Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Progranulin Promotes the Formation and Development of Capsules Caused by Silicone in Sprague-Dawley Rats
Authors Zhou Y, Pang H, Wang J, Wu H, Xu Z, Liu X, Xiao Z
Received 15 May 2022
Accepted for publication 28 July 2022
Published 6 August 2022 Volume 2022:15 Pages 1561—1573
DOI https://doi.org/10.2147/CCID.S374128
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Yongting Zhou, Hao Pang, Jie Wang, Hao Wu, Zidi Xu, Xueyi Liu, Zhibo Xiao
Department of Plastic Surgery, the Second Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China
Correspondence: Zhibo Xiao, Email [email protected]
Background: Silicone implants are currently the most widely used artificial materials in plastic surgery. Capsule formation following implant application is unavoidable. When the capsule is excessively thick and strongly contracted, it can lead to obvious symptoms, clinically known as capsular contracture. Biological factors have always been the focus of research on the capsule formation. As a growth factor, progranulin (PGRN) plays an important regulatory role in wound healing, tissue fibrosis, tumor proliferation and invasion, and inflammation regulation. At present, the research on the capsule mainly involves the regulation of tissue healing and fibrosis under the influence of inflammation. Because PGRN has a regulatory role in these processes, we believe that the study of both can provide a new theoretical basis and intervention sites for monitoring and inhibiting the development of the capsule.
Methods: In this experiment, the effects of different surgical operations on the content of PGRN in the surgical site and plasma of rats were detected. Sprague-Dawley (SD) rat dermal fibroblasts were co-cultured by recombinant PGRN. The effects of r-PGRN on fibroblasts were detected by 5-ethynyl-2’-deoxyuridine (EdU) assay, wound healing assay and Western blot assay. Finally, the effect of PGRN on capsule formation and contracture was studied by changing the content of PGRN in the prosthesis in rats after operation.
Results: Surgical trauma and silicone implant increased plasma and local PGRN levels in SD rats. PGRN can activate the TGF-β/SMAD signaling pathway in a dose-dependent manner, thereby promoting fibroblast proliferation, differentiation and migration and inhibiting apoptosis and enhancing cell function, thereby promoting capsule formation and contracture.
Conclusion: PGRN promotes the formation and contracture of the silicone implant capsule in SD rats by activating the TGF-β/SMAD signaling pathway. This discovery may provide new therapeutic targets and detection indicators.
Keywords: fibrosis, fibroblast, silicone implants, implantation, TGF-β
Introduction
As the main clinical method to treat soft tissue volume insufficiency, implantation plays a very important role in plastic surgery.1 Among many artificial graft materials, silicone implants have become the most widely used because of their good histocompatibility, stable and long-lasting effects, and low price.2 When silicone implants are implanted in the human body, the body will react by forming a capsule to wrap it.3–5 This series of host responses can be summarized into the following steps: bleeding and factor release, formation of temporary matrix, development of acute and chronic inflammation, granulation formation, and capsule development. As a protective physiological response, the capsule cuts off contact between the implants and the tissues. During the process of capsule formation, a layer of host proteins is recruited on the surface of the biomaterial, and this layer of proteins determines the type and quantity of adsorbed cytokines.6 Chemokines released by platelets and blood clots, such as TGF-β, PDGF, LT, and IL, can direct macrophages to wounds.7 When macrophages adhere and become activated, they secrete various inflammatory mediators, such as IL-1, IL-6, IL-10, IL-12, IL-18, TNF-α, TGF-β, IL-8 and then promote the production of capsules. Capsule formation, a fibrotic process similar to wound healing, has a signal transduction process similar to that of wound healing, in which TGF-β signaling pathway plays an important role in capsule evolution. However, once this implant reaction exceeds a certain limit, it can produce obvious somatosensory symptoms and an abnormal appearance,8 clinically known as capsular contracture. This abnormal phenomenon involves mutual cross-talk of a variety of cytokines and cells.9,10 Myofibroblasts act as contractile fibroblasts, and myofibroblasts are localized near the capsule-tissue interface in the capsule tissue.11 The percentage of myofibroblasts in the contracted capsule was significantly increased compared to the non-contracted capsule.12
PGRN is a soluble glycoprotein growth factor with a molecular weight of approximately 88 kDa. Intact PGRN proteins can be split into individual granule protein motifs (GRNs) and have different biological effects than PGRN.13 As an exocrine protein, PGRN can be expressed in a variety of cells, such as leukocytes, neurons, glia, chondrocytes, and epithelial cells,14 mainly involving inflammation, cell-cycle and cell-function adjustment and playing different biological roles in different diseases. PGRN can activate multiple pathways in various tumors, such as ERK and TNFR2/Akt signalling,15 PI3K16 or Wnt signalling17 to stimulate the proliferation and invasion of tumor entities.18,19 In addition to its role in tumors, PGRN is also involved in various fibrotic and immune system diseases (wound healing,20,21 scleroderma,22 pulmonary fibrosis23 and rheumatoid arthritis24).Moreover, changes in serum PGRN levels reflect the development and outcome of certain diseases.25,26 Formation of an implant capsule is a typical repair and fibrosis process after surgical injury. Whether PGRN is involved in it has not been clearly reported.
In this study, the changes in PGRN content in the plasma and it in the different surgical sites of rats receiving different surgical procedures and the effect of recombinant PGRN protein (r-PGRN) on fibroblasts and SD rat capsules were investigated by establishing rat animal models and performing in vitro experiments. We hope that the biological mechanism of capsule formation can be better understood and that this study can provide new therapeutic targets and monitoring indicators related to capsules.
Materials and Methods
Animals
Experiments with Sprague-Dawley (SD) rats (purchased from the Animal Experiment Center of the Second Affiliated Hospital of Harbin Medical University) were approved by the Animal Ethics Committee (SYDW2020-042) of the Second Affiliated Hospital of Harbin Medical University, and the rats were raised and used in accordance with the “Administrative Measures for Laboratory Animals of Harbin Medical University”. The animals were housed in a temperature- and light-controlled room at a constant temperature of approximately 24 °C with a 12-hour light/dark cycle. Animals were fed standard animal pellets and tap water ad libitum.
Operation Stage 1
Sixty female SD rats were randomly divided into three groups (control group, sham-operated group and implanted group, n = 20 in each group) when their body weight reached approximately 120 g. On day 0, after anesthesia with isoflurane, the backs of the rats were shaved and sterilized. A 1.5-cm transverse incision was made at the intersection of the line connecting the subscapular angles on both sides of the back of the rats and the midline of the spine in both the sham-operated group and the implanted group. The deep surface of the superficial muscle fascia was exposed. Then, two equivalent pockets of a suitable size were created along the spine to the caudal direction in this plane. A silicone implant with a volume of 4 cm x 1 cm x 1 cm was placed in the backs of the rats in the implanted group, while the sham-operated group had no implant. The incision was sutured closed layer by layer with 6–0 nylon thread. It has been pointed out that PGRN mRNA content can be rapidly and significantly increased in the damaged area of the skin for 10 days or more.27 So five rats were randomly selected from each of the three groups at 3, 7, 14, and 112 days after the operation. After full anesthesia, blood was collected from the heart (2 mL/rat) and placed in an anticoagulation tube. On day 14, the tissue on the surface of the silicone implant in the implanted group and the tissue in the same part of the sham-operated group and the control group were harvested.
Enzyme-Linked Immunosorbent Assay
Blood samples from stage 1 SD rats were taken and centrifuged at 1000 x g/min at 4 °C for 15 min, and plasma was extracted. Fifty microliters of plasma samples from rats in different groups were added to wells coated with rat PGRN antibody. Then, 100 μL of horseradish peroxidase (HRP)-labelled detection antibodies was added to each well, and the plate was incubated at 37 °C for 60 min. After incubation, the liquid was discarded and the plate was dried. A total of 350 μL of washing solution was used to wash each well, and the washing was repeated five times. The liquid was discarded and the plate was dried. Then, 50 μL each of substrates A and B was added to each well, and the plate was incubated at 37 °C for 15 min. Then, 50 μL of stop solution was added to each well, and the absorbance (OD value) of each well was measured at a wavelength of 450 nm within 15 min.
Acquisition and Culture of Fibroblasts
Three female SD rats weighing approximately 100 g were euthanized via carbon dioxide inhalation. Then, their backs were shaved and disinfected. The dorsal skin tissue 3 cm x 3 cm below the line connecting the subscapular angles was excised on both sides. The subcutaneous soft tissue was trimmed with scissors to expose the dermis and further divided into tissue blocks with an area of 1 mm x 2 mm. The tissue pieces were placed in a petri dish with the dermis facing down and cultured in fresh complete high-glucose DMEM (Sigma–Aldrich, USA) containing 10% fetal bovine serum (FBS; Biological Industries, USA). The dishes were incubated in a humidified incubator at 37 °C with 5% CO2. After 7–10 days, the cells grew around the tissue block, the tissue block was removed, the cells were subcultured and cells at passages 3–5 were used for subsequent experiments.
Immunofluorescence Staining Detection
The extracted cells were seeded in 24-well plates. The next day, the cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 15 min at room temperature, and then permeabilized with 0.5% Triton-X-100 for 20 min. After blocking with goat serum for 30 min at 37 °C, the cells were incubated with vimentin antibody (1:200, Waneibio, China) overnight at 4 °C. The next day, the cells were incubated with fluorescent secondary antibodies for 1 hour at 37 °C. Cell nuclei were then stained with DAPI. Immunofluorescence photographs were obtained via confocal microscopy (Olympus FluoView 1000, JPN).
Fibroblasts Co-Culture
Fibroblasts were co-cultured with r-PGRN (BioVision, USA) at different concentrations (0 ng/mL, 20 ng/mL, 50 ng/mL, 100 ng/mL, 200 ng/mL) for 24 hours in complete medium. After protein extraction, Western blotting was performed to explore the effect of PGRN on fibroblast function. In another set of experiments, we pretreated fibroblasts with the TGF-β inhibitor HY-10431 (1:1000, MedChemExpress, USA), followed by co-culture with r-PGRN (200 ng/mL) to study the molecular mechanism of PGRN and the molecular mechanism between fibroblasts.
EdU Cell Proliferation Assay
An appropriate amount of fibroblasts was added to a 96-well plate, and 10% FBS high glucose DMEM medium was used to prepare PGRN solutions of different concentrations and then to prepare the EdU mixture. The corresponding EdU mixture was added to the control group and the experimental group and incubated overnight. On the second day, 4% paraformaldehyde was added and fixed for 30 min. After washing, 100 μL of 0.5% Triton X-100 solution was added to each well, and after incubation on a shaker for 10 min, 100 μL of the freshly prepared staining reaction mixture was added to each well and incubated at room temperature for 30 min in the dark. After incubation, wells were washed with PBS. Then, 100 μL of 1×Hoechst33342 was added to each well, followed by incubation in the dark for 30 min and washing with PBS after incubation. Subsequent observations and recordings were made using a fluorescence microscope.
Wound Healing Experiment
Equal numbers of fibroblasts were evenly seeded in a six-well plate and washed with PBS on the second day, and a 200-μL pipette tip was used to make scratches in the cell monolayer with a width of approximately 500 μm. In the control group, the medium was replaced with high-glucose DMEM without FBS, and the experimental group was treated with different concentrations (20 ng/mL, 50 ng/mL, 100 ng/mL, 200 ng/mL) of r-PGRN mixed with high-glucose DMEM without FBS. Photos were taken at 0 h and 24 h. All photographs were obtained with an inverted optical microscope (ZEISS Axio Vert.A1, Germany).
Western Blot Analysis
Soluble proteins from co-cultured fibroblasts and capsule samples were extracted using RIPA lysis buffer (Beyotime, China) and 1% phenylmethanesulfonyl fluoride (PMSF) (Beyotime). The protein concentration was determined using a BCA protein concentration kit (Beyotime). Equivalent amounts of protein (20–50 μg) were separated via SDS–PAGE and transferred to PVDF membranes. After blocking in blocking solution (EpiZyme, China) for 30 min at room temperature, the membranes were incubated with the appropriate primary antibody overnight at 4 °C and then with the corresponding secondary antibody (1:5000, Beyotime) for 1 h at room temperature on the second day. Finally, antigen-antibody complexes were detected with enhanced chemiluminescence reagent (ECL). GAPDH and β-actin were used as loading controls. Primary antibodies against the following proteins were used: GAPDH (1:500, Waneibio), β-actin (1:500, Waneibio), collagen I (1:500, Waneibio), collagen III (1:500, Waneibio), MMP-1 (1:500, Waneibio), p-SMAD2&3 (1:500, Waneibio), SMAD2&3 (1:500, Waneibio), α-SMA (1:500, Waneibio): 500, Waneibio), BCL-2 (1:500, Waneibio), BAX (1:500, Waneibio), TGF-β1 (1:500, Waneibio), PGRN (1:500, Biorbyt, UK) and TIMP-1 (1:500, Waneibio).
Operation Stage 2
Twenty female SD rats with a body weight of approximately 120 g were divided into a control group and experimental group, with n = 10 in each group. Both groups then underwent the same surgery as described for the implanted group in stage 1. Beginning on the 1st day after surgery, the experimental group was injected with r-PGRN (BioVision) solution (100 ng/mL, 1 mL/day) on the surface of the implant every day for 10 days. In the control group, an equal volume of saline was injected on the surface of the implant for 10 days. After 112 days,28 the rats were euthanized via carbon dioxide inhalation, and the capsule tissue surrounding the implant was obtained.
Histological Staining
Tissues obtained on day 112 after stage 2 were fixed in 4% paraformaldehyde for 36 hours. The fixed tissue was dehydrated in graded alcohol and embedded in paraffin blocks, after which serial, longitudinal, 3-μm paraffin-embedded sections were prepared and stained with hematoxylin and eosin (H&E) (Solarbio China) and Masson’s trichrome (Solarbio). Both stainings were performed according to the instructions. Capsule thickness measurements were performed by two blinded physicians, and the average of the measurements was recorded.
Statistical Analysis
All data were analyzed using GraphPad Prism 9.0 software and are presented as the mean ± SEM, with p-values less than 0.05 (p < 0.05) considered to indicate a significant difference.
Results
Surgical Trauma and Silicone Implants Can Synergistically Increase the Local and Plasma PGRN Levels in Rats, and the Plasma PGRN Concentration is Positively Correlated with the Local PGRN Concentration
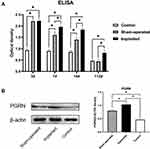
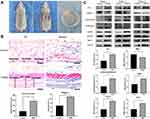
In this study, we used ELISA to detect the changes of PGRN levels in the plasma of rats after stage 1 surgery and compared them with the rats in the control group with sham surgery. The results showed that the plasma PGRN content in the two groups after surgery (sham-operated group and implanted group) reached a higher level within 0–3 days after surgery. Although there was no significant difference in plasma PGRN concentration between the sham-operated group and the implanted group at 3 days, compared with the control group, the plasma PGRN concentration of the two groups after surgery were significantly increased. At 7 days, there was a statistical difference between the two groups treated with surgery. The plasma PGRN concentration in the implanted group was continuously higher than that in the sham-operated group, and the elevated state of PGRN after surgery lasted for at least 14 days after surgery. At 112 days, the plasma PGRN content of rats in the sham-operated group fell back to the level of the control group at the same period. The content of PGRN in the plasma of the rats in the implanted group also decreased gradually over time, but it was still higher than the level in the control group at the same period. This high-level state continued until the end of the experiment (Figure 1A).
In order to further understand whether postoperative PGRN can be locally enriched in implants and the corresponding relationship between peripheral blood and surgical sites, we measured PGRN protein in local tissue by Western blotting at 14 days. The results showed that, corresponding to the results observed in the ELISA study, the local PGRN content of the implants in the implanted group was higher than that in the sham-operated group at 14 days. The local PGRN content of the two groups which receiving operations was higher than that in the control group (Figure 1B). It can be seen that there is a certain positive correlation between plasma PGRN concentration and local PGRN concentration.
Identification of Rat Fibroblasts
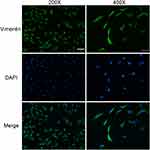
Fibroblasts were isolated from rat dermal tissue. Under an inverted microscope, rat dermal fibroblasts were attached to the bottom of the culture flask. The adherent cells had larger cell bodies, mostly spindle shaped or star shaped, with large and prominent nucleoli. The growth curve of vigorous fibroblasts was s-shaped. Vimentin immunofluorescence staining showed obvious green fluorescence in the cytoplasm (positive vimentin staining), and the cell nuclei were stained blue (Figure 2).
r-PGRN Promotes Fibroblast Migration
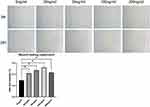
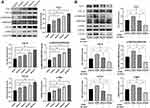
In the wound healing experiment, defects of equal width were created in each group, and the rate of change of the defect area per unit time was compared, thereby quantifying the cell migration ability. We found that r-PGRN promoted the migration of fibroblasts in the experimental group, with values of 0.4167 ± 0.032, 0.4705 ± 0.02451, 0.5193 ± 0.02790, and 0.4282 ± 0.01435, compared with 0.2928 ± 0.03589 in the control group. This promotion was dose-dependent in a concentration range of 0–100 ng/mL (Figure 3).
r-PGRN Promotes Fibroblast Proliferation and Inhibits Cell Apoptosis
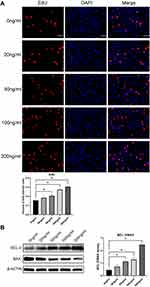
In the EdU fluorescence staining experiment, EdU(+) cells display red fluorescence, indicating cell division behavior. Nuclei exhibited blue fluorescence. The ratio of EDU(+) cells to all the cells in the field of view indicates the strength of cell proliferation. Statistical results obtained by fluorescence microscopy showed that r-PGRN promoted the proliferation of fibroblasts in a dose-dependent manner. Compared with the proportion of EDU(+) cells (15.53 ± 1.062%) in the control group (0 ng/mL), the proportion of EDU(+) cells increased sequentially as the concentration of r-PGRN increased during co-culture in the experimental group, with values of 18.35 ± 1.5%, 20.55 ± 1.413%, 27.37 ± 1.291%, and 30.43 ± 0.4994% (Figure 4A).
In Western blot experiments, the relative extent of apoptosis was determined by comparing the ratio of the apoptosis-inhibiting protein BCL-2 to the apoptosis-promoting protein BAX (BCL-2/BAX). We found that r-PGRN inhibited apoptosis of fibroblasts in a dose-dependent manner. Compared with the ratio of 0.4492 ± 0.09456 in the control group, the expression of the anti-apoptotic protein BCL-2 in the experimental group was increased, while the expression of the pro-apoptotic protein BAX was decreased, and the BCL-2/BAX ratio was upregulated (0.7199 ± 0.1576, 1.126 ± 0.07989, 1.277 ± 0.1638, and 2.462 ± 0.1956) (Figure 4B).
r-PGRN Promotes the Functional Activation and Differentiation of Fibroblasts by Activating the TGF-β/SMAD Signaling Pathway
Western blot results showed that r-PGRN promoted the expression of type I and type III collagen in the fibroblasts in the experimental group in a dose-dependent manner compared with the control group. In addition, PGRN had a certain promoter effect on the differentiation of fibroblasts. As a marker of myofibroblasts, α-SMA showed increased expression in the experimental group in a dose-dependent manner (Figure 5A). To further investigate the potential mechanism by which PGRN enhances fibroblast function, we analyzed the protein expression of TGF-β1 and p-SMAD2&3 in the experimental group via Western blot. We found that r-PGRN stimulated TGF-β1 and p-SMAD2&3 protein expression in fibroblasts in the experimental group in a dose-dependent manner (Figure 5A). In the following experiments, fibroblasts were pretreated with a TGF-β signaling pathway inhibitor (HY-10431) for 1 hour before co-culture with r-PGRN. Western blot experiments were subsequently performed. The results showed that the promoting effect of r-PGRN on fibroblasts could be blocked by HY-10431, as demonstrated by the decreased expression of collagen types I and III, α-SMA, TGF-β1 and p-SMAD2&3 (Figure 5B).
An Increase in Local PGRN Content Can Promote the Formation and Contracture of Prosthesis Capsules
We placed the implant into the back of the rats via implantation surgery. The growth and development of the rats were stable after the operation. The position of the prosthesis was stable, and there was no obvious displacement. There were no postoperative complications, such as infection, hematoma, ulceration, or prosthesis exposure, in the surgical area (Figure 6A). Through H&E staining of the capsules, we found that the thickness of the capsules in the experimental group under r-PGRN stimulation was thicker than that in the control group. Using Masson’s trichrome staining, we found that the number of collagen fibers in the capsules in the experimental group was larger, the fibers were thicker, the arrangement was tight, and the direction was regular. (Figure 6B). Through Western blot experiments, we found that the expression levels of type I collagen (COL-I), the myofibroblast marker α-SMA, and matrix metalloproteinase inhibitor (TIMP-1) were increased in the capsule in the experimental group. Matrix metalloproteinase-1 (MMP-1) expression was decreased. In addition, the activation degree of the TGF-β/SMADsignaling pathway in the capsules of the experimental group was higher than that of the control group, which showed that the expressions of TGF-β1 and p-SMAD2&3 in the capsules of the experimental group were increased (Figure 6C).
Discussion
Capsular tissues are dense fibrous connective tissues, of which fibroblasts and collagen fibers are the main components.29 Fully elaborating the effects of various factors on the function and differentiation of fibroblasts will help to explain the evolution of capsule formation and contracture and provide a new direction for the diagnosis and treatment of serious complications caused by abnormal capsule hyperplasia.
It has been pointed out that as a secreted protein, PGRN mRNA content can be rapidly and significantly increased in the damaged area of the skin. In contrast, PGRN mRNA is barely expressed in undamaged skin.27 In our study, we found that the increase in PGRN expression induced by implantation was not only local but also systemic. This may be related to the characteristics of implantation. In the clinical application of implants a deeper dissection is often required to allow more soft tissue to cover the surface of the prosthesis and reduce the visibility of the prosthesis edge. The degree of injury is significantly different between implantation and skin injury, so we believe that the degree of injury determines the degree of PGRN elevation. This was also reflected at 3 days, because there was no difference in the surgical site, depth, or range between the sham-operated group and the implanted group. Moreover, there was no significant difference in early plasma PGRN content.
We also found that the presence of implants affected the changes in plasma PGRN content in rats. This was mainly reflected on day 7, day 14 and day 112, when the plasma PGRN content of the implanted group was higher than that of the other two groups. Studies have shown that PGRN expression in fibroblasts, endothelial cells, and infiltrating inflammatory cells is elevated in the response phase after injury.13 Due to the presence of the prosthesis, the wound healing process is prolonged. Macrophages, lymphocytes, fibroblasts and other cellular components are enriched in the capsule. Moreover, persistent activation of acute and chronic inflammation occurs locally. This results in a sustained release of PGRN.
We also noticed that due to the persistence of the implant, the elevated state of plasma PGRN content in the implanted group could last until the end of the experiment. At this time, the injury in the sham-operated group had been healed, and the plasma PGRN content dropped to the level of the control group at the same period. This suggests that the implant will continue to stimulate the increase of PGRN content for a longer period of time, so that PGRN can regulate the formation of local capsules in the long term. In addition, we found by Western blotting that PGRN could be enriched in the injured local tissue on the surgical local tissue on day 14, which provided a basis for PGRN participation in the local capsular formation. The changing trend in local and plasma PGRN content is consistent, which suggests the possibility of detecting the local PGRN content by measuring the plasma PGRN content.
In human fibrosis, there are two interrelated processes, fibrosis and fibrinolysis. In in vivo and in vitro experiments, PGRN showed promotion of fibroblast fibrotic function and inhibition of the fibrinolytic process, because the capsule is mainly composed of fibroblasts and collagen fibers. Therefore, PGRN mainly affects the composition and function of cells in the capsule, thereby promoting capsule thickening and increasing the risk of capsule contracture. In terms of cellular composition, because PGRN promoted fibroblast proliferation and migration and inhibited fibroblast apoptosis in a dose-dependent manner, higher doses of PGRN played a greater role in increasing the number of fibroblasts within the capsule, not only in terms of cell number but also in terms of cell differentiation; PGRN promotes fibroblast-to-myofibroblast differentiation in a dose-dependent manner.
As an important activated form of fibroblasts, myofibroblasts not only have stronger collagen secretion ability but also have contractile ability similar to that of smooth muscle cells. α-SMA has been used as an important indicator to predict the possibility and severity of capsular contracture in several studies.12,30 Some studies have noted that PGRN can induce the differentiation of fibroblasts into myofibroblasts.31 In the present study, higher concentrations of r-PGRN increased the expression of α-SMA both in vivo and in vitro. This indicated that r-PGRN could stimulate the differentiation of fibroblasts into myofibroblasts. Therefore, we believe that increasing the concentration of PGRN can increase the proportion of myofibroblasts in the capsule, thereby increasing the collagen content and contractility of the capsule tissue and promoting the formation and contraction of the capsule.
In terms of cell function, PGRN promoted the expression of COL-I and COL-III in a dose-dependent manner, increased the content of collagen fibers in the capsule tissue, and increased the thickness and strength of the capsule, thereby promoting the contracture of the capsule. During the process of fibrinolysis, the dynamic balance of MMPs and TIMPs jointly maintains the normal physiological process of envelope development, and the imbalance of the two ratios can alter the process of envelope formation.32,33 PGRN inhibits fibrinolysis by inhibiting the expression of MMP-1 and promoting the expression of TIMP-1 in the capsule tissue, thereby promoting collagen deposition and capsule formation and contracture.
Many fibrosis processes are often accompanied by enhanced transduction of the TGF-β signaling pathway.34,35 Similarly, the TGF-β/SMAD signaling pathway has always been the focus of research on capsule formation and contracture.36 Activation of this pathway can stimulate fibroblasts to synthesize collagen and promote fibroblast differentiation. Western blot analysis of co-cultured fibroblasts and capsule specimens showed that r-PGRN promoted TGF-β1 expression and SMAD2&3 phosphorylation, resulting in enhanced transduction of this signaling pathway in fibroblasts. In a related study of scleroderma, it was shown that PGRN could increase the expression of TβR1 in fibroblasts.37 In the current study, r-PGRN also partially reversed the pathway blockade caused by pretreatment with the pathway inhibitor HY-10431. This may be related to PGRN increasing the expression of TβR1 in fibroblasts.
Based on the biological effects of r-PGRN on fibroblasts, we believe that PGRN can promote the formation and contracture of silicone implant capsules. The regulation of fibroblasts and fibrinolysis by PGRN is the functional basis for its promotion of capsular development. The dose dependence of the PGRN-promoting function relies on the concentration to stimulate capsule development. Considering the consistency of the changes of PGRN content in local capsules and plasma after implantation, we believe that after implantation surgery, the content of PGRN in plasma can represent the degree of local PGRN enrichment in capsules. Depending on its concentration, the future effects of PGRN on the capsule can be predicted. This allows more accurate and rational prediction of the outcome of the capsule’s future evolution and provides new targets for capsule diagnosis and therapy.
Conclusion
The present study demonstrates that surgical trauma and implants can increase local and plasma PGRN levels in rats and that PGRN can promote the formation and contracture of silicone implant capsules in a dose-dependent manner. We believe that using PGRN as a detectable systemic marker to monitor capsule development will help us better predict capsule formation and outcome. In addition, as a cytokine that can stimulate capsular formation and contracture, PGRN can also be targeted for drug therapy. Given the large number of people undergoing implantation procedures (eg breast augmentation) each year, the presence of PGRNs can provide an important aid in monitoring and treating adverse effects.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Champaneria MC, Wong WW, Hill ME, et al. The evolution of breast reconstruction: a historical perspective. World J Surg. 2012;36:730–742.
2. Baino F. Scleral buckling biomaterials and implants for retinal detachment surgery. Med Eng Phys. 2010;32:945–956.
3. Anderson JM. Multinucleated giant cells. Curr Opin Hematol. 2000;7:40–47.
4. Gretzer C, Emanuelsson L, Liljensten E, et al. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17:669–687.
5. Luttikhuizen DT, Harmsen MC, Van Luyn MJ. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006;12:1955–1970.
6. Wilson CJ, Clegg RE, Leavesley DI, et al. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1–18.
7. Broughton GN, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S–34S.
8. Lossing C, Hansson HA. Peptide growth factors and myofibroblasts in capsules around human breast implants. Plast Reconstr Surg. 1993;91:1277–1286.
9. Blum A, Abboud W, Shajrawi I, et al. Prolonged fever due to silicone granulomatosis. Isr Med Assoc J. 2007;9:121–122.
10. Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast Reconstr Surg. 2007;120:275–284.
11. Hwang K, Sim HB, Huan F, et al. Myofibroblasts and capsular tissue tension in breast capsular contracture. Aesthetic Plast Surg. 2010;34:716–721.
12. Baker JJ, Chandler ML, LeVier RR. Occurrence and activity of myofibroblasts in human capsular tissue surrounding mammary implants. Plast Reconstr Surg. 1981;68:905–912.
13. Toh H, Chitramuthu BP, Bennett HP, et al. Structure, function, and mechanism of progranulin; the brain and beyond. J Mol Neurosci. 2011;45:538–548.
14. Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009;31:1245–1254.
15. Yang D, Li R, Wang H, et al. Clinical implications of progranulin in gastric cancer and its regulation via a positive feedback loop involving AKT and ERK signaling pathways. Mol Med Rep. 2017;16:9685–9691.
16. Daya M, Loilome W, Techasen A, et al. Progranulin modulates cholangiocarcinoma cell proliferation, apoptosis, and motility via the PI3K/pAkt pathway. Onco Targets Ther. 2018;11:395–408.
17. Tian R, Li Y, Yao X. PGRN Suppresses Inflammation and Promotes Autophagy in Keratinocytes Through the Wnt/beta-Catenin Signaling Pathway. Inflammation. 2016;39:1387–1394.
18. Yue S, Ye X, Zhou T, et al. PGRN(-/-) TAMs-derived exosomes inhibit breast cancer cell invasion and migration and its mechanism exploration. Life Sci. 2021;264:118687.
19. Greither T, Steiner T, Bache M, et al. GP88/PGRN Serum Levels Are Associated with Prognosis for Oral Squamous Cell Carcinoma Patients. Biology. 2021;10:1045.
20. Li SS, Zhang MX, Wang Y, et al. Reduction of PGRN increased fibrosis during skin wound healing in mice. Histol Histopathol. 2019;34:765–774.
21. Ding Y, Wei J, Hettinghouse A, et al. Progranulin promotes bone fracture healing via TNFR pathways in mice with type 2 diabetes mellitus. Ann N Y Acad Sci. 2021;1490:77–89.
22. Miyagawa T, Ichimura Y, Nakamura K, et al. Progranulin overproduction due to constitutively activated c-Abl/PKC-delta/Fli1 pathway contributes to the resistance of dermal fibroblasts to the anti-fibrotic effect of tumor necrosis factor-alpha in localized scleroderma. J Dermatol Sci. 2018;92:207–214.
23. Xie T, Han L, Chen Y, et al. Progranulin and Activin A Concentrations are Elevated in Serum from Patients with Acute Exacerbations of Idiopathic Pulmonary Fibrosis. Lung. 2021;199:467–473.
24. Cerezo LA, Kuklova M, Hulejova H, et al. Progranulin Is Associated with Disease Activity in Patients with Rheumatoid Arthritis. Mediators Inflamm. 2015;2015:740357.
25. Luo Q, He X, Zheng Y, et al. Elevated progranulin as a novel biomarker to predict poor prognosis in community-acquired pneumonia. J Infect. 2020;80:167–173.
26. Rao L, Song Z, Yu X, et al. Progranulin as a novel biomarker in diagnosis of early-onset neonatal sepsis. Cytokine. 2020;128:155000.
27. Liu C, Li J, Shi W, et al. Progranulin Regulates Inflammation and Tumor. Antiinflamm Antiallergy Agents Med Chem. 2020;19:88–102.
28. Vieira VJ, D’Acampora A, Neves FS, et al. Capsular Contracture In Silicone Breast Implants: insights From Rat Models. An Acad Bras Cienc. 2016;88:1459–1470.
29. Domanskis E, Owsley JJ. Histological investigation of the etiology of capsule contracture following augmentation mammaplasty. Plast Reconstr Surg. 1976;58:689–693.
30. Rohrich RJ, Kenkel JM, Adams WP. Preventing capsular contracture in breast augmentation: in search of the Holy Grail. Plast Reconstr Surg. 1999;103:1759–1760.
31. Dong T, Yang D, Li R, et al. PGRN promotes migration and invasion of epithelial ovarian cancer cells through an epithelial mesenchymal transition program and the activation of cancer associated fibroblasts. Exp Mol Pathol. 2016;100:17–25.
32. Ulrich D, Lichtenegger F, Eblenkamp M, et al. Matrix metalloproteinases, tissue inhibitors of metalloproteinases, aminoterminal propeptide of procollagen type III, and hyaluronan in sera and tissue of patients with capsular contracture after augmentation with Trilucent breast implants. Plast Reconstr Surg. 2004;114:229–236.
33. Ulrich D, Ulrich F, Pallua N, et al. Effect of tissue inhibitors of metalloproteinases and matrix metalloproteinases on capsular formation around smooth and textured silicone gel implants. Aesthetic Plast Surg. 2009;33:555–562.
34. Weng T, Yan D, Shi D, et al. The MSP-RON pathway regulates liver fibrosis through transforming growth factor beta-dependent epithelial-mesenchymal transition. Liver Int. 2021;41:1956–1968.
35. Douglas HE. TGF-ss in wound healing: a review. J Wound Care. 2010;19:403–406.
36. Katzel EB, Koltz PF, Tierney R, et al. The impact of Smad3 loss of function on TGF-beta signaling and radiation-induced capsular contracture. Plast Reconstr Surg. 2011;127:2263–2269.
37. Yang T, Zhang X, Chen A, et al. Progranulin Promotes Bleomycin-Induced Skin Sclerosis by Enhancing Transforming Growth Factor-beta/Smad3 Signaling through Up-Regulation of Transforming Growth Factor-beta Type I Receptor. Am J Pathol. 2019;189:1582–1593.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.