Back to Journals » Infection and Drug Resistance » Volume 15
Progress in Multidisciplinary Treatment of Fournier’s Gangrene
Authors Zhang KF, Shi CX, Chen SY , Wei W
Received 15 September 2022
Accepted for publication 10 November 2022
Published 28 November 2022 Volume 2022:15 Pages 6869—6880
DOI https://doi.org/10.2147/IDR.S390008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Ke-Fan Zhang,1,* Chuan-Xin Shi,1,* Si-Yu Chen,2,* Wei Wei1
1Department of General Surgery, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China; 2Department of Cardiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Wei, Department of General Surgery, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China, Email [email protected]
Abstract: Fournier’s gangrene (FG) is a life-threatening and special form of necrotizing fasciitis, characterized by occult onset, rapid progress and high mortality, occurring mainly in men over 50 years of age. Risk factors of FG include diabetes, HIV infection, chronic alcoholism and other immunosuppressive state. FG was previously considered as an idiopathic disease, but in fact, three quarters of the infections originated from the skin, urethra and gastrointestinal tract. Initial symptoms of FG are often inconsistent with severity and can progress promptly to fatal infection. Although the treatment measures of FG have been improved in recent years, the mortality does not seem to have decreased significantly and remains at 20% – 30%. The time to identify FG and the waiting period before surgical debridement are directly related to the prognosis. Therefore, in addition to the combination of intensive fluid resuscitation and broad-spectrum antibiotics, treatment of FG should particularly emphasize the importance of early surgical debridement assisted with fecal diversion and skin reconstruction when necessary. This paper is to briefly summarize the progress in the definition, epidemiology, clinical manifestations, diagnosis, treatment and prognosis of Fournier’s gangrene in recent years, more importantly, illustrates the importance of multidisciplinary cooperation in the management of FG.
Keywords: Fournier’s gangrene, necrotizing fasciitis, lethal infection, surgical debridement, multidisciplinary cooperation
Introduction
Fournier’s gangrene (FG) is a rare and dangerous necrotizing soft tissue infection (NSTI), characterized by obliterative endarteritis and arteriolar thrombosis of subcutaneous tissue caused by bacterial infection. FG mainly occurs in the external genitalia and perineal areas, and can spread rapidly along the fascia plane, eventually leading to sepsis and multiple organ failure.1 Genital gangrene was first mentioned in the middle-ages medical works of famous Arab physician Avicenna. Baurienne published the first case of FG in early modern medical literature in 1784. A boy developed scrotal swelling and tissue damage on the fourth day after being bitten by a cow. This disease was eventually named after French eminent venereologist Dr. Alfred Fournier after he reported a case series in 1883, containing five cases of previously healthy young men with rapidly progressive necrotizing infection.2 Willison introduced the term “necrotizing fasciitis” to describe the characteristic symptoms of FG.3 Many terms have been used to describe the clinical condition including “idiopathic gangrene of the scrotum”, “periurethral phlegmon”, “streptococcal scrotal gangrene”, “phagedena” and “synergistic necrotizing cellulitis.”4
Epidemiology
Incidence and Demographics
FG is a relatively rare surgical emergency, which was initially considered as an idiopathic disease of male. The latest epidemiological investigation found that this disease can occur at any age and gender. The overall incidence is about 1.6/100,000 males, and male incidence increased with age reaching the peak of 3.3/100,000 between the age of 50 and 79.5,6 Epidemiological study based on State Inpatient Database of United States identified 1641 male FG patients (97.7%) and 39 female FG patients (2.3%), accounting for only 0.02% of hospitalizations.5,6 Male cases are far more than female cases (male:female is 10:1), and the proportion of patients younger than 40 years old is very small.7,8 Contemporary study based on National Inpatient Sample from 2004 to 2012 found that the proportion of FG patients aged 40 to 59 years was the highest, reaching 50.7%.8 The large-scale case analysis showed that the female incidence of FG is far lower than that of male, but the severity of female patients seems to be greater than male.6 Recent studies showed that the female incidence rate is increasing, the proportion of female patients was as high as 23% and 55% in the two recent case studies.9,10 However, with the increase of the average age of the population and the number of patients receiving immunosuppressive therapy or infected with human immunodeficiency virus (HIV), the incidence of FG incidence rate has increased in some areas.11
Mortality and Prognosis Predication
A study systematic reviewed 6152 cases published from 1993 to 2018, and found that the case mortality was between 0–42% respectively, and the total mortality was 19.8%, which did not seem to have improved significantly over the twenty-five years.12 However, a recent study found that the mean mortality of FG patients from 2000 to 2021 was 7.5%, which has decreased compared with previous years.13
There are many factors significantly related to the prognosis of FG patients, including early diagnosis, waiting period to surgery and comorbidity. Surgery is the cornerstone of the treatment of FG, any factors delaying surgical intervention predicts a poorer prognosis. A retrospective study found that the mortality will rise when the time for making a definitive diagnosis exceeds 136 minutes in the emergency department.14 Goh et al found that as the waiting period before debridement after admission increased from 24 hours to 48 hours, the survival rate decreased from 93.2% to 75.2%.15 Comorbidity is not only an important risk factor of FG, but also affects the prognosis. Relevant study found that the mortality of FG patients with diabetes, heart disease, renal failure and kidney disease was significantly higher, but there was no significant correlation between liver and malignant diseases and mortality.16
Fournier's gangrene severity index(FGSI), including body temperature, respiratory rate, heart rate and serum sodium, was used to predict the prognosis of FG patients. When the FGSI score is >9, the mortality is 75%. When the FGSI score is ≤ 9, the survival rate is 78%.17 Although several studies support different thresholds, most studies have demonstrated that higher FGSI scores are helpful to identify high-risk patients and indicate poor prognosis.18,19 Considering the significant relationship between comorbidity and prognosis, adding comorbidity to FGSI may more accurately predict prognosis.16 There are many scoring systems that can predict the prognosis of FG patients, but little attention was paid to the length of stay, which is associated with hospitalization expenses and treatment methods.
Based on the correlation between each univariate and the length of stay, Ghodoussipour et al finally chose age and eight laboratory indicators to constitute a multivariable model, namely Combined Urology and Plastics Index (CUPI), for predicting the length of stay (LOS) (Table 1). The average LOS in patients with CUPI score ≤ 5 was 25 days (SD 15.6), while average LOS in patients with CUPI score >5 was 71 days (SD 49.8).20 CUPI is a supplement to the existing prognosis prediction model, high CUPI score being useful to detect high-risk FG patients and urge multidisciplinary participation, which can shorten LOS and improve the prognosis. However, there is a lack of prospective and multicenter studies to validate this scoring system.
 |
Table 1 CUPI Scoring System.20 |
Pathogenic Microorganism and Mechanism
FG was once considered idiopathic because of the lack of clear etiology. Modern systematic review found that only about a quarter of patients can be classified as idiopathic. The majority of infections of FG patients originated from genitourinary tract, anorectal and external genital soft tissues.21,22 According to the pathogenic microorganisms, necrotizing fasciitis can be roughly divided into four types: Type I (polymicrobial), Type II (monomicrobial), Type III (Clostridium) and Type IV (fungal) (Table 2). 23–28 Type I was most common, accounting for about 80%, which always endangers the elderly with comorbidity (such as diabetes, chronic kidney disease and alcoholism).25,28 Most FG was a mixed infection of multiple microorganisms, including Gram-positive and Gram-negative bacteria, anaerobic bacteria and/or spindle spore rods. Tang et al reviewed 2265 patients in the database in recent 30 years and found that 54% of patients had multiple microbial infections.29 Clara m et al retrospectively studied 131 FG patients underwent bacterial culture in a tertiary medical center in the United States from 2011 to 2018. The results showed that the median number of microorganisms was 3, and the most common pathogens were Staphylococcus (66; 46%), Streptococcus (53; 37%), Bacteroides (34; 24%), Candida (31,22%), and Escherichia coli (28; 20%).30
 |
Table 2 Classification of Necrotizing Fasciitis Based on Pathogenic bacteria23–28 |
The exotoxins and enzymes produced by aerobic and anaerobic bacteria can destroy tissues at the speed of 1 inch per hour and prolong the infection time. The lethal infection spreads rapidly from the genital area to the anterior abdominal wall and other important organs.31,32 Platelet aggregation and complement fixation induced by aerobic bacteria together with heparanase and collagenase produced by anaerobic bacteria promotes the thrombosis of local microvessels and severe ischemia in this area, which eventually progresses to Fournier’s gangrene.4
Risk Factors
FG related risk factors impair the function of the patient’s immune system, creating a favorable environment for the occurrence and progress of infection (Table 3).33 When the host in a state of low immunity, common symbiotic bacteria in human perineum play a synergistic role in the occurrence and progression of FG.
 |
Table 3 Related Risk Factors for the Development of Fournier’s Gangrene |
Comorbidity is an important risk factor of FG. Comorbidity such as diabetes causes persistent immune disorder and enhances the susceptibility of patients to sepsis by weakening innate immunity, adaptive immunity and immune regulation.34–36 The data show that about 52–88% of patients have at least one comorbidity.37,38 Patients with comorbidities such as diabetes and alcoholism, atherosclerosis, peripheral arterial disease, malnutrition, prostate cancer, human immunodeficiency virus (HIV) infection, leukemia and liver disease are likely to develop into FG.16,39–46 If the patient has more than one comorbidity at the same time, the incidence and severity of FG will rise.47
Diabetes has been considered as the most important causative factors of FG. Based on 1641 FG patients in 35 states in the United States, evidence showed that 37% of patients were complicated with diabetes, and the increase in diabetes prevalence is related to the increase in Fournier’s gangrene incidence.5 The FG incidence rate will increase by 0.2 / 100,000 for every 1% increase in the prevalence of diabetes.5 It is also pointed out that the use of sodium glucose cotransporter-2 (SGLT2) inhibitors in diabetes patients may be related to FG.48,49 However, there is no statistical evidence to support the correlation between SGLT2 inhibitors and FG, so this correlation needs to be strictly verified.50
Some local diseases around the perineum, especially in immunosuppressed people, are also important risk factors of the FG including urinary system diseases (renal abscess, urinary calculi, and urethral stricture),51–54 anorectal diseases (perianal abscess, thrombotic external hemorrhoids, and strangulated inguinal hernia)55–58 and local skin diseases (necrotic ulcer).59,60 In addition, pathogens that commonly cannot penetrate the skin can quickly reach deeper tissues through invasive operations, such as medical operations (catheterization,61,62 prostate biopsy63) and intravenous injection (drug abuse64,65).
Diagnosis
Clinical Manifestation
The early diagnosis of FG mainly depends on clinical manifestations and past history, but the early symptoms are subtle and unspecific. Study have showed that the interval from the initial symptoms to skin gangrene is 5.1 ± 3.1d, and about three quarters of cases were misdiagnosed.42,66 A population-based longitudinal study found the prodromal period of FG before diagnosis was about 21-day and nearly 50% of the 8098 patients got a symptomatically similar diagnosis (such as scrotal swelling, cellulitis and genital pain), which resulted in diagnostic delay.67 Early diagnosis of FG patients without risk factors and inducing conditions is very difficult, which require clinician to sufficiently understand the early manifestations.37,68
The earliest clinical manifestation of skin in FG patients includes perineum and perianal pain, pruritus, edema and unclear boundary patchy erythema. As the infection continues to spread along the fascia plane at the speed of 1 inch/h, the erythema color become deepen and bullae appear.16,31,66,69,70 Because of the local nerve injury, the pain in the lesion is reduced. When combined with anaerobic infection, subcutaneous twisting and malodorous purulent drainage may occur, and the final skin manifestation is gangrene.42 In addition to typical local skin manifestations, systemic symptoms include fever, chills and tachycardia. Goh et al systematically reviewed 1463 patients from 1980 to 2013 and determined several manifestations with diagnostic significance, including pain inconsistent with physical examination, deteriorate despite broad-spectrum antibiotics, bullae in the skin, and gas in the soft tissue on plain X-ray.15
The genital organ of most patients was affected by FG. In female patients, vulva or labia (95–100%) were almost all encroached by pathogen. In men, scrotum (71–76%) was easily involved, while testicles were less involved (47–53%), benefiting from independent blood supply.10 Isolated penis FG is more rarely, and its early manifestations are genital pain and fever, which can quickly develop into penis swelling, necrosis, ulcer and stench.71
Imaging Examination
The specific imaging features of FG, especially gas in the fascia, can help clinicians make a clear diagnosis and determine the extent of FG.
Computed tomography (CT) is the first choice of imaging examination to evaluate the FG, which has the advantages of high sensitivity (88.5%), high specificity (93.3%), and rapid acquisition.72 Contrast enhanced CT can further estimate the degree of fascia involvement before operation and determine whether the lesion is from the rectum.73,74 Some CT scoring system is also helpful. McGillicuddy developed a computed tomography-based scoring system (5 points for fascia air, 4 points for muscle/fascia edema, 3 points for fluid formation between soft tissues, 2 points for local lymphadenopathy and 1 point for subcutaneous edema), which can help clinicians to make the diagnosis of necrotizing fasciitis when the total score is greater than or equal to 6.10,75
The imaging feature of FG on MRI are extensive perineal inflammation, fascia thickening and soft tissue gas, with or without effusion or fistula.74 Because long acquisition time may delay surgery, some studies advocated MRI as a postoperative evaluation.76 The imaging feature of FG on Ultrasound are subcutaneous emphysema, inflammation and exudation, which is very useful in differentiating incarcerated indirect inguinal hernia, testicular torsion or orchitis.77 Fine-needle aspiration biopsy under Ultrasound guidance can be used to assist diagnosis.78 However, small field of vision and pain caused by direct compression limit the use of US in FG.79 X-ray seems not to be a good imaging examination choice for suspected FG patients, because of the overlapping gas in the pelvic cavity organs may lead to misdiagnosis.74 A research containing 5982 patients found that the sensitivity of CT to necrotizing fasciitis (93%) is far better than that of X-ray (49%).72
Laboratory Examination-Dependent Diagnostic and Predictive Scoring System
There is no specific laboratory index or biomarker, so the difficulty of diagnosis lies mainly in how to early distinguish FG from other soft tissue infections.80,81 Based on the blood routine and biochemical test of 89 necrotizing fasciitis patients, researchers developed the laboratory risk indicator for necrotizing fasciitis (LRINEC) (Table 4), which can detect early necrotizing fasciitis. Suspected patients with LRINEC score ≥6 should be attentively evaluated for the risk of necrotizing fasciitis.82 The LRINEC scoring system has been subsequently verified and show great reliability (the sensitivity: 43.2–80%, positive predictive value: 57–64% and negative predictive value: 42–86%) to effectively assist in detecting early clinical necrotizing fasciitis.83 The high false negative rate (35.71%) of LRINEC in the retrospective study reminded clinicians not to rely solely on the scoring system to exclude suspected cases.84 Many clinicians try to continuously improve LRINEC. For example, after adding comorbidity (chronic hepatitis) to LRINEC, the false negative rate can be reduced by 30%, the sensitivity can be increased by 11%, and the specificity can be reduced by only 1%.85
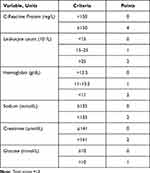 |
Table 4 The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC Score)82 |
Combined Treatment
The management of FG often requires multidisciplinary participation. The cornerstone of treatment is rapid resuscitation of critical patients, broad-spectrum antibiotic and complete surgical debridement, of which early surgical debridement is the most important. Some adjunctive treatments (such as HBOT, VAC and UPH) have been proved to have positive effects in practice, which should not be ignored in the management of FG.
Fluid Resuscitation
FG patients may soon progress to severe systemic infection even death due to the rapid progress, the data shows the final direct cause of death was sepsis (76%) and multiple organ failure (66%).16 Inadequate fluid intake, coupled with body fluid loss at the wound site and capillary leakage to the stroma caused by endothelial injury, reduced venous reflux and microcirculation perfusion.86 Fluid resuscitation is an effective way to improve venous return, cardiac output (CO), and oxygen transport and create opportunities for surgical intervention.87 SSC guidelines for adults recommend starting resuscitation immediately after identification and using at least 30 mL/kg of liquid within 3 hours. Crystalloid fluid is the first choice for fluid resuscitation.88,89 Under the premise of full fluid resuscitation, the prognosis can be significantly improved by properly handling the comorbidity of patients. A retrospective study found that some comorbidities significantly affect the prognosis and increased the mortality of FG patients, including diabetes, heart disease, renal failure and kidney disease.16
Source Control (Operation)
Source control is very important for managing FG, including abscess drainage, removal of potentially infectious devices, and thorough debridement of necrotic tissue, which can prevent the sepsis and septic shock.90 Characteristic findings during operation, including the gray soft tissue, pus and turbid “dishwashing water” like liquid, odor, no bleeding, and the tissue lacks resistance, can further clarify the diagnosis.42,85 The mortality can be significantly reduced when the operation was performed within 6 hours after admission.91 Kabay et al also found that the delay of surgical debridement was significantly related to the increased mortality.92 After identifying the degree of the infection in soft tissue, including zone 1 (necrotic), zone2 (inflamed epidermis/dermis without necrosis), and zone 3 (normal skin), appropriate skin and soft tissue sparing surgery can prepare for subsequent primary closure.93 Some clinicians attach great importance to the ‘60-minute rule’ in debridement, which includes 20 mins for debridement and 40 mins for complete hemostasis until the entire necrosis tissue is excised.94 After the operation, the exposed wounds need to be properly covered with gauze (such as saline gauze and biologic dressings), which need to be replaced frequently every day.95 FG patients often need repeated debridement, Sam N’s retrospective study containing 19 FG patients found that the average number of operations for complete debridement was 3.5, including 2.3 for survivors and 5.2 for dead patients.96
Combined Broad-Spectrum Antibiotics
Due to the diversity of pathogenic microorganisms, clinicians must empirically select sufficient broad-spectrum antibiotics, and their antibacterial spectrum should cover for gram-positive, gram-negative, aerobic and anaerobic bacteria.97 Classic selection of broad-spectrum antibiotics includes carbapenems or β-Lactamase inhibitor plus Clindamycin, when methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected vancomycin or linezolid should be added; Patients with allergy to β-Lactamase inhibitor antibiotics should choose aminoglycosides or fluoroquinolones plus metronidazole. For patients with obvious risk of fungal infection (type I, IV), amphotericin B or fluconazole should be added.30,42
The selection of antibiotics should be adjusted in time according to the results of bacterial culture and drug sensitivity. Deep samples taken by surgeons at the interface between healthy and necrotic tissues in first operation can help to identify the pathogens in about 90% of cases.98 The de-escalation of antibiotics is usually safe and important, which can save costs, reduce the risk of antibiotic resistance and reduce toxicity and side effects, because of that the use of antibiotics is related to antibiotic resistance.99 So, it is necessary to adjust the use of antibiotics in time once the result of bacterial culture is clear, including stopping unnecessary antibiotics and narrowing the antibacterial spectrum.
Hyperbaric Oxygen Therapy (HBOT)
Hypoxia is the main factor leading to delayed wound healing in patients with FG. HBOT can increase the oxygen partial pressure of tissues and organs, and promote wound recovery through various mechanism.100 HBOT is especially suitable for FG patients who are unresponsive to conventional treatment, complicated with Clostridium or anaerobes and deep tissue involvement.101
Although few studies reported an increase in mortality, most studies showed that HBOT has a beneficial effect in the prognosis of FG. Several current systematic and meta-analysis on the assessment of HBOT in FG patients showed that HBOT can reduce the mortality of FG patients without significantly increasing the length of hospital stay.102,103 HBOT and early debridement are independent predictor of the lower mortality of FG patients.104
Vacuum Assisted Closure (VAC)
VAC can promote debridement and increase wound perfusion through continuous negative pressure suction, which is help to wound vascularization, fibroblast migration, and cell proliferation.105,106 VAC has been demonstrated to reduce the wound area, frequency of dressing change, and the dose of analgesics, thereby ultimately increasing the quality of life of FG patients.107,108 However, the use of VAC may prevent clinicians from observing wounds clearly and prolong the stay of hospital, so the equipment should be changed every 72 to 96 hours.95,109,110 VAC was often used in patients with large skin defects, which can act as temporary closure method to prepare for underlying secondary reconstruction. Moreover, latest retrospective multi-institutional cohort study found that the number of pathogens in FG patients is positively related to the use of VAC, which further supported the use of VAC in FG patients.111
Fecal Diversion
FG patients need strict wound management including fecal diversion after the aggressive debridement because of fecal pollution can affect wound healing even cause serious sepsis.112 Appropriate fecal diversion includes colostomy and rectal catheter. The traditional method is to perform colostomy after debridement and drainage. Although the actual application frequency of diversional colostomy in the literature varies greatly due to the absence of a consensus, and most of the time rely on the personal experience of the surgeon, but it can bring great benefit to patients who are complicated with anal sphincter dysfunction and fecal incontinence.113 Enterostomy has been proved in practice that can decrease the length of stay and the fatality rate of patients with FG, but what cannot be ignored is that colostomy has some postoperative complications such as anastomotic leak, bowel obstruction, and surgical site infection, worse still some temporary stoma may not be reversed due to some prohibitive comorbidity.114,115 The flexible seal fecal management system (FMS) is a non-surgical fecal diversion method, which transfers feces to the fecal bag through the soft catheter in the rectum to avoid fecal pollution.116,117 This method not only avoids the complications related to colostomy, but also reduces the psychological and economic burden of patients, but the contraindications such as rectal perforation and ulcer should be excluded before use.117 In several years, occasional complications such as tenesmus and rectal bleeding may bring some risks to the implementation of FMS.118
Unprocessed Honey Therapy (UPH)
Fungi (such as Candida) can lead to systemic infection when the mucosal or skin barrier is damaged or the host is in a state of hypoimmunity.119 Unprocessed honey has been proved has antibacterial effect on many bacteria and fungi in vitro due to its low pH value, high permeability and enzyme activity. UPH is cheap and easy to access, but it is only recommended to be used in patients with small skin lesions and no complications.120 Although many studies have proved the benefits of UPH, several studies found that the therapeutic effect of UPH is still controversial. Sufya n et al applied unprocessed honey locally in 25 FG patients, the results showed that UPH could not promote the healing of wound and even delayed wound healing in some cases.121
Postoperative Skin Reconstruction
The invasive pathological process of Fournier’s gangrene can lead to skin defects of scrotum, perineum and penis. Although the component separation primary wound closure after debridement was demonstrated to be safe, large defects or severe infections often need to be reconstructed.122 Based on the location, size and depth of defects and the availability of local tissues, surgical reconstruction methods can be divided into skin grafts, local advancement flaps, scrotal flaps, multiple fasciocutaneous and myocutaneous flaps, and testicular transposition.123 Meanwhile, for the reconstruction of defect larger than half of the scrotum area, systematic review recommended split-thickness skin grafting (STSG) or flaps.124 Both flaps and skin grafts have been proved to have satisfactory aesthetic effects for genital reconstruction of FG patients.125 Thanks to the good elasticity of scrotal skin, even if the remaining healthy scrotum skin is less than half, it can still be closed and sutured to cover the entire scrotum to complete reconstruction or secondary intention.95 Unlike the scrotum, if the skin defect of penile is larger than 25%, grafts were usually needed.95 The first objective of skin reconstruction is to cover the exposed soft tissues with skin. At the same time, this ideal reconstruction should sufficiently preserve the function, restore the appearance and reduce postoperative complications.
Conclusion
FG is a rare and life-threatening necrotic infection, often associated with mixed infection of aerobic and anaerobic bacteria, mainly involving external genitalia and perianal area. The susceptible population is middle-aged and elderly patients with comorbidity and predisposing factors. Definite diagnosis needs the combination between identification of susceptible population and familiarity with early clinical manifestations beside the assistance of laboratory and imaging examinations, because early clinical manifestations of skin are obscure. In addition, predictive scoring systems such as LRINEC can provide reference for clinicians. Early and decisive surgical debridement and empirical application of broad-spectrum antibiotics can greatly improve the prognosis of patients.
Due to multi-disciplinary cooperation, the improvement of medical level and postoperative nursing level, the survival rate of FG has been continuously improved. But multiple debridement often leads to large-area skin defects, therefore clinical workers still need to continue to explore better diagnosis, treatment and nursing modes of FG. Multidisciplinary collaborative diagnosis and treatment is very important in the management of FG. At present, there is a diagnosis and treatment mode dominated by department of burn plastic surgery, which can fully evaluate the damaged skin area before operation to determine the operation mode, and determine the reconstruction mode after debridement, which can improve the prognosis and quality of life of patients.
Acknowledgments
Thanks for the technical support provided by the WUTAI experimental center of the Second Affiliated Hospital of Nanjing Medical University.
Funding
This project is funded by the 789 Excellent Talents Training Program of The Second Affiliated Hospital of Nanjing Medical University (Grant number: 789ZYRC202070210).
Disclosure
The authors declare no conflict of interest.
References
1. Shyam DC, Rapsang AG. Fournier’s gangrene. Surgeon. 2013;11(4):222–232. doi:10.1016/j.surge.2013.02.001
2. Fournier JA. Gangrene foudroyante de la verge. Semaine Medicale. 1883;3:345–348.
3. Wilson B. Necrotizing fasciitis. Am Surg. 1952;18(4):416–431.
4. Thwaini A, Khan A, Malik A, et al. Fournier’s gangrene and its emergency management. Postgrad Med J. 2006;82(970):516–519. doi:10.1136/pgmj.2005.042069
5. Sorensen MD, Krieger JN, Rivara FP, et al. Fournier’s Gangrene: population based epidemiology and outcomes. J Urol. 2009;181(5):2120–2126. doi:10.1016/j.juro.2009.01.034
6. Sorensen MD, Krieger JN. Fournier’s Gangrene: epidemiology and outcomes in the general US population. Urol Int. 2016;97(3):249–259. doi:10.1159/000445695
7. Eke N. Fournier’s gangrene: a review of 1726 cases. Br J Surg. 2000;87(6):718–728. doi:10.1046/j.1365-2168.2000.01497.x
8. Furr J, Watts T, Street R, et al. Contemporary trends in the inpatient management of Fournier’s Gangrene: predictors of length of stay and mortality based on population-based sample. Urology. 2017;102:79–84. doi:10.1016/j.urology.2016.09.021
9. Beecroft NJ, Jaeger CD, Rose JR, et al. Fournier’s Gangrene in females: presentation and management at a tertiary center. Urology. 2021;151:113–117. doi:10.1016/j.urology.2020.05.056
10. Ballard DH, Raptis CA, Guerra J, et al. Preoperative CT findings and interobserver reliability of Fournier Gangrene. AJR Am J Roentgenol. 2018;211(5):1051–1057. doi:10.2214/AJR.18.19683
11. Ugwumba FO, Nnabugwu I, Ozoemena OF. Fournier’s gangrene – analysis of management and outcome in south-eastern Nigeria. S Afr J Surg. 2012;50(1):16–19.
12. Radcliffe RS, Khan MA. Mortality associated with Fournier’s gangrene remains unchanged over 25 years. BJU Int. 2020;125(4):610–616. doi:10.1111/bju.14998
13. Bowen D, Juliebø-Jones P, Somani BK. Global outcomes and lessons learned in the management of Fournier’s gangrene from high-volume centres: findings from a literature review over the last two decades. World J Urol. 2022;40(10):2399–2410. doi:10.1007/s00345-022-04139-4
14. Yilmaz Baser H, Zumrutbas AE, Yilmaz A, et al. Importance of emergency department waiting period in Fournier’s Gangrene; 10 years of experience. Int J Clin Pract. 2021;75(9):e14361. doi:10.1111/ijcp.14361
15. Goh T, Goh LG, Ang CH, et al. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):e119–e125. doi:10.1002/bjs.9371
16. El-Qushayri AE, Khalaf KM, Dahy A, et al. Fournier’s gangrene mortality: a 17-year systematic review and meta-analysis. Int J Infect Dis. 2020;92:218–225. doi:10.1016/j.ijid.2019.12.030
17. Laor E, Palmer LS, Tolia BM, et al. Outcome prediction in patients with Fournier’s gangrene. J Urol. 1995;154(1):89–92. doi:10.1016/S0022-5347(01)67236-7
18. Bozkurt O, Sen V, Demir O, et al. Evaluation of the utility of different scoring systems (FGSI, LRINEC and NLR) in the management of Fournier’s gangrene. Int Urol Nephrol. 2015;47(2):243–248. doi:10.1007/s11255-014-0897-5
19. Elsaket AE, Maharajh S, Urry RJ. The presentation, management and outcomes of Fournier’s gangrene at a tertiary urology referral centre in South Africa. S Afr Med J. 2018;108(8):671–676. doi:10.7196/SAMJ.2018.v108i8.13100
20. Ghodoussipour SB, Gould D, Lifton J, et al. Surviving Fournier’s gangrene: multivariable analysis and a novel scoring system to predict length of stay. J Plast Reconstr Aesthet Surg. 2018;71(5):712–718. doi:10.1016/j.bjps.2017.12.005
21. Vick R, Carson CC. Fournier’s disease. Urol Clin North Am. 1999;26(4):841–849. doi:10.1016/S0094-0143(05)70224-X
22. Smith GL, Bunker CB, Dinneen MD. Fournier’s gangrene. Br J Urol. 1998;81(3):347–355. doi:10.1046/j.1464-410x.1998.00532.x
23. Esposito S, Bassetti M, Concia E, et al. Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: an update. J Chemother. 2017;29(4):197–214. doi:10.1080/1120009X.2017.1311398
24. Stevens DL, Bryant AE. Necrotizing soft-tissue infections. N Engl J Med. 2018;378(10):971. doi:10.1056/NEJMc1800049
25. Elliott D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg. 2000;179(5):361–366. doi:10.1016/S0002-9610(00)00360-3
26. Goodell KH, Jordan MR, Graham R, et al. Rapidly advancing necrotizing fasciitis caused by Photobacterium (Vibrio) damsela: a hyperaggressive variant. Crit Care Med. 2004;32(1):278–281. doi:10.1097/01.CCM.0000104920.01254.82
27. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249–257. doi:10.1016/j.jhin.2010.01.028
28. Garcia NM, Cai J. Aggressive Soft Tissue Infections. Surg Clin North Am. 2018;98(5):1097–1108. doi:10.1016/j.suc.2018.05.001
29. Tang LM, Su YJ, Lai YC. The evaluation of microbiology and prognosis of Fournier’s gangrene in past five years. Springerplus. 2015;4(1):14. doi:10.1186/s40064-014-0783-8
30. Castillejo Becerra CM, Jaeger CD, Rose JR, et al. Microorganisms and antibiogram patterns in Fournier’s Gangrene: contemporary experience from a single tertiary care center. J Urol. 2020;204(6):1249–1255. doi:10.1097/JU.0000000000001194
31. Thayer J, Mailey BA. Two-stage neoscrotum reconstruction using porcine bladder extracellular Matrix after Fournier’s Gangrene. Plast Reconstr Surg Glob Open. 2020;8(8):e3034. doi:10.1097/GOX.0000000000003034
32. El-Shazly M, Aziz M, Aboutaleb H, et al. Management of equivocal (early) Fournier’s gangrene. Ther Adv Urol. 2016;8(5):297–301. doi:10.1177/1756287216655673
33. Sarkis P, Farran F, Khoury R, et al. Gangrène de Fournier : revue de la littérature récente[Fournier’s gangrene: a review of the recent literature]. Prog Urol. 2009;19(2):75–84. French. doi:10.1016/j.purol.2008.09.050
34. Xiu F, Stanojcic M, Diao L, et al. Stress hyperglycemia, insulin treatment, and innate immune cells. Int J Endocrinol. 2014;2014:486403. doi:10.1155/2014/486403
35. Trevelin SC, Carlos D, Beretta M, et al. Diabetes mellitus and sepsis: a challenging association. Shock. 2017;47(3):276–287. doi:10.1097/SHK.0000000000000778
36. Hatanaka E, Monteagudo PT, Marrocos MS, et al. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol. 2006;146(3):443–447. doi:10.1111/j.1365-2249.2006.03229.x
37. Voelzke BB, Hagedorn JC. Presentation and Diagnosis of Fournier Gangrene. Urology. 2018;114:8–13. doi:10.1016/j.urology.2017.10.031
38. Martinschek A, Evers B, Lampl L, et al. Prognostic aspects, survival rate, and predisposing risk factors in patients with Fournier’s gangrene and necrotizing soft tissue infections: evaluation of clinical outcome of 55 patients. Urol Int. 2012;89(2):173–179. doi:10.1159/000339161
39. Ferretti M, Saji AA, Phillips J. Fournier’s Gangrene: a review and outcome comparison from 2009 to 2016. Adv Wound Care. 2017;6(9):289–295. doi:10.1089/wound.2017.0730
40. Hatipoglu E, Demiryas S, Şimşek O, et al. Fournier’s gangrene: five years’ experience from a single center in Turkey. Ulus Travma Acil Cerrahi Derg. 2020;26(2):235–241. doi:10.14744/tjtes.2020.66805
41. David R, Traeger L, Kahokehr A, et al. A prospective case series of Fournier’s gangrene at a tertiary centre involving adjacent organs. ANZ J Surg. 2021;91(12):2817–2823. doi:10.1111/ans.17005
42. Montrief T, Long B, Koyfman A, et al. Fournier Gangrene: a review for emergency clinicians. J Emerg Med. 2019;57(4):488–500. doi:10.1016/j.jemermed.2019.06.023
43. Herrera Ortiz AF, Arámbula JG, Del Castillo V, et al. Fournier’s Gangrene with retroperitoneal extension as the first manifestation of the Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency Syndrome (AIDS). Cureus. 2021;13(12):e20517. doi:10.7759/cureus.20517
44. Çalışkan S, Özsoy E, Sungur M, et al. Fournier’s gangrene: review of 36 cases. Ulus Travma Acil Cerrahi Derg. 2019;25(5):479–483. doi:10.14744/tjtes.2019.30232
45. Koyama M, Kitazawa M, Ehara T, et al. 直腸癌に対する化学療法中に発症した会陰部壊疽性筋膜炎の2 例 [Two cases of Fournier’s gangrene that occurred during chemotherapy for rectal cancer]. Gan To Kagaku Ryoho. 2017;44(2):169–171. Japanese.
46. Imamura S, Ogura N, Aoki Y, et al. 二次治療IRIS+Bevacizumab 投与中にフルニエ壊疽を発症した直腸肛門管癌の1 例 [A case of Fournier’s gangrene during second-line IRIS plus bevacizumab chemotherapy for rectal and anal canal cancer]. Gan To Kagaku Ryoho. 2022;49(5):597–599. Japanese.
47. Joury A, Mahendra A, Alshehri M, et al. Extensive necrotizing fasciitis from Fournier’s gangrene. Urol Case Rep. 2019;26:100943. doi:10.1016/j.eucr.2019.100943
48. García-García A, Galeano-Valle F, Nuevo-González JA, et al. Fournier’s gangrene and SGLT2 inhibitors: a case study. Endocrinol Diabetes Nutr. 2020;67(6):423–425. doi:10.1016/j.endinu.2019.12.007
49. Bersoff-Matcha SJ, Chamberlain C, Cao C, et al. Fournier Gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann Intern Med. 2019;170(11):764–769. doi:10.7326/M19-0085
50. Taylor L, Asmar O, Mandal A, et al. Perspectives from a regional plastic surgery centre on evidence for the purported link between SGLT2 inhibitors and Fournier’s Gangrene. Front Surg. 2021;8:754101. doi:10.3389/fsurg.2021.754101
51. Duarte I, Outerelo C, Santana A, et al. Fournier Gangrene as a complication of a perinephric abscess after kidney transplant: a case report. Transplant Proc. 2021;53(4):1281–1283. doi:10.1016/j.transproceed.2021.02.009
52. Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol. 2007;177(5):1667–1674. doi:10.1016/j.juro.2007.01.041
53. Fialkov JM, Watkins K, Fallon B, et al. Fournier’s gangrene with an unusual urologic etiology. Urology. 1998;52(2):324–327. doi:10.1016/S0090-4295(98)00195-2
54. Mundy AR, Andrich DE. Urethral strictures. BJU Int. 2011;107(1):6–26. doi:10.1111/j.1464-410X.2010.09800.x
55. Louro JM, Albano M, Baltazar J, et al. Fournier’s Gangrene: 10-year experience of a plastic surgery and burns department at a tertiary hospital. Acta Med Port. 2019;32(5):368–374. doi:10.20344/amp.11003
56. Yücel M, Özpek A, Başak F, et al. Fournier’s gangrene: a retrospective analysis of 25 patients. Ulus Travma Acil Cerrahi Derg. 2017;23(5):400–404. doi:10.5505/tjtes.2017.01678
57. Numoto S, Kurahashi H, Azuma Y, et al. Fournier’s gangrene during ACTH therapy. Brain Dev. 2017;39(5):435–438. doi:10.1016/j.braindev.2016.11.012
58. Ameh EA, Dauda MM, Sabiu L, et al. Fournier’s gangrene in neonates and infants. Eur J Pediatr Surg. 2004;14(6):418–421. doi:10.1055/s-2004-821138
59. Fukui K, Fujioka M, Ishiyama S. Sacral pressure ulcer-induced Fournier’s Gangrene extending to the retroperitoneum: a case report. Wounds. 2018;30(1):E5–E8.
60. Zakariya-Yousef Breval I, Trujillo Díaz N, De La Herranz Guerrero P. [Fournier’s gangrene secondary to inguinoperineal abscess caused by Acidaminococcus intestini and Streptococcus gallolyticus spp. pasteurianus]. Rev Esp Quimioter. 2021;34(6):679–681. Spanish. doi:10.37201/req/070.2021
61. Sihombing AT, Palgunadi IN, Stefanus D. Complete urethral disruption as a complication of urethral catheterization presenting as scrotal mass: a rare case. Urol Case Rep. 2020;33:101378. doi:10.1016/j.eucr.2020.101378
62. López Pacios JC, Sánchez Merino JM, Piñeiro Fernández MC, et al. Gangrena de Fournier secundaria a cateterismo uretral [Fournier’s gangrene secondary to urethral catheterization]. Arch Esp Urol. 2005;58(2):167–170. Spanish. doi:10.4321/s0004-06142005000200012
63. Kumagai A, Ogawa D, Koyama T, et al. 経直腸的前立腺生検後にフルニエ壊疽を発症した管理不良な糖尿病患者の1例 [A case report of Fournier’s gangrene in a diabetic patient induced by transrectal prostate biopsy (TRPB)]. Nihon Hinyokika Gakkai Zasshi. 2002;93(5):648–651. Japanese. doi:10.5980/jpnjurol1989.93.648
64. Zingaro MD, Boni A, Vermandois JAR, et al. Fournier’s Gangrene and intravenous drug abuse: an unusual case report and review of the literature. Open Med. 2019;14:694–710. doi:10.1515/med-2019-0114
65. Ghazanfar H, Espinosa PV, Zeana C, et al. Penile necrosis associated with local intravenous injection of cocaine. Am J Case Rep. 2022;23:e935250. doi:10.12659/AJCR.935250
66. Ferreira PC, Reis JC, Amarante JM, et al. Fournier’s gangrene: a review of 43 reconstructive cases. Plast Reconstr Surg. 2007;119(1):175–184. doi:10.1097/01.prs.0000244925.80290.57
67. Erickson BA, Miller AC, Warner HL, et al. Understanding the prodrome of necrotizing soft tissue infections of the genitalia (Fournier’s Gangrene) and the incidence, duration, and risk factors associated with potential missed opportunities for an earlier diagnosis: a population-based longitudinal study. J Urol. 2022;25:10–97.
68. Lahouar R, Naouar S, Ben Khalifa B, et al. Isolated Penile Fournier’s gangrene: a very rare entity. Urol Case Rep. 2021;37:101608.
69. Abass-Shereef J, Kovacs M, Simon EL. Fournier’s Gangrene masking as perineal and scrotal cellulitis. Am J Emerg Med. 2018;36(9):1719.e1–1719.e2. doi:10.1016/j.ajem.2018.05.067
70. Singh A, Ahmed K, Aydin A, et al. Fournier’s gangrene. A clinical review. Arch Ital Urol Androl. 2016;88(3):157–164. doi:10.4081/aiua.2016.3.157
71. Moussa M, Abou Chakra M. Isolated Penile Fournier’s gangrene: a case report and literature review. Int J Surg Case Rep. 2019;62:65–68. doi:10.1016/j.ijscr.2019.08.012
72. Fernando SM, Tran A, Cheng W, et al. Necrotizing soft tissue infection: diagnostic accuracy of physical examination, imaging, and LRINEC score: a systematic review and meta-analysis. Ann Surg. 2019;269(1):58–65. doi:10.1097/SLA.0000000000002774
73. Zacharias N, Velmahos GC, Salama A, et al. Diagnosis of necrotizing soft tissue infections by computed tomography. Arch Surg. 2010;145(5):452–455. doi:10.1001/archsurg.2010.50
74. Ballard DH, Mazaheri P, Raptis CA, et al. Fournier Gangrene in men and women: appearance on CT, Ultrasound, and MRI and what the surgeon wants to know. Can Assoc Radiol J. 2020;71(1):30–39. doi:10.1177/0846537119888396
75. Mcgillicuddy EA, Lischuk AW, Schuster KM, et al. Development of a computed tomography-based scoring system for necrotizing soft-tissue infections. J Trauma. 2011;70(4):894–899. doi:10.1097/TA.0b013e3182134a76
76. Yoneda A, Fujita F, Tokai H, et al. MRI can determine the adequate area for debridement in the case of Fournier’s gangrene. Int Surg. 2010;95(1):76–79.
77. Sweet DE, Feldman MK, Remer EM. Imaging of the acute scrotum: keys to a rapid diagnosis of acute scrotal disorders. Abdom Radiol. 2020;45(7):2063–2081. doi:10.1007/s00261-019-02333-4
78. Lee PC, Turnidge J, Mcdonald PJ. Fine-needle aspiration biopsy in diagnosis of soft tissue infections. J Clin Microbiol. 1985;22(1):80–83. doi:10.1128/jcm.22.1.80-83.1985
79. Avery LL, Scheinfeld MH. Imaging of penile and scrotal emergencies. Radiographics. 2013;33(3):721–740. doi:10.1148/rg.333125158
80. Auerbach J, Bornstein K, Ramzy M, et al. Fournier Gangrene in the emergency department: diagnostic dilemmas, treatments and current perspectives. Open Access Emerg Med. 2020;12:353–364. doi:10.2147/OAEM.S238699
81. Johnson LJ, Crisologo PA, Sivaganesan S, et al. Evaluation of the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score for detecting necrotizing soft tissue infections in patients with diabetes and lower extremity infection. Diabetes Res Clin Pract. 2021;171:108520. doi:10.1016/j.diabres.2020.108520
82. Wong CH, Khin LW, Heng KS, et al. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535–1541.
83. Abdullah M, Mcwilliams B, Khan SU. Reliability of the Laboratory Risk Indicator in Necrotising Fasciitis (LRINEC) score. Surgeon. 2019;17(5):309–318.
84. García-Tarriño R, Ballesteros-Betancourt J, Soriano-Viladomiu A, et al. Necrotizing fasciitis: usefulness of the LRINEC score in a third-level hospital. Injury. 2021;52(Suppl 4):S8–S15.
85. Van Stigt S, Knubben M, Schrooten T, et al. Prognostic factors for mortality in 123 severe cases of necrotizing fasciitis in 5 hospitals in the Netherlands between 2003 and 2017. Eur J Trauma Emerg Surg. 2022;48(2):1189–1195.
86. Bakker J, Kattan E, Annane D, et al. Current practice and evolving concepts in septic shock resuscitation. Intensive Care Med. 2022;48(2):148–163.
87. Berlin DA, Bakker J. Understanding venous return. Intensive Care Med. 2014;40(10):1564–1566.
88. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247.
89. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222–231.
90. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45(3):486–552.
91. Nawijn F, Smeeing DPJ, Houwert RM, et al. Time is of the essence when treating necrotizing soft tissue infections: a systematic review and meta-analysis. World J Emerg Surg. 2020;15:4.
92. Kabay S, Yucel M, Yaylak F, et al. The clinical features of Fournier’s gangrene and the predictivity of the Fournier’s Gangrene Severity Index on the outcomes. Int Urol Nephrol. 2008;40(4):997–1004.
93. Perry TL, Kranker LM, Mobley EE, et al. Outcomes in Fournier’s Gangrene using skin and soft tissue sparing flap preservation surgery for wound closure: an alternative approach to wide radical debridement. Wounds. 2018;30(10):290–299.
94. Hagedorn JC, Wessells H. A contemporary update on Fournier’s gangrene. Nat Rev Urol. 2017;14(4):205–214.
95. Erickson BA, Flynn KJ. Management of necrotizing soft tissue infections (Fournier’s Gangrene) and surgical reconstruction of debridement wound defects. Urol Clin North Am. 2022;49(3):467–478.
96. Chawla SN, Gallop C, Mydlo JH. Fournier’s Gangrene: an analysis of repeated surgical debridement. Eur Urol. 2003;43(5):572–575.
97. Tarasconi A, Perrone G, Davies J, et al. Anorectal emergencies: WSES-AAST guidelines. World J Emerg Surg. 2021;16(1):48.
98. Urbina T, Razazi K, Ourghanlian C, et al. Antibiotics in necrotizing soft tissue infections. Antibiotics. 2021;10(9):1104.
99. Arulkumaran N, Routledge M, Schlebusch S, et al. Antimicrobial-associated harm in critical care: a narrative review. Intensive Care Med. 2020;46(2):225–235.
100. Tutino R, Colli F, Rizzo G, et al. Which role for hyperbaric oxygen therapy in the treatment of Fournier’s Gangrene? A retrospective study. Front Surg. 2022;9:850378.
101. Tanaka T, Minami A, Uchida J, et al. Potential of hyperbaric oxygen in urological diseases. Int J Urol. 2019;26(9):860–867.
102. Raizandha MA, Hidayatullah F, Kloping YP, et al. The role of hyperbaric oxygen therapy in Fournier’s Gangrene: a systematic review and meta-analysis of observational studies. Int Braz J Urol. 2022;48(5):771–781.
103. Schneidewind L, Anheuser P, Schönburg S, et al. Hyperbaric oxygenation in the treatment of Fournier’s gangrene: a systematic review. Urol Int. 2021;105(3–4):247–256.
104. Creta M, Longo N, Arcaniolo D, et al. Hyperbaric oxygen therapy reduces mortality in patients with Fournier’s Gangrene. Results from a multi-institutional observational study. Minerva Urol Nefrol. 2020;72(2):223–228.
105. Syllaios A, Davakis S, Karydakis L, et al. Treatment of Fournier’s Gangrene with vacuum-assisted closure therapy as enhanced recovery treatment modality. In Vivo. 2020;34(3):1499–1502.
106. Silberstein J, Grabowski J, Parsons JK. Use of a vacuum-assisted device for Fournier’s gangrene: a new paradigm. Rev Urol. 2008;10(1):76–80.
107. Iacovelli V, Cipriani C, Sandri M, et al. The role of vacuum-assisted closure (VAC) therapy in the management of FOURNIER’S gangrene: a retrospective multi-institutional cohort study. World J Urol. 2021;39(1):121–128.
108. Yanaral F, Balci C, Ozgor F, et al. Comparison of conventional dressings and vacuum-assisted closure in the wound therapy of Fournier’s gangrene. Arch Ital Urol Androl. 2017;89(3):208–211.
109. Czymek R, Schmidt A, Eckmann C, et al. Fournier’s gangrene: vacuum-assisted closure versus conventional dressings. Am J Surg. 2009;197(2):168–176.
110. Franco-Buenaventura D, Garcia-Perdomo HA. Vacuum-assisted closure device in the postoperative wound care for Fournier’s gangrene: a systematic review. Int Urol Nephrol. 2021;53(4):641–653.
111. Cipriani C, Iacovelli V, Sandri M, et al. The microbiological profile of patients with Fournier’s gangrene: a retrospective multi-institutional cohort study. Urologia. 2022;89(3):437–443.
112. Ozturk E, Sonmez Y, Yilmazlar T. What are the indications for a stoma in Fournier’s gangrene? Colorectal Dis. 2011;13(9):1044–1047.
113. Sarofim M, Di RA, Descallar J, et al. Relationship between diversional stoma and mortality rate in Fournier’s gangrene: a systematic review and meta-analysis. Langenbecks Arch Surg. 2021;406(8):2581–2590.
114. Sherman KL, Wexner SD. Considerations in Stoma Reversal. Clin Colon Rectal Surg. 2017;30(3):172–177.
115. Li YD, Zhu WF, Qiao JJ, et al. Enterostomy can decrease the mortality of patients with Fournier gangrene. World J Gastroenterol. 2014;20(24):7950–7954.
116. Padmanabhan A, Stern M, Wishin J, et al. Clinical evaluation of a flexible fecal incontinence management system. Am J Crit Care. 2007;16(4):384–393.
117. Goh M, Chew MH, Au-Yong PS, et al. Nonsurgical faecal diversion in the management of severe perianal sepsis: a retrospective evaluation of the flexible faecal management system. Singapore Med J. 2014;55(12):635–639.
118. Page BP, Boyce SA, Deans C, et al. Significant rectal bleeding as a complication of a fecal collecting device: report of a case. Dis Colon Rectum. 2008;51(9):1427–1429.
119. Kühbacher A, Burger-Kentischer A, Rupp S. Interaction of candida species with the skin. Microorganisms. 2017;5(2):32.
120. De Groot T, Janssen T, Faro D, et al. Antifungal activity of a medical-grade honey formulation against candida auris. J Fungi. 2021;7(1):50.
121. Sufya N, Matar N, Kaddura R, et al. Evaluation of bactericidal activity of Hannon honey on slowly growing bacteria in the chemostat. Drug Healthc Patient Saf. 2014;6:139–144.
122. Sandberg JM, Warner HL, Flynn KJ, et al. Favorable outcomes with early component separation, primary closure of necrotizing soft tissue infections of the genitalia (Fournier’s Gangrene) debridement wound defects. Urology. 2022;166:250–256.
123. Insua-Pereira I, Ferreira PC, Teixeira S, et al. Fournier’s gangrene: a review of reconstructive options. Cent European J Urol. 2020;73(1):74–79.
124. Karian LS, Chung SY, Lee ES. Reconstruction of defects after Fournier gangrene: a systematic review. Eplasty. 2015;15:e18.
125. Michael P, Peiris B, Ralph D, et al. Genital reconstruction following Fournier’s Gangrene. Sex Med Rev. 2022;10(4):800–812.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
