there is a need for a perioperative period that can be prepared successfully and address issues such as hemostasis, proper adhesion, and copolymer-induced inflammation. For anti-adhesive agents in the abdomen, heart, and tendons, issues such as low tissue adhesion, rapid degradation, and poor in vivo retention need to be addressed. In addition, a process for monitoring wound healing needs to be developed to establish a guideline for each stage of the wound to determine what treatment is needed when and how to treat it. After obtaining dynamic data on the wound, the structure and function of the hydrogel will be further designed to enable personalized treatment.
In recent years, mitochondria have attracted increasing attention in wound healing, and mitochondrial involvement in wound repair and regeneration is mainly focused on ROS levels,77,78 mitochondrial respiration,79 mitochondrial fracture,80 mitochondrial metabolism,81 and mitochondrial transfer,82 etc. Nanomaterials targeting cell-loaded drugs are one of the hot spots of research in recent years.83 Among the nanomaterials (mesoporous polydopamine nanoparticles,84 Au nanocage)85 and mitochondria for diabetic wound healing have also been reported. In the future, it is possible to target cells near the wound (fibroblasts,27 neutrophils,86 and macrophages,87 etc.) or mitochondria for wound repair by F127 nanoscaffolds for wound repair. This can be achieved by several methods: 1. Mitochondrial removal by modified F127, which causes apoptosis in the vicinity of the wound for wound healing but leads to both mitochondrial dysfunction and delayed wound healing. 2. F127 responds accordingly to target cells by adjusting ROS levels, mitochondrial respiration, mitochondrial breakage, mitochondrial metabolism and mitochondrial translocation to achieve wound healing. 3. F127 loaded with drugs and ligands, targeting wound cells to further localize to mitochondria, releasing drugs and thus healing the wound. 3. F127 is loaded with drug, stimulus response agent (photosensitizer, acoustic sensitizer, photothermal imaging contrast agent, photothermal transducer and pH responsive material), and mitochondria target the stimulus response agent, and in response to the stimulus response, the drug is released to the target cells where the mitochondria are located to achieve wound healing.
As researchers continue to practice and accumulate knowledge, materials including F127 derivatives, which are believed to be increasingly available, will be widely used in the near future, not only for wound healing and scar repair, but also for bone repair,88 periodontitis,89 otitis externa,90 conjunctivitis,91 analgesia,92 and HIV infection93 to further improve the quality of life of patients.
Databases
We used the PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of Science (https://www.webofscience.com/wos/alldb/basic-search) databases in this review, with “F127” and “wound” as keywords and any year, the last five years were chosen as far as possible, and ultimately novelty and relevance as the principle to determine whether to introduce articles.
Author Contributions
Shanshan Li, Cheng Yang, and Junqiang Li contributed equally to this review. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the [The Second Affiliated Hospital, Air Force Medical University] under Grant [number 2021SHRCC063], project leader: Shanshan Li.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tang Q, Xue N, Ding X, et al. Role of wound microbiome, strategies of microbiota delivery system and clinical management. Adv Drug Deliv Rev. 2023;192:114671. doi:10.1016/j.addr.2022.114671
2. Markiewicz-Gospodarek A, Koziol M, Tobiasz M, et al. Burn wound healing: clinical complications, medical care, treatment, and dressing types: the current state of knowledge for clinical practice. Int J Environ Res Public Health. 2022;19(3):1338. doi:10.3390/ijerph19031338
3. Stewart BT, Yankson IK, Afukaar F, et al. Road traffic and other unintentional injuries among travelers to developing countries. Med Clin North Am. 2016;100(2):331–343. doi:10.1016/j.mcna.2015.07.011
4. Doss ER, Popejoy LL. Informal family caregiving of patients with diabetic extremity wounds: an integrative review. West J Nurs Res. 2023;45(3):272–281. doi:10.1177/01939459221115694
5. Gao L, Liu X, Zhao W, et al. Extracellular-matrix-mimicked 3D nanofiber and hydrogel interpenetrated wound dressing with a dynamic autoimmune-derived healing regulation ability based on wound exudate. Biofabrication. 2022;15(1):015021. doi:10.1088/1758-5090/acaa01
6. Han C, Zhang X, Pang G, et al. Hydrogel microcapsules containing engineered bacteria for sustained production and release of protein drugs. Biomaterials. 2022;287:121619. doi:10.1016/j.biomaterials.2022.121619
7. Yang B, Liang C, Chen D, et al. A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. Bioact Mater. 2022;15:103–119. doi:10.1016/j.bioactmat.2021.11.032
8. Tolabi H, Davari N, Khajehmohammadi M, et al. Progress of microfluidic hydrogel-based scaffolds and organ-on-chips for the cartilage tissue engineering. Adv Mater. 2023;35:e2208852. doi:10.1002/adma.202208852
9. Li Z, Li G, Xu J, et al. Hydrogel transformed from nanoparticles for prevention of tissue injury and treatment of inflammatory diseases. Adv Mater. 2022;34(16):e2109178. doi:10.1002/adma.202109178
10. Chen J, Chen L, Wu Y, et al. A H2O2-activatable nanoprobe for diagnosing interstitial cystitis and liver ischemia-reperfusion injury via multispectral optoacoustic tomography and NIR-II fluorescent imaging. Nat Commun. 2021;12(1):6870. doi:10.1038/s41467-021-27233-4
11. Zou S, He Q, Wang Q, et al. Injectable Nanosponge-loaded Pluronic F127 hydrogel for pore-forming toxin neutralization. Int J Nanomedicine. 2021;16:4239–4250. doi:10.2147/IJN.S315062
12. Pradines B, Djabourov M, Vauthier C, et al. Gelation and micellization behaviors of pluronic((R)) F127 hydrogel containing poly(isobutylcyanoacrylate) nanoparticles specifically designed for mucosal application. Colloids Surf B Biointerfaces. 2015;135:669–676. doi:10.1016/j.colsurfb.2015.08.021
13. Cao J, Su M, Hasan N, et al. Nitric oxide-releasing thermoresponsive Pluronic F127/Alginate hydrogel for enhanced antibacterial activity and accelerated healing of infected wounds. Pharmaceutics. 2020;12(10):926. doi:10.3390/pharmaceutics12100926
14. Dung TH, Huong LT, Yoo H. Morphological feature of Pluronic F127 and its application in burn treatment. J Nanosci Nanotechnol. 2018;18(2):829–832. doi:10.1166/jnn.2018.14880
15. Lee SY, Jeon SI, Sim SB, et al. A supramolecular host-guest interaction-mediated injectable hydrogel system with enhanced stability and sustained protein release. Acta Biomater. 2021;131:286–301. doi:10.1016/j.actbio.2021.07.004
16. Wang C, Wang M, Xia K, et al. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact Mater. 2021;6(8):2523–2534. doi:10.1016/j.bioactmat.2021.01.029
17. Chen Y, Khan AR, Yu D, et al. Pluronic F127-functionalized molybdenum oxide nanosheets with pH-dependent degradability for chemo-photothermal cancer therapy. J Colloid Interface Sci. 2019;553:567–580. doi:10.1016/j.jcis.2019.06.066
18. Zhou H, Qi Z, Pei P, et al. Biocompatible nanomicelles for sensitive detection and photodynamic therapy of early-stage cancer. Biomater Sci. 2021;9(18):6227–6235. doi:10.1039/D1BM00847A
19. Klouda L and Mikos AG. (2008). Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm, 68(1), 34–45. 10.1016/j.ejpb.2007.02.025
20. Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346(6212):941–945. doi:10.1126/science.1253836
21. Guo B, Dong R, Liang Y, et al. Haemostatic materials for wound healing applications. Nat Rev Chem. 2021;5(11):773–791. doi:10.1038/s41570-021-00323-z
22. Li Q, Wang D, Jiang Z, et al. Advances of hydrogel combined with stem cells in promoting chronic wound healing. Front Chem. 2022;10:1038839. doi:10.3389/fchem.2022.1038839
23. Qu J, Zhao X, Liang Y, et al. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi:10.1016/j.biomaterials.2018.08.044
24. Zhang L, Zhang Y, Ma F, et al. A low-swelling and toughened adhesive hydrogel with anti-microbial and hemostatic capacities for wound healing. J Mater Chem B. 2022;10(6):915–926. doi:10.1039/D1TB01871J
25. Wang S, Zheng H, Zhou L, et al. Nanoenzyme-reinforced injectable hydrogel for healing diabetic wounds infected with multidrug resistant bacteria. Nano Lett. 2020;20(7):5149–5158. doi:10.1021/acs.nanolett.0c01371
26. Choi JS, Yoo HS. Pluronic/chitosan hydrogels containing epidermal growth factor with wound-adhesive and photo-crosslinkable properties. J Bimed Mater Res A. 2010;95A(2):564–573. doi:10.1002/jbm.a.32848
27. Latif A, Fisher LE, Dundas AA, et al. Microparticles decorated with cell-instructive surface chemistries actively promote wound healing. Adv Mater;2022. e2208364. doi:10.1002/adma.202208364
28. Roy R, Zayas J, Mohamed MF, et al. IL-10 dysregulation underlies chemokine insufficiency, delayed macrophage response, and impaired healing in diabetic wounds. J Invest Dermatol. 2022;142(3 Pt A):692–704.e14. doi:10.1016/j.jid.2021.08.428
29. Mofazzal JM, Sahandi ZP, Moosavi BS, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33–64. doi:10.1016/j.addr.2017.08.001
30. Schmolka IR. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J Biomed Mater Res. 1972;6(6):571–582. doi:10.1002/jbm.820060609
31. Nalbandian RM, Henry RL, Wilks HS. Artificial skin. II. Pluronic F-127 Silver nitrate or silver lactate gel in the treatment of thermal burns. J Bimed Mater Res. 1972;6(6):583. doi:10.1002/jbm.820060610
32. Wang S, Li J, Ma Z, et al. A sequential therapeutic hydrogel with injectability and antibacterial activity for deep burn wounds’ cleaning and healing. Front Bioeng Biotechnol. 2021;9:794769. doi:10.3389/fbioe.2021.794769
33. Li Z, Zhou F, Li Z, et al. Hydrogel cross-linked with dynamic covalent bonding and micellization for promoting burn wound healing. ACS Appl Mater Inter. 2018;10(30):25194–25202. doi:10.1021/acsami.8b08165
34. Alvarado-Gomez E, Martínez-Castañon G, Sanchez-Sanchez R, Ganem-Rondero A, Yacaman MJ, Martinez-Gutierrez F. Evaluation of anti-biofilm and cytotoxic effect of a gel formulation with Pluronic F-127 and silver nanoparticles as a potential treatment for skin wounds. Mat Sci Eng C-Bio S. 2018;92:621–630. doi:10.1016/j.msec.2018.07.023
35. Kant V, Gopal A, Kumar D, et al. Topical pluronic F-127 gel application enhances cutaneous wound healing in rats. Acta Histochem. 2014;116(1):5–13. doi:10.1016/j.acthis.2013.04.010
36. Muszanska AK, Busscher HJ, Herrmann A, van der Mei HC, Norde W. Pluronic-lysozyme conjugates as anti-adhesive and antibacterial bifunctional polymers for surface coating. Biomaterials. 2011;32(26):6333–6341. doi:10.1016/j.biomaterials.2011.05.016
37. Zhou D, Li S, Pei M, et al. Dopamine-modified hyaluronic acid hydrogel adhesives with fast-forming and high tissue adhesion. ACS Appl Mater Inter. 2020;12(16):18225–18234. doi:10.1021/acsami.9b22120
38. Liu G, Wang L, He Y, et al. Polydopamine nanosheets doped injectable hydrogel with nitric oxide release and photothermal effects for bacterial ablation and wound healing. Adv Healthc Mater. 2021;10(23):e2101476. doi:10.1002/adhm.202101476
39. Mei L, Zhang D, Shao H, et al. Injectable and self-healing probiotics-loaded hydrogel for promoting superbacteria-infected wound healing. ACS Appl Mater Inter. 2022;14(18):20538–20550. doi:10.1021/acsami.1c23713
40. Yang B, Song J, Jiang Y, et al. Injectable adhesive self-healing multicross-linked double-network hydrogel facilitates full-thickness skin wound healing. ACS Appl Mater Inter. 2020;12(52):57782–57797. doi:10.1021/acsami.0c18948
41. Li S, Pei M, Wan T, et al. Self-healing hyaluronic acid hydrogels based on dynamic Schiff base linkages as biomaterials. Carbohydr Polym. 2020;250:116922. doi:10.1016/j.carbpol.2020.116922
42. Wei Z, Zhao J, Chen YM, et al. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci Rep. 2016;6:37841. doi:10.1038/srep37841
43. Deng P, Chen F, Zhang H, et al. Multifunctional double-layer composite hydrogel conduit based on chitosan for peripheral nerve repairing. Adv Healthc Mater. 2022;11(13):e2200115. doi:10.1002/adhm.202200115
44. Gong X, Luo M, Wang M, et al. Injectable self-healing ceria-based nanocomposite hydrogel with ROS-scavenging activity for skin wound repair. Regen Biomater. 2022;9(1):rbab074. doi:10.1093/rb/rbab074
45. Hu Q, Xie N, Liao K, et al. An injectable thermosensitive Pluronic F127/hyaluronic acid hydrogel loaded with human umbilical cord mesenchymal stem cells and asiaticoside microspheres for uterine scar repair. Int J Biol Macromol. 2022;219:96–108. doi:10.1016/j.ijbiomac.2022.07.161
46. Engel AK, Yoden T, Sanui K, et al. Synthesis of aromatic Schiff base oligomers at the air/water interface. J Am Chem Soc. 1985;107(26):8308–8310. doi:10.1021/ja00312a108
47. Yu H, Liu Y, Yang H, et al. An injectable self-healing hydrogel based on chain-extended PEO-PPO-PEO multiblock copolymer. Macromol Rapid Commun. 2016;37(21):1723–1728. doi:10.1002/marc.201600323
48. Wang P, Deng G, Zhou L, et al. Ultrastretchable, self-healable hydrogels based on dynamic covalent bonding and triblock copolymer micellization. ACS Macro Lett. 2017;6(8):881–886. doi:10.1021/acsmacrolett.7b00519
49. Pathak K, Misra SK, Sehgal A, et al. Biomedical applications of quaternized chitosan. Polymers. 2021;13(15):2514. doi:10.3390/polym13152514
50. Zhang H, Qiu T, Bai Y, et al. Enhanced antibacterial activity of lysozyme loaded quaternary ammonium chitosan nanoparticles functionalized with cellulose nanocrystals. Int J Biol Macromol. 2021;191:71–78. doi:10.1016/j.ijbiomac.2021.09.027
51. Mi Y, Chen Y, Tan W, et al. The influence of bioactive glyoxylate bearing Schiff base on antifungal and antioxidant activities to chitosan quaternary ammonium salts. Carbohydr Polym. 2022;278:118970. doi:10.1016/j.carbpol.2021.118970
52. Liu W, Wang M, Cheng W, et al. Bioactive antiinflammatory antibacterial hemostatic citrate-based dressing with macrophage polarization regulation for accelerating wound healing and hair follicle neogenesis. Bioact Mater. 2021;6(3):721–728. doi:10.1016/j.bioactmat.2020.09.008
53. Serracanta J, Baena J, Martinez-Mendez JR, et al. Bromelain-based enzymatic burn debridement: Spanish multidisciplinary consensus. Eur J Plast Surg. 2022;2022:1–9.
54. Zhou L, Xi Y, Xue Y, et al. Injectable self-healing antibacterial bioactive polypeptide-based hybrid nanosystems for efficiently treating multidrug resistant infection, skin-tumor therapy, and enhancing wound healing. Adv Funct Mater. 2019;29(22):1806883. doi:10.1002/adfm.201806883
55. He Y, Liu K, Guo S, et al. Multifunctional hydrogel with reactive oxygen species scavenging and photothermal antibacterial activity accelerates infected diabetic wound healing. Acta Biomater. 2023;155:199–217. doi:10.1016/j.actbio.2022.11.023
56. Wu H, Li F, Wang S, et al. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials. 2018;151:66–77. doi:10.1016/j.biomaterials.2017.10.018
57. Li Z, Liu L, Chen Y. Dual dynamically crosslinked thermosensitive hydrogel with self-fixing as a postoperative anti-adhesion barrier. Acta Biomater. 2020;110:119–128. doi:10.1016/j.actbio.2020.04.034
58. Cellesi F, Tirelli N, Hubbell JA. Materials for cell encapsulation via a new tandem approach combining reverse thermal gelation and covalent crosslinking. Macromol Chem Phys. 2002;203(10–11):1466–1472. doi:10.1002/1521-3935(200207)203:10/11<1466::AID-MACP1466>3.0.CO;2-P
59. Zheng Z, Eglin D, Alini M, et al. Visible light-induced 3D bioprinting technologies and corresponding bioink materials for tissue engineering: a review. Engineering-Prc. 2021;7(7):966–978.
60. Ren P, Zhang H, Dai Z, et al. Stiff micelle-crosslinked hyaluronate hydrogels with low swelling for potential cartilage repair. J Mater Chem B. 2019;7(36):5490–5501. doi:10.1039/C9TB01155B
61. Shen C, Li Y, Meng Q. Adhesive polyethylene glycol-based hydrogel patch for tissue repair. Colloid Surface B. 2022;218:112751. doi:10.1016/j.colsurfb.2022.112751
62. Hiendlmeier L, Zurita F, Vogel J, et al. 4D printed soft and stretchable self-folding cuff electrodes for small-nerve interfacing. Adv Mater. 2023;35:e2210206. doi:10.1002/adma.202210206
63. Liu Y, Zhang Z, Zhang Y, et al. Construction of adhesive and bioactive silk fibroin hydrogel for treatment of spinal cord injury. Acta Biomater. 2022. doi:10.1016/j.actbio.2022.12.048
64. Zhang N, Wang Y, Zhang J, Guo J, He J. Controlled domain gels with a biomimetic gradient environment for osteochondral tissue regeneration. Acta Biomater. 2021;135:304–317. doi:10.1016/j.actbio.2021.08.029
65. Xu C, Chang Y, Xu Y, et al. Silicon-phosphorus-nanosheets-integrated 3D-printable hydrogel as a bioactive and biodegradable scaffold for vascularized bone regeneration. Adv Healthc Mater. 2022;11(6):e2101911. doi:10.1002/adhm.202101911
66. Du X, Wu L, Yan H, et al. Multifunctional hydrogel patch with toughness, tissue adhesiveness, and antibacterial activity for sutureless wound closure. ACS Biomater Sci Eng. 2019;5(5):2610–2620. doi:10.1021/acsbiomaterials.9b00130
67. Davidson EC. Tannic Acid in the Treatment of Burns. Surg Gynecol Obstet. 1928;545:351.
68. Du X, Hou Y, Wu L, et al. An anti-infective hydrogel adhesive with non-swelling and robust mechanical properties for sutureless wound closure. J Mater Chem B. 2020;8(26):5682–5693. doi:10.1039/D0TB00640H
69. Chatterjee S, Upadhyay P, Mishra M, et al. Advances in chemistry and composition of soft materials for drug releasing contact lenses. Rsc Adv. 2020;10(60):36751–36777. doi:10.1039/D0RA06681H
70. Wang L, Shen M, Hou Q, et al. 3D printing of reduced glutathione grafted gelatine methacrylate hydrogel scaffold promotes diabetic bone regeneration by activating PI3K/Akt signaling pathway. Int J Biol Macromol. 2022;222(Pt A):1175–1191. doi:10.1016/j.ijbiomac.2022.09.236
71. Zhao X, Guo B, Wu H, et al. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat Commun. 2018;9(1):2784. doi:10.1038/s41467-018-04998-9
72. Choi JS, Yoo HS. Chitosan/pluronic hydrogel containing bFGF/heparin for encapsulation of human dermal fibroblasts. J Biomater Sci Polym Ed. 2013;24(2):210–223. doi:10.1163/156856212X630267
73. Wu J, Xiao Z, Chen A, et al. Sulfated zwitterionic poly(sulfobetaine methacrylate) hydrogels promote complete skin regeneration. Acta Biomater. 2018;71:293–305. doi:10.1016/j.actbio.2018.02.034
74. Zhao Z, Pan M, Qiao C, et al. Bionic engineered protein coating boosting anti-biofouling in complex biological fluids. Adv Mater. 2022;35:e2208824. doi:10.1002/adma.202208824
75. Ibrahim G, Isloor AM, Asiri AM, et al. Novel, one-step synthesis of zwitterionic polymer nanoparticles via distillation-precipitation polymerization and its application for dye removal membrane. Sci Rep. 2017;7(1):15889. doi:10.1038/s41598-017-16131-9
76. Fang K, Wang R, Zhang H, et al. Mechano-responsive, tough, and antibacterial zwitterionic hydrogels with controllable drug release for wound healing applications. ACS Appl Mater Inter. 2020;12(47):52307–52318. doi:10.1021/acsami.0c13009
77. Xu Z, Liu Y, Ma R, et al. Thermosensitive hydrogel incorporating Prussian blue nanoparticles promotes diabetic wound healing via ROS scavenging and mitochondrial function restoration. ACS Appl Mater Inter. 2022;14(12):14059–14071. doi:10.1021/acsami.1c24569
78. Xu S, Li S, Bjorklund M, et al. Mitochondrial fragmentation and ROS signaling in wound response and repair. Cell Regen. 2022;11(1):38. doi:10.1186/s13619-022-00141-8
79. Schiffmann LM, Werthenbach JP, Heintges-Kleinhofer F, et al. Mitochondrial respiration controls neoangiogenesis during wound healing and tumour growth. Nat Commun. 2020;11(1):3653. doi:10.1038/s41467-020-17472-2
80. Fu H, Zhou H, Yu X, et al. Wounding triggers MIRO-1 dependent mitochondrial fragmentation that accelerates epidermal wound closure through oxidative signaling. Nat Commun. 2020;11(1):1050. doi:10.1038/s41467-020-14885-x
81. Willenborg S, Sanin DE, Jais A, et al. Mitochondrial metabolism coordinates stage-specific repair processes in macrophages during wound healing. Cell Metab. 2021;33(12):2398–2414.e9. doi:10.1016/j.cmet.2021.10.004
82. Jin P, Pan Q, Lin Y, et al. Platelets facilitate wound healing by mitochondrial transfer and reducing oxidative stress in endothelial cells. Oxid Med Cell Longev. 2023;2023:2345279. doi:10.1155/2023/2345279
83. Khalilov R. A COMPREHENSIVE REVIEW OF ADVANCED NANO-BIOMATERIALS IN REGENERATIVE MEDICINE AND DRUG DELIVERY. ABES. 2023;8(1):5–18.
84. Deng QS, Gao Y, Rui BY, et al. Double-network hydrogel enhanced by SS31-loaded mesoporous polydopamine nanoparticles: symphonic collaboration of near-infrared photothermal antibacterial effect and mitochondrial maintenance for full-thickness wound healing in diabetes mellitus. Bioact Mater. 2023;27:409–428. doi:10.1016/j.bioactmat.2023.04.004
85. Ding J, Gao B, Chen Z, et al. An NIR-triggered au nanocage used for photo-thermo therapy of chronic wound in diabetic rats through bacterial membrane destruction and skin cell mitochondrial protection. Front Pharmacol. 2021;12:779944. doi:10.3389/fphar.2021.779944
86. Wang W, Gao Y, Zhang M, et al. Neutrophil-like biomimic AIE nanoparticles with high-efficiency inflammatory cytokine targeting enable precise photothermal therapy and alleviation of inflammation. ACS Nano. 2023;17(8):7394–7405. doi:10.1021/acsnano.2c11762
87. Xiong Y, Lin Z, Bu P, et al. A whole-course-repair system based on neurogenesis-angiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv Mater. 2023;35(19):e2212300. doi:10.1002/adma.202212300
88. Ahmadian E, Eftekhari A, Janas D, et al. Nanofiber scaffolds based on extracellular matrix for articular cartilage engineering: a perspective. Nanotheranostics. 2023;7(1):61–69. doi:10.7150/ntno.78611
89. Almoshari Y, Ren R, Zhang H, et al. GSK3 inhibitor-loaded osteotropic Pluronic hydrogel effectively mitigates periodontal tissue damage associated with experimental periodontitis. Biomaterials. 2020;261:120293. doi:10.1016/j.biomaterials.2020.120293
90. Al-Mahallawi AM, Abdelbary AA, El-Zahaby SA. Norfloxacin loaded nano-cubosomes for enhanced management of otitis externa: in vitro and in vivo evaluation. Int J Pharm. 2021;600:120490. doi:10.1016/j.ijpharm.2021.120490
91. Zafar A, Imam SS, Yasir M, et al. Preparation of NLCs-based topical erythromycin gel: in vitro characterization and antibacterial assessment. Gels. 2022;8(2):116. doi:10.3390/gels8020116
92. Zhang J, Zhu S, Zhao M, et al. Analgesic and potentiated photothermal therapy with ropivacaine-loaded hydrogels. Theranostics. 2023;13(7):2226–2240. doi:10.7150/thno.81325
93. Enggi CK, Isa HT, Sulistiawati S, et al. Development of thermosensitive and mucoadhesive gels of cabotegravir for enhanced permeation and retention profiles in vaginal tissue: a proof of concept study. Int J Pharm. 2021;609:121182. doi:10.1016/j.ijpharm.2021.121182
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.