Back to Journals » International Journal of General Medicine » Volume 16
Prognostic Value of Systemic Immune-Inflammation Index-Based Nomogram in Patients with Extrahepatic Cholangiocarcinoma Treated by Percutaneous Transhepatic Biliary Stenting Combined with 125I Seed Intracavitary Irradiation
Authors Yang J, Shu C, Shang X, Xu H, Wei N
Received 9 March 2023
Accepted for publication 15 May 2023
Published 29 May 2023 Volume 2023:16 Pages 2081—2094
DOI https://doi.org/10.2147/IJGM.S411577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jing Yang, Chengsen Shu, Xianfu Shang, Hao Xu, Ning Wei
Department of Interventional Radiology, the Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221006, People’s Republic of China
Correspondence: Ning Wei, Email [email protected]
Purpose: This study aimed to investigate the prognostic value of systemic immune-inflammation index (SII) in patients with extrahepatic cholangiocarcinoma (EHCC) treated by percutaneous transhepatic biliary stenting (PTBS) combined with 125I seed intracavitary irradiation and further develop a predictive model related to SII.
Methods: A total of 145 patients with EHCC who received PTBS combined with 125I seed implantation were retrospectively analyzed. The optimal cut-off value of SII was identified by receiver operating characteristic (ROC) curve analysis. Kaplan–Meier curves and Cox regression were applied to estimate the prognostic value of SII and identify other significant factors of overall survival (OS). Additionally, a novel nomogram was constructed. The concordance index (C-index), calibration plots and decision curve analysis were used to evaluate the performance of the nomogram model.
Results: The optimal cut-off value for preoperative SII of 890.2 stratified the patients into High-SII (H-SII) and Low-SII (L-SII) groups. Univariate and multivariate analyses demonstrated that SII was an independent factor for OS. We also found that better therapeutic effect could be obtained with combined postoperative chemotherapy (P < 0.001). Moreover, we revealed that elevated preoperative CA19-9 (P = 0.038) and TBIL level (P = 0.024) were reason for poor prognosis of EHCC. A well-discriminated and calibrated nomogram was developed to predict the 1-year and 2-year OS of EHCC (C-index: 0.709).
Conclusion: The SII may be a feasible and convenient prognosis predictor for EHCC. The comprehensive nomogram based on SII presented in this study is a promising model for predicting OS in EHCC patients after PTBS combined with 125I seed intracavitary irradiation.
Keywords: extrahepatic cholangiocarcinoma, biliary stenting, 125I seed, systemic immune-inflammation index, nomogram
Introduction
Cholangiocarcinoma (CCA) is a highly malignant and aggressive tumor that originates from the epithelial cells of bile duct.1 According to the anatomical location, CCA is classified into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (EHCC), and of which EHCC accounts for 75–90%.2 EHCC is further divided into hilar cholangiocarcinoma (hCCA) and distal cholangiocarcinoma (dCCA).2 Surgical resection is recommended as the preferred therapy for early EHCC.3 But few patients are candidates for surgery, mainly owing to the advanced tumor stage at diagnosis and the high risk of complications, recurrence, and metastasis.4 Previous studies had reported that palliative surgery, radiotherapy, chemotherapy, targeted therapy could be considered as optional treatments for unresectable EHCC.5–7 With the rapid development and progress of minimally invasive technology, interventional therapy has gradually become the first clinical choice for unresectable EHCC. Recent studies have revealed that biliary drainage and biliary stenting can relieve biliary obstruction and restore bile drainage for EHCC.8 It is worth mentioning that the ingrowth of tumor or epithelial hyperplasia would cause further restenosis within 3–6 months,9 doubts about the efficacy of biliary stenting come up. Fortunately, as the implantation of radioactive 125I seed, interstitial brachytherapy combined with biliary stenting has been identified as a viable and effective palliative therapy, which can improve stent patency and prolong survival time.10,11 At present, the studies of PTBS combined with 125I seed intracavitary irradiation for EHCC mostly focused on the clinical efficacy, and fewer researches about related factors affecting prognosis have been reported. Therefore, it is crucial to identify effective prognostic markers for interventional treatment to optimize adjuvant therapies.
Various previous studies have shown that immune inflammatory indicators have predictive value for postoperative survival outcomes in several cancers, including EHHC.12–14 The systemic immune inflammatory index (SII) is calculated by platelet, neutrophil and lymphocyte counts, which has been served as useful prognostic biomarkers for several solid tumors.15,16 The clinical utility of SII as a prognostic indicator has been verified in EHCC patients who received radical resection.14 To our knowledge, the prognostic value of SII in patients with unresectable EHCC has not yet been explored.
In this study, we intended to analyze the prognostic value of preoperative SII in patients with EHCC treated by PTBS combined with 125I seed intracavitary irradiation and to further construct a nomogram model for prognosis on the basis of SII.
Methods
Study Population
Patients who underwent PTBS combined with 125I seed implantation for EHCC between August 2018 and December 2021 in department of interventional radiology were included in our study. Inclusion criteria: (1) pathologically or radiologically (contrast-enhanced magnetic resonance imaging (MRI) or computed tomography (CT) confirmed EHCC). (2) with no surgical indication. (3) all implanted biliary stent combined with 125I seed strip. Exclusion criteria: (1) combined with severe cardiac, hepatic, and renal dysfunction. (2) ICC or benign bile duct stricture. (3) previously underwent radical surgery, chemotherapy, radiotherapy, or other therapeutic methods for EHCC. (4) incomplete admission or follow-up data (Figure 1). Informed consent was signed before interventional therapy by all patients. Our study was carried out in accordance with the principles of the Declaration of Helsinki17 and approved by the ethics committee of Affiliated Hospital of Xuzhou Medical University.
 |
Figure 1 Flow diagram of patients enrolled. |
Materials
Percutaneous transhepatic cholangial drainage (PTCD) was executed by pigtail catheters; 125I seed (Ningbo Junan Pharmaceutical Technology limited company), specification: 4.5mm*0.8mm, with a half-life of 59.6 days; Biliary metal stent (Bard Company, 8 mm×6 cm, 8 mm×8 cm); balloon dilation catheter (Cook Company, length: 40–80mm, diameter: 6–8cm); guidewire (Terumo company); PTCD tube (Cook company).
Surgical Procedures
All patients received PTCD before stent implantation. About a week later, cholangiography through drainage tube was first made to confirm the location of bile duct obstruction and measure the length and diameter of the obstructive bile duct. The guidewire was introduced through drainage tube, which was then replaced by 6F catheter sheath. Subsequently, the catheter was inserted through the guidewire and further reached the duodenum via repeatedly adjusting the direction of guidewire. Under the guidance of DSA, the biliary stent was slowly released at the bile duct stricture. At the same time of withdrawing the 6F catheter sheath, the prepared 125I seed strip was placed between the stent and bile duct wall. Finally, a drainage tube was placed indwelling above the stent. If the stent expanded unsatisfied, the balloon dilation was performed.
The amount of 125I seed was calculated as follows: N = Length of the biliary obstruction segment (mm)/4.5+4.18
Data Collection
Blood characteristics were extracted from peripheral blood within 3 days prior to the onset of interventional surgery. The variables measured included Neutrophil counts, Lymphocyte counts, Platelet counts, prealbumin (PAB), albumin (ALB), total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), a lkaline phosphatase (ALP), Carbohydrate antigen199 (CA19-9), high-sensitivity C-reactive protein (hsCRP), carcinoembryonic antigen (CEA), prothrombin time (PT). Neutrophil, lymphocyte, and platelet counts were collected to calculate SII: SII = platelet (P)×neutrophil (N)/lymphocyte (L). Body mass index (BMI) was defined as weight (kg)/square height (m2).
Follow-Up and Outcomes
In our study, the survival status of all patients was obtained through outpatient follow-up or telephone interview. Follow-up was performed every 3 months from the end of interventional surgery and lasted until September 2022 or death. Each follow-up visit consisted of a clinical interview, routine biochemical examinations, tumor landmarker tests, and imaging investigations such as abdominal ultrasound, CT, and (or) MRI. The major outcome of the study was overall survival (OS). OS was determined as the duration from the date of interventional therapy to death or the last follow-up.
Statistical Analysis
Statistical analyses were conducted using SPSS software version 24.0 and R software (version 4.1.3, https:www.r-project.org/). Continuous variables were converted into categorical variables and expressed as numbers and percentages. On the ground of receiver operating characteristic (ROC) curve analysis, the optimal cut-off value of SII was obtained by calculating the maximum Youden’s index. Then the patients were split into high and low groups. The discrepancy between groups was compared by the chi-square (x2) test. Spearman correlation analysis was applied to analyse correlations between SII and above significantly variables. Cox proportional hazards regression model was applied to perform univariate and multivariate analysis to evaluate clinical factors associated with OS. The predictive value of SII for OS was assessed using the Kaplan–Meier method and Cox proportional hazards model. Variables with P value less than 0.05 in the multivariate analysis were selected to plot the nomogram. The calibration plots and concordance indexes (C-index) were applied to evaluate the performance of the established nomogram model in predicting prognosis. Finally, decision curve analyses (DCA) were generated to determine the clinical effectiveness of the nomogram. A two-sided P value <0.05 was considered as statistical significance.
Results
Patients Characteristics
A consecutive series of 145 EHCC patients who underwent percutaneous transhepatic biliary stenting combined with 125I seed intracavitary irradiation were enrolled in this study (Figure 1). Up to the deadline for follow-up, a total of 27 patients were alive and 118 patients died. The median follow-up time was 25.5 months (range 2.5–30 months). Of all these patients, 91 (62.8%) patients were male, 54 (37.2%) patients were female and the mean age was 67.9±11.8 years. Ninety-two cases were clinically and (or) pathologically diagnosed as hCCA and 53 cases were dCCA. Basic clinical data and laboratory tests are presented in Table 1.
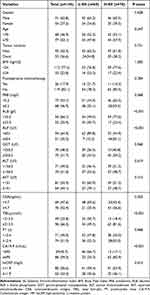 |
Table 1 Baseline Clinical Characteristics of the Participants |
Biliary stent and 125I seed went on smoothly in all patients. The symptom of obstructive jaundice ameliorated significantly after the operation. The postoperative complication occurred in 15 cases (10.3%): 8 had hyperamylasemia (diastase was more than 3 times the reference value within 24 h) and 7 had biliary tract infection. All of them were effectively controlled under symptomatic treatment.
By the end of follow-up, a total of 32 patients had stent restenosis, of which 18 patients received re-intervention, including 5 patients underwent secondary stent placement, 13 patients received percutaneous hepatic biliary drainage and the remaining 14 patients had no treatment after stent obstruction due to basal intolerance, subjects refusal, and other reasons.
Thirty-one pathologically confirmed patients had combined with postoperative chemotherapy after interventional therapy. Five patients failed to the entire process of chemotherapy owing to poor physical condition or other factors. The regimen of perfusion chemotherapy was administered: gemcitabine 600–1000 mg/m2 and oxaliplatin 60–100 mg/m2; The perfusion time was 20 min, 3–4 weeks apart for the next perfusion chemotherapy, a total of 5 courses. Twenty-six patients completed full course of chemotherapy and markedly prolonged survival.
Determination of the Optimal Cut-off Values for SII
The ROC curves of SII indicated that 890.2 was optimal cut-off values. SII was a statistically significant indicator for distinguishing between deceased and surviving cases, with the corresponding area under the curve (AUC) value 0.751 (95% CI: 0.652–0.850). Accordingly, these patients were categorized into the Low-SII group (L-SII, n = 69, 47.6%) and the High-SII group (H-SII, n = 76, 52.4%) in the light of the optimal cut-off value.
Association of SII with EHCC
As presented in Table 1, no notable differences were observed between groups in the aspect of baseline characteristics, such as gender, age, tumor location, BMI, ALT, AST, GGT, postoperative chemotherapy, PAB, PT, and hsCRP (all P > 0.050). SII was statistically related with several clinical features, including ALB, ALP, TBIL and CA19-9 (all P < 001), as well as CEA (P = 0.002). The elaborated correlations between the SII and other variables are depicted in Table 1. By means of the Spearman correlation analysis, SII was associated weakly with these variables, and the confounding effect on SII was negligible (Table 2).
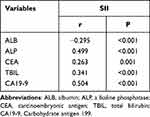 |
Table 2 Correlation Between SII and Clinical Variables in EHCC Patients |
Correlations Between SII and Survival
The median survival time of patients was 13.2 months (95% CI: 11.1–15.3), with the 1-year and 2-year OS rates were 55.2% and 13.1%, respectively. Figure 2A reveals the Kaplan–Meier survival curves of all patients. As presented in Figure 2B, higher preoperative SII scores tended to generate worse OS in patients with EHHC [hazard ratio (HR)=17.587, 95% CI: 6.960–13.240, P < 0.001]. Subsequently, the prognostic value of SII level was analyzed in the subgroups based on location of EHCC. As further described in Figure 2C, higher SII was associated with lower OS rates for hCCA patients (HR = 10.683, 95% CI: 8.448–13.552, P = 0.001). Similarly, higher SII was correlated with lower OS rates for dCCA patients (HR = 12.612, 95% CI: 7.821–9.579, P < 0.001), which is demonstrated in Figure 2D.
Figure 2 Continued.
Cox Regression Analysis of Risk Factors of OS
Univariate analysis revealed that BMI [HR = 1.904, 95% CI: 1.256–2.885, P = 0.002], preoperative ALT level (HR = 0.580, 95% CI: 0.400–0.842, P = 0.004), postoperative chemotherapy (HR=4.912,95% CI: 2.695–8.952, P < 0.001), preoperative CA19-9 level (HR=2.772,95% CI: 1.841–4.173, P < 0.001), preoperative TBIL level (HR=2.537,95% CI: 1.644–3.913, P < 0.001), and preoperative SII level (HR=2.423,95% CI: 1.650–3.557, P < 0.001) were associated with OS in EHCC patients (Table 3). Subsequently, BMI, ALT, postoperative chemotherapy, CA19-9, TBIL, SII entered into multivariate analysis model. The preoperative CA19-9 (HR = 0.600, 95% CI: 0.366–0.985, P = 0.038), preoperative TBIL level (HR = 0.570, 95% CI: 0.347–0.935, P = 0.024), postoperative chemotherapy (HR=4.961,95% CI: 2.673–9.208, P < 0.001), and preoperative SII level (HR = 0.558, 95% CI: 0.360–0.566, P = 0.009) were independently factors correlated with OS for patients with EHCC treated by percutaneous transhepatic biliary stenting combined with 125I seed intracavitary irradiation. The results of multivariate analysis were depicted in the forest plot (Figure 3).
 |
Table 3 Univariate Analyses of Features Correlated with OS of EHCC Patients |
 |
Figure 3 Forest plot based on the results of multivariate analysis of the indicators associated with OS. |
A Nomogram Predicting OS Based on SII
On the basis of the significant indicators of multivariate analysis, a predictive nomogram for 1-year and 2-year OS in patients with EHCC was built. As shown in Figure 4, each feature was assigned points according to its risk contribution to OS and a higher total score was associated with a lower OS rate. The performance of the model was borne out internally. The time-dependent receiver operating characteristic (ROC) curve of the prognostic model is depicted in Figure 5C. The C-index of the nomogram for prediction of the OS was 0.709 in patients with EHCC. The calibration curves for 1-year, 2-year nomogram displayed great agreement between the predicted and real probability of OS (Figure 5A and B). Decision curve analysis revealed that our prognostic model integrating preoperative SII, CA19-9, TBIL and postoperative chemotherapy had preferable clinical utility (Figure 5D).
 |
Figure 4 Survival nomogram based on SII.The total points of every patient can be used to predict the OS of EHCC patients. |
 |
Figure 5 Continued. |
Discussion
As is well-known, inflammation may be conducive to the cancer microenvironment and promote tumor cells proliferation.19,20 Accumulating evidence has demonstrated that inflammation-based prognostic scores, such as NLR, PLR and SII, are related to the prognosis of patients with resectable EHCC.14,21–23 However, to date, little is known about the utility of inflammatory-based indicator in unresectable EHCC. In the current study, we explored that SII, a novel developed serum inflammatory marker, was recognized as an independent dangerous factor for prognosis in patients with EHCC treated by percutaneous transhepatic biliary stenting combined with 125I seed intracavitary irradiation. The study showed that the higher SII before interventional therapy in patients was associated with worse prognosis. The study identified CA19-9, TBIL, postoperative chemotherapy, along with SII, which were associated with the OS of EHCC. On the basis of those significant indicators, the prognostic nomogram prediction model of the interventional therapy for EHCC patients was established, this is, to the best of our knowledge, the first nomogram was used to predict the prognosis of such patients.
The biological reason for the prognostic value of SII should be elucidated by the function of neutrophil, platelet and lymphocytes, respectively. On the one hand, neutrophils are involved in stimulating tumor progression by secreting numerous inflammatory mediators, such as interleukin (IL)-6, IL-8, which may be essential for tumor angiogenesis.24 On the other hand, neutrophils can lead to DNA damage of epithelial cells and activate oncogenes by releasing noxious factors.25 The platelet, covered in previous researches, can protect circulating tumor cells from shear stress in the circulation to escape injury.26 While platelets activated by tumor cells will in turn facilitate tumor cell diffusion by relaxing the endothelial barrier to allow transendothelial migration.26,27 Lymphocytes play a critical role in surveilling and inhibiting proliferation and migration of cancer cells.28 Activated B lymphocytes are able to alter the levels of circulating cytokines and (or) chemokines to regulate cancer progression. Not only could T lymphocytes directly destroy cancer cells but also cause apoptosis via binding to Fas-FasL on the surface of tumor cells.29 Decreased lymphocyte numbers and quality might result in weakening the immune system and proliferation of cancer cells. Taken together, the SII is promising for predicting the prognosis of unresectable EHCC, to some extent.
Elevated levels of TBIL or CA19-9 were other prognostic parameter for lower OS in the present study. This can be explained by the fact that the higher TBIL, the longer obstruction of biliary tract may be, and the more serious tumor invasion.30 If the preoperative serum TBIL is rapidly increased to a higher level in the short term, it indicates that the cancer cells grow faster, their degree may be less differentiated, and the invasion ability is stronger.31 CA19-9, a tumor marker, is essential in screening, differentiating diagnosis, and judging prognosis of tumors, including EHCC.32–34 Yang et al suggested that preoperative CA19-9 levels were vital for prognosis of intrahepatic cholangiocarcinoma after hepatectomy.35 Tella et al found that CA19-9 is an important risk factor for OS in EHCC (HR: 1.72; 95% CI=1.462.02; P < 0.01).34
Recently, the effect of adjuvant chemotherapy for CCA has been widely demonstrated clinically.36–38 In this study, 26 patients completed the full course of chemotherapy and significantly prolonged survival. Cox regression model analysis suggested that postoperative chemotherapy was a protective factor affecting OS (P < 0.001). This indicated that the interventional therapy combined with neoadjuvant chemotherapy could provide preferable therapeutic efficacy. Regarding the optional time for chemotherapy, some scholars proposed that it should be performed within 3 and 4 days after the implantation of 125I seed, owing to the radiative function of 125I particles at this time can improve the permeability and the partial oxygen pressure of the tumor vessel, which can further enhance chemotherapeutic effectualness.39 However, more evidence is required to gather to confirm that.
Nomogram has been widely used as a visualization instrument to conduct individualized assessments, optimize adjuvant therapies and postoperative monitor in tumor patients. In this study, a nomogram consisting of SII, CA19-9, postoperative chemotherapy, and TBIL, which were the independent predictors of OS, was established. Data from the C-index and calibration plots certified that the nomogram had an outstanding predictive value on predicting oncological outcomes in patients with EHCC undergoing PTBS combined with 125I seed intracavitary irradiation. As the first nomogram of unresectable EHCC, our model may help screen preoperative high-risk groups, monitor the disease status individually to follow-up intervals, and optimize the postoperative adjuvant treatment of EHCC, which has potential clinical application value and worthy of further study and development.
There are some notable limitations in our study. First, the study was retrospective in a single-center and single-region, with limited sample and inevitable selection bias. In the future, a large number of internal and external samples are still required to validate and optimize the model. Second, the dynamic changes of the SII and other factors during follow-up may also associate with patient outcomes, which requires further research. Third, the differences in the prognostic value of SII with other inflammatory markers after intervention of EHCC patients have not been compared, such as NLR, PLR, COUNT and so on. Fourth, in the future, the nomogram model can be further improved to explore the prognostic impact of different postoperative adjuvant therapy regimen on EHCC patients. Last but not least, as fewer cases with a clear pathological diagnosis, the impact of pathological classification and differentiation on patient prognosis has not been considered.
Conclusion
In summary, we investigated that SII might be an accessible and reliable indicator to predict the short-term prognosis of patients with EHCC. The SII-based nomogram integrating CA19-9, postoperative chemotherapy and TBIL, may be a reliable tool to help doctors conduct personalized assessments. High-quality multicenter, prospective studies are required to further estimate the precision of the above nomogram model.
Data Sharing Statement
The raw data of the study will be acquired on request from the corresponding author.
Acknowledgments
We owe our thanks to the patients enrolled in our study.
Funding
The authors declared that no source of funding was used for the manuscript.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi:10.1038/s41575-020-0310-z
2. Doherty B, Nambudiri VE, Palmer WC. Update on the Diagnosis and Treatment of Cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19(1):2. doi:10.1007/s11894-017-0542-4
3. Cai Y, Cheng N, Ye H, Li F, Song P, Tang W. The current management of cholangiocarcinoma: a comparison of current guidelines. Biosci Trends. 2016;10(2):92–102. doi:10.5582/bst.2016.01048
4. Lewis HL, Rahnemai-Azar AA, Dillhoff M, Schmidt CR, Pawlik TM. Current Management of Perihilar Cholangiocarcinoma and Future Perspectives. Chirurgia. 2017;112(3):193–207. doi:10.21614/chirurgia.112.3.193
5. Ricci AD, Rizzo A, Brandi G. Immunotherapy in Biliary Tract Cancer: worthy of a Second Look. Cancer Control. 2020;27(3):1073274820948047. doi:10.1177/1073274820948047
6. Viscardi G, Tralongo AC, Massari F, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi:10.1016/j.ejca.2022.09.031
7. Rizzo A, Ricci AD, Brandi G. Durvalumab: an investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expert Opin Investig Drugs. 2021;30(4):343–350. doi:10.1080/13543784.2021.1897102
8. Squadroni M, Tondulli L, Gatta G, Mosconi S, Beretta G, Labianca R. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2017;116:11–31. doi:10.1016/j.critrevonc.2016.11.012
9. Indar AA, Lobo DN, Gilliam AD, et al. Percutaneous biliary metal wall stenting in malignant obstructive jaundice. Eur J Gastroenterol Hepatol. 2003;15(8):915–919. doi:10.1097/00042737-200308000-00013
10. Li ZM, Jiao DC, Han XW, Lei QY, Zhou XL, Xu M. Preliminary application of brachytherapy with double-strand 125I seed and biliary drainage for malignant obstructive jaundice. Surg Endosc. 2022;36(7):4932–4938. doi:10.1007/s00464-021-08848-6
11. Xiang Y, Lu S, Li Y, Liu Z, Wang W. Iodine-125 seed Combined With Biliary Stent Placement Versus Stent Placement Alone For Unresectable Malignant Biliary Obstruction: a Meta-Analysis Of Randomized Controlled Trials. J Cancer. 2021;12(5):1334–1342. doi:10.7150/jca.49663
12. Zhou W, Kuang T, Han X, et al. Prognostic role of lymphocyte-to-monocyte ratio in pancreatic neuroendocrine neoplasms. Endocr Connect. 2020;9(4):289–298. doi:10.1530/EC-19-0541
13. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-Lymphocyte Ratio Is a Prognostic Marker in Patients with Locally Advanced (Stage IIIA and IIIB) Non-Small Cell Lung Cancer Treated with Combined Modality Therapy. Oncologist. 2017;22(6):737–742. doi:10.1634/theoncologist.2016-0443
14. Toyoda J, Sahara K, Maithel SK, et al. ASO Visual Abstract: prognostic Utility of Systemic Immune-Inflammation Index After Resection of Extrahepatic Cholangiocarcinoma-Results from the US Extrahepatic Biliary Malignancy Consortium. Ann Surg Oncol. 2022;29(12):7617–7618. doi:10.1245/s10434-022-12269-7
15. Olmez OF, Bilici A, Gursoy P, et al. Impact of systemic inflammatory markers in patients with ALK-positive non-small cell lung cancer treated with crizotinib. Pulmonology. 2022:21. doi: 10.1016/j.pulmoe
16. Yang C, Hu BW, Tang F, et al. Prognostic Value of Systemic Immune-Inflammation Index (SII) in Patients with Glioblastoma: a Comprehensive Study Based on Meta-Analysis and Retrospective Single-Center Analysis. J Clin Med. 2022;11:24. doi:10.3390/jcm11247514
17. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
18. Chen Y, Wang XL, Yan ZP, et al. The use of ¹²5I seed strands for intraluminal brachytherapy of malignant obstructive jaundice[J]. Cancer Biother Radiopharm. 2012;27(5):317–323. doi:10.1089/cbr.2011.0999
19. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
20. Hu D, Lin Y, Liu F, et al. Elevated Preoperative Platelet to Lymphocyte Ratio Indicates Poor Survival in Patients with Resected High-grade Serous Ovarian Carcinoma. Clin Lab. 2016;62(8):1443–1449. doi:10.7754/Clin.Lab.2016.151137
21. YaKitano Y, Yamashita YI, Yamamura K, et al. Effects of Preoperative Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios on Survival in Patients with Extrahepatic Cholangiocarcinoma. Anticancer Res. 2017;37(6):3229–3237. doi:10.21873/anticanres.11685
22. Terasaki F, Sugiura T, Okamura Y, et al. Systemic immune-inflammation index as a prognostic marker for distal cholangiocarcinoma. Surg Today. 2021;51(10):1602–1609. doi:10.1007/s00595-021-02312-7
23. Kumamoto Y, Kaizu T, Tajima H, et al. Neutrophil-to-lymphocyte ratio as a predictor of postoperative morbidity in patients with distal cholangiocarcinoma. Mol Clin Oncol. 2018;9(4):362–368. doi:10.3892/mco.2018.1698
24. Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28(2):187–196. doi:10.1016/j.smim.2016.03.018
25. Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. [Corrigendum] Neutrophils in cancer development and progression: roles, mechanisms, and implications (Review). Int J Oncol. 2017;50(2):745. doi:10.3892/ijo.2016.3764
26. Schumacher D, Strilic B, Sivaraj KK, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24(1):130–137. doi:10.1016/j.ccr.2013.05.008
27. Stanger BZ, Kahn ML. Platelets and tumor cells: a new form of border control. Cancer Cell. 2013;24(1):9–11. doi:10.1016/j.ccr.2013.06.009
28. Chumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1016/j.ccr.2013.05.008
29. Tan DW, Fu Y, Su Q, et al. Prognostic Significance of Neutrophil to Lymphocyte Ratio in Oncologic Outcomes of Cholangiocarcinoma: a Meta-analysis. Sci Rep. 2016;6:33789. doi:10.1038/srep33789
30. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi:10.1016/j.jhep.2005.10.013
31. Coelen RJ, Wiggers JK, Nio CY, et al. Preoperative computed tomography assessment of skeletal muscle mass is valuable in predicting outcomes following hepatectomy for perihilar cholangiocarcinoma. HPB (Oxford). 2015;17(6):520–528. doi:10.1111/hpb.12394
32. Zhang D, Zeng H, Pan Y, et al. Liver Tumor Markers, HALP Score, and NLR: simple, Cost-Effective, Easily Accessible Indexes for Predicting Prognosis in ICC Patients after Surgery. J Pers Med. 2022;12:12. doi:10.3390/jpm12122041
33. Sinha SR, Prakash P, Singh RK, Sinha DK. Assessment of tumor markers CA 19-9, CEA, CA 125, and CA 242 for the early diagnosis and prognosis prediction of gallbladder cancer. World J Gastrointest Surg. 2022;14(11):1272–1284. doi:10.4240/wjgs.v14.i11.1272
34. Tella SH, Kommalapati A, Yadav S, et al. Novel staging system using carbohydrate antigen (CA) 19-9 in extra-hepatic cholangiocarcinoma and its implications on overall survival. Eur J Surg Oncol. 2020;46(5):789–795. doi:10.1016/j.ejso.2020.01.016
35. Yang H, Wang J, Li Z, et al. Risk Factors and Outcomes of Early Relapse After Curative Resection of Intrahepatic Cholangiocarcinoma. Front Oncol. 2019;9:854. doi:10.3389/fonc.2019.00854
36. Kato A, Shimizu H, Ohtsuka M, et al. Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer Patients Treated with Gemcitabine Plus Cisplatin Combination Therapy Followed by Radical Surgery. Ann Surg Oncol. 2015;22:S1093–1099. doi:10.1245/s10434-015-4768-9
37. Neuzillet C, Casadei-Gardini A, Brieau B, et al. Fluropyrimidine single agent or doublet chemotherapy as second line treatment in advanced biliary tract cancer. Int J Cancer. 2020;147(11):3177–3188. doi:10.1002/ijc.33146
38. Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328–2338. doi:10.1093/annonc/mdu162
39. Cron GO, Beghein N, Crokart N, et al. Changes in the tumor microenvironment during low-dose-rate permanent seed implantation iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2005;63(4):1245–1251. doi:10.1016/j.ijrobp.2005.07.971
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


