Back to Journals » Cancer Management and Research » Volume 11
Prognostic Value Of Preoperative Systemic Inflammatory Biomarkers In Patients With Gallbladder Cancer And The Establishment Of A Nomogram
Authors Deng Y, Zhang F , Yu X, Huo CL, Sun ZG, Wang S
Received 3 June 2019
Accepted for publication 16 September 2019
Published 21 October 2019 Volume 2019:11 Pages 9025—9035
DOI https://doi.org/10.2147/CMAR.S218119
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Beicheng Sun
Yan Deng,1 Feng Zhang,2 Xiao Yu,1 Cheng-Long Huo,1 Zhen-Gang Sun,1 Shuai Wang1
1Department of Hepatobiliary Surgery, Jing Zhou Central Hospital, The Second Clinical Medical College, Yangtze University, Jing Zhou, Hubei 434020, People’s Republic of China; 2Department of Ophthalmology, Jing Zhou Central Hospital, The Second Clinical Medical College, Yangtze University, Jing Zhou, Hubei 434020, People’s Republic of China
Correspondence: Shuai Wang; Zhen-Gang Sun
Department of Hepatobiliary Surgery, Jing Zhou Central Hospital, The Second Clinical Medical College, Yangtze University, Jing Zhou, Hubei 434020, People’s Republic of China
Tel +86-18107167230
Email [email protected]; [email protected]
Background and aim: Preoperative systemic inflammatory biomarkers, including neutrophil to lymphocyte ratio (NLR), derived neutrophil to lymphocyte ratio (dNLR), platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR) have been developed to predict patient outcome in several types of carcinomas. The aim of this study was to investigate the potential prognostic value of NLR, dNLR, PLR, and LMR, and establish a prognostic nomogram in postoperative GBC patients who underwent radical cholecystectomy.
Methods: 169 GBC patients were retrospectively enrolled in the present study. ROC curve analysis was used to determine the optimal cut-off values of systemic inflammatory biomarkers. The prognostic value of those biomarkers was investigated according to the Kaplan-Meier method and Cox regression model. A relevant prognostic nomogram was established.
Results: Results showed that NLR, dNLR, PLR, and LMR were significantly associated with overall survival (OS); whereas, NLR and LMR were retained as independent indicators. Based on these independent predictors including tumor differentiation, T stage, N stage, CEA, NLR, and LMR, a nomogram was generated with an accuracy of 0.801.
Conclusion: Based on our findings, the predictive nomogram could accurately predict individualized survival probability of postoperative GBC patients, and might support clinicians in treatment optimization and clinical decision-making.
Keywords: gallbladder carcinoma, systemic inflammatory biomarker, prognosis, overall survival, nomogram
Introduction
Gallbladder cancer (GBC) is one of the most common primary biliary tract malignancies.1–4 Based on the data obtained from the Surveillance, Epidemiology and End Results (SEER) program, the incidence of GBC is estimated at 2.5 per 100,000 persons.5,6 Symptoms related to GBC can be vague and nonspecific, further complicating early detection.7,8 Besides, due to aggressive biological behavior of the cancer and the aberrant anatomical characteristics of the gallbladder, prognosis of GBC patients is poor.9,10 Undoubtedly, if the condition of patients allowed, surgical intervention might render probability of long-term overall survival (OS).11 However, despite recent continuous development of diagnosis and treatment of the disease, it is still a highly lethal cancer.12
Currently, histopathologic classifications and clinicopathological parameters such as tumor grade, stage, histologic type, lymph node status, and CEA are primarily used to draw correlations with survival. The prognosis of certain GBC patients who have undergone radical surgical resection remains appalling.13 Recently, those clinicopathological parameters and some prognostic models are difficult to exactly evaluate the prognosis.14,15 Hence, it is expected that a combination of some specific GBC indicators into conventional clinicopathological characters will exactly predict prognosis.
Increasing research has indicated that systemic inflammatory response plays an important role in the initiation, progression, metastasis, and treatment resistance in a variety of malignancies including GBC.16–18 Some cancer patients usually present with systemic inflammatory response, which could be detected by change of peripheral blood cell amounts.17 Based on the count of circulating inflammatory cells, several prognostic biomarkers such as neutrophil to lymphocyte ratio (NLR),19,20 derived neutrophil to lymphocyte ratio (dNLR),21 platelet to lymphocyte ratio (PLR),22 and lymphocyte to monocyte ratio (LMR)23 have been developed to predict patient outcome in various carcinomas. According to previous research, the roles of those prognostic biomarkers in GBC were less well known and no more responding prognostic nomogram was established.
Therefore, we aimed to verify the relationships of pretreatment inflammatory biomarkers including NLR, dNLR, PLR, and LMR with prognosis of GBC patients who have undergone radical surgery and attempted to establish a prognostic nomogram with improved predictive capacity.
Materials And Methods
Patients
The study enrolled all GBC patients who underwent radical cholecystectomy at Jing Zhou Central Hospital, the second clinical medical college, Yangtze University between January 2010 and May 2017. Eligible patients were included according to the following criteria: 1) patient underwent radical cholecystectomy; 2) patient with histological diagnosis of GBC; 3) patient with complete clinical and histological information as well as follow-up data; 4) patient aged > 18 years old; 5) patient has complete peripheral blood cell count data. The exclusion criteria were : 1) patient had other malignancies; 2) patient had perioperative surgery-associated mortality; 3) patient had systemic infection, autoimmune disease or inflammation. At last, 169 GBC patients remained and were analyzed in our study. Written informed consent was obtained from every eligible patient or their family members. The study was approved by the Ethical Committee of Jing Zhou Central Hospital, the second clinical medical college, Yangtze University. The methods were carried out in accordance with the relevant guidelines and regulations. Patients' records were anonymized and de-identified prior to analysis. This study was conducted in accordance with the Declaration of Helsinki.
Follow-Up
Patients were routinely followed up every 3 months for the first year, every 4 months for the second year, and every 6 months thereafter in our institute. The post-treatment surveillance program consisted of physical examination, cytological assessment, and abdominal computed tomography or ultrasound. Follow-up was terminated in February 2019. The endpoint was OS during the interval surgery and death or the last follow-up. To maximally reduce the extent of bias, two clinicians performed follow-up and review, respectively.
Data Collection
Baseline clinicopathological parameters were obtained from medical records: age at surgery, gender, presence of a concomitant disease (hypertension, diabetes mellitus or cystic liver), tumor-node-metastasis (TNM) stage, and pathological report. The hematological parameters including CEA, hemoglobin, monocyte, albumin, platelet, leukocyte and neutrophil were extracted from blood tests within 2 weeks prior to surgery. Preoperative anemia was defined as a baseline hemoglobin level of < 120 g/L for male or < 110 g/L for female. The eighth edition of the American Joint Committee on Cancer (AJCC-8th) TNM classification was used to define TNM stage. The definitions of inflammatory response biomarkers are described as follows: NLR = neutrophil to lymphocyte ratio; dNLR = neutrophil to (white cell count – neutrophil count) ratio; PLR = platelet count to lymphocyte ratio; and LMR = lymphocyte to monocyte ratio.
Surgical Management
The detailed surgical approach was determined by primary tumor invasion based on the AJCC staging criteria. For patients with incidental GBC detected by histopathological examination, a second radical resection was performed except for Tis and T1a. In Tis and T1a patients, simple cholecystectomy was the radical treatment. Cholecystectomy with radical resection which encompasses 3 cm of liver parenchyma segment IVb and V plus adequate lymphadenectomy is the main procedure for T1b gallbladder cancer. Cholecystectomy with a more formal resection of segments IVb and V plus adequate lymphadenectomy is the method of choice for T2 lesions of GBC. In T3 cancer, radical surgery includes an extended right hepatectomy with possible caudate lobectomy, regional lymphadenectomy, extirpation of other affected structures and even pancreaticoduodenectomy or adjacent organs' resection. In T4 disease, tumor has vascular invasion such as portal vein or hepatic artery. If the condition of patients allowed, radical surgery for T3 cancer plus vascular reconstruction is the radical treatment for T4 cancer.
Statistical Analysis
The optimal cut-off level of biomarker was determined by receiver operating curve (ROC) analysis. Kolmogorov–Smirnov test was used to reveal a non-normal distribution (each P < 0.050). Thus, they were shown as median and range. Nonparametric Wilcoxon rank-sum test or Kruskal-Wallis test was used to determine associations of NLR, dNLR, PLR, and LMR with other categorized clinicopathological factors. Survival analysis was calculated using Kaplan-Meier method and significant difference was identified by log rank test. Variables shown to have significant prognostic value by univariate analysis were further analyzed based on multivariate Cox proportional hazards model. A nomogram for possible prognostic variables associated with OS was established by R software version 3.3.1 using the package of rms. Calibration plots were performed to examine the performance characteristics of predictive nomogram. The predictive accuracy of nomogram was evaluated by Harrell’s concordance index (C-index), which is a measure of discrimination. C-index ranges from 0.5 (no predictive power) to 1 (perfect prediction). All the statistical analyses were performed using PASW Statistics 23.0 software (SPSS Inc., Chicago, IL, US) or R version 3.3.1 software (Vienna, Austria). Two-sided P value of less than 0.05 was considered to indicate a significant difference.
Results
Patient Characteristics
Baseline characteristics of 169 GBC patients were summarized in Table 1. Among these patients, the median age at diagnosis was 64 years old (range 30–87 years old). 55 (32.544%) patients were male. Based on the TNM staging system, 16 patients presented with stage I, followed by 37 with II, 76 with stage III, and 40 with stage IV. Regarding the pathological differentiation level of tumor, 17.160%, 40.237%, and 42.603% of patients were histologically diagnosed with well, moderately, and poor differentiated disease, respectively. A minority of the patients had concomitant diabetes mellitus, hypertension or hepatic cysts. About 48.521% had a previous history of gallstones. The mean of monocyte, lymphocyte, platelet, leukocyte, and neutrophil count was 0.45 k/mm2 (range 0.01–2.17k/mm2), 1.28 k/mm2 (range 0.19–3.51 k/mm2), 174 k/mm2 (range 37–525 k/mm2), 5.80 k/mm2 (range 1.60–16.91 k/mm2), and 3.90 k/mm2 (range 1.00–15.20 k/mm2), respectively. After follow-up, 120 (71.006%) patients died of GBC, with an estimated median OS of 14 months (range 1–97 months).
 |
Table 1 Baseline Characteristics Of GBC Patients |
The Optimal Cut-Off Values For NLR, dNLR, PLR, And LMR
The ROC curves of these inflammatory biomarkers were depicted in Figure 1. The areas under curve (AUC) for PLR, NLR, dNLR, and LMR were 0.600, 0.643, 0.648, and 0.645, respectively. The optimal cut-off value was 145.33 for PLR; 2.61 for NLR 1.78 for dNLR, and 2.66 for LMR by ROC curve analysis. Based on the optimal cut-off values, patients were subsequently divided into two groups.
 |
Figure 1 Optimal cut-off values for NLR (A), dNLR (B), PLR (C) and LMR (D) were applied with ROC curves for OS. |
Correlations Of NLR, dNLR, PLR, And LMR With Other Clinicopathological Features
Potential associations of NLR, dNLR, PLR, and LMR with other clinicopathological variables were explored (Table 2). Results revealed that poor tumor differentiation was correlated with elevated NLR, dNLR, and PLR, as well as descended LMR. Besides, advanced TNM stage and N stage were significantly correlated with elevated PLR and decreased LMR. In addition, LMR was significantly lower in patients with advanced T stage or anemia. No significant difference of the other clinicopathological variables with these inflammatory biomarkers was found.
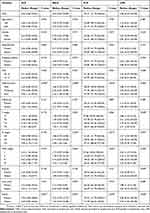 |
Table 2 Associations Of Inflammation-Based Markers With Clinicopathologic Characteristics Of GBC Patients |
Associations Of Inflammatory Biomarkers With OS
The 1-, 3-, and 5-year survival rates were 55.621%, 31.361%, and 19.523%, respectively (Figure 2A). Kaplan–Meier curve revealed that conventional tumor differentiation and TNM stage were significantly associated with OS (Figure 2B and C). In addition, poor postoperative outcome was correlated to high NLR, dNLR, and PLR as well as low LMR (Figure 3A–D).
 |
Figure 2 Kaplan-Meier curves for cumulative OS of the study population (A) and OS of patients stratified according to tumor differentiation (B) and TNM stage (C). |
 |
Figure 3 Kaplan-Meier curves for overall survival probability according to the preoperative NLR (A), dNLR (B), PLR (C), and LMR (D), respectively. |
As shown in Table 3, results from univariate analysis indicated that CEA [HR: 1.463; 95% CI: 1.011–2.118; P = 0.044], tumor differentiation [HR: 2.029; 95% CI: 1.560–2.639; P < 0.001], T stage [HR: 2.278; 95% CI: 1.773–2.928; P < 0.001], N stage [HR: 2.166; 95% CI: 1.680–2.792; P < 0.001], TNM stage [HR: 2.190; 95% CI: 1.734–2.765; P < 0.001], NLR [HR: 2.268; 95% CI: 1.525–3.373; P < 0.001], dNLR [HR: 1.906; 95% CI: 1.294–2.808; P = 0.001], PLR [HR: 2.152; 95% CI: 1.485–3.118; P < 0.001], and LMR [HR: 2.048; 95% CI: 1.421–2.951; P < 0.001] were significant prognostic factors for OS. To avoid the occurrence of collinearity of TNM stage with T stage and N stage, TNM stage was not enrolled into multivariate Cox regression modeling because it was calculated based on T stage, N stage, and M stage. Multivariate analysis indicated that tumor differentiation [HR: 1.459; 95% CI: 1.064–2.002; P = 0.019], T stage [HR: 1.850; 95% CI: 1.389–2.463; P < 0.001], N stage [HR: 1.516; 95% CI: 1.116–2.059; P = 0.008], CEA [HR: 1.704; 95% CI: 1.156–2.513; P = 0.007], NLR [HR: 3.298; 95% CI: 1.359–8.000; P = 0.008], and LMR [HR: 1.549; 95% CI: 1.051–2.283; P = 0.027] were independent prognostic parameters for OS.
 |
Table 3 Univariate Cox Proportional Hazards Regression Analysis For Overall Survival (OS) In Patients With Gallbladder Carcinoma (GBC) |
Nomogram For The Predication Of OS
A responding prognostic nomogram was established using all the significant independent parameters for OS consisting of tumor differentiation, T stage, N stage, CEA, NLR, and LMR. Each variable was assigned a weighted number of points in the nomogram, then the sum of points for each GBC patient was in accordance with a specific 1-, 3-, and 5-year OS (Figure 4A). In the nomogram, a lower point indicated a better OS. For internal validation, the bootstrapped calibration plot of the nomogram predicting 1-, 3-, and 5-year survival performed well with the ideal model (Figure 4B–D). C-index of tumor TNM stage and differentiation was 0.650, and 0.747, respectively. Whereas, the value of the nomogram improved to 0.801.
Discussion
Like the results of prior studies,24,25 several GBC prognostic models such as tumor differentiation and TNM stage were confirmed as independent predictors. However, those tumor-related factors only reflect the degree of cancer progression and partially explain the prognostic heterogeneity.26 Hence, it is expected that a combination of some specific GBC indicators into conventional tumor-related factors such as TNM stage will exactly predict prognosis. With that objective in mind, we aimed to probe into the prognosis of pretreatment inflammatory biomarkers and attempted to establish a prognostic nomogram with improved predictive capacity in GBC patients. To the best of our knowledge, this study is the first to screen more powerful predictive biomarkers in those systemic inflammatory indexes and establish a prognostic nomogram based on those powerful biomarkers and conventional prognostic markers in GBC patients who have undergone radical cholecystectomy.
Recently, emerging evidence has shown that an inflammatory microenvironment plays an important role in carcinogenesis, tumor growth, invasion, and metastasis.27–29 Contemporary studies have led to a general acceptance that prognosis of a cancer patient is determined not solely by tumor-related factors, but also by host response to systemic inflammation.30 A better understanding of the prognostic relationship of systemic inflammation and cancer patients might contribute to prevention and treatment of cancer. Host systemic inflammation could be reflected in routine hematological tests in clinical practice.17 Blood cell count alone might not be significantly associated with overall survival, but systemic inflammatory biomarkers, which are produced by combination of those cell counts, have been considered as independent prognostic factors for various carcinomas.
In the present study, we showed that preoperative systemic inflammatory biomarkers including NLR, dNLR, PLR, and LMR could be used as prognostic factors for GBC patients after radical cholecystectomy. Those markers were significantly associated with OS of GBC patients in univariate analysis. According to multivariate analysis, NLR and LMR were retained as independent indicators.
Although systemic inflammatory biomarkers are useful parameters for predicting outcome of cancer patients, the underlying mechanism largely remains to be undefined. Following, several mechanisms of inflammatory reaction to tumor might explain the results. Firstly, lymphocyte is a basic component of the cellular basis of immunosurveillance and immunoediting as well as the innate immune system, which could destroy residual cancer cells and micro metastases and then inhibit tumor cells' proliferation and migration.31,32 It is known that host anti-tumor activity is mediated by cellular immune reaction related to lymphocytes.33–35 Gooden et al36 indicated that increasing infiltration of lymphocytes has been significantly associated with good prognosis and better response to treatment in patients with cancer. Lymphocytopenia is also related to tumor burden, metastatic sites, inflammatory syndrome, and host characteristics.34,35 Secondly, inflammation can active monocyte to peripheral blood and then promote their differentiation into tumor-related macrophages after they have been recruited to tumor tissues.37,38 Tumor-related macrophages interact with tumor cells, and then produce various types of cytokines and chemokines including IL-6 and tumor necrosis factor-α (TNF-α) leading to tumor progression.39,40 What is more, monocytes and their progeny could function as immunosuppressive, which also contributes to carcinogenesis, tumor growth, invasion, and metastasis.41 Thirdly, cancer-associated inflammatory factors such as TNF-α, IF-6, and myeloid growth factors could trigger neutrophils, whereas elevated neutrophils have been reported to contain and prompt secretion of the potent angiogenesis cytokine including vascular endothelial growth factor (VEGF) and reactive oxygen species (ROS).42–44 ROS might cause host normal cell DNA damage and genetic instability; thus, it plays an important role in tumor microenvironment.45 Besides, neutrophilia could suppress activity of immune cells including lymphocyte, activated T cell, and natural killer cell by inhibiting the immune system.46 Like prior studies, elevated NLR and decreased LMR were associated with poor survival time in GBC patients who have undergone radical surgery in this study.
The correlations between inflammatory biomarkers and clinicopathological characteristics were analyzed and results showed that adverse systemic inflammatory biomarkers were significantly related to poor differentiation and advanced stage including T stage, N stage, and TNM stage. These correlations were supported by the explanation that terminal cancer patients are prone to have immunosuppressive status and to suffer from inflammatory response.47,48
The present study attempted to establish a predictive nomogram using all the significant independent predicators including NLR, LMR, CEA, tumor differentiation, T stage, and N stage to predict the probability of postoperative patients. Calibration plots of the nomogram performed well in predicted 1-, 3-, and 5-year OS with the ideal model, which indicated that our nomogram was well calibrated to predict OS. Besides, internal validation indicated that the nomogram had better discrimination power (C-index, 0.801) compared to conventional tumor TNM stage (C-index, 0.650) and differentiation (C-index, 0.747).
The findings of the present study should be interpreted in consideration of its possible limitations. To begin with, the study had a retrospective design with relatively small-sized sample, which might have had a negative impact on the findings. The prognostic significance remains to be confirmed by multicenter clinical studies. Besides, the nomogram lacks external validation cohort, which requires further investigation to confirm its robustness. Thirdly, although the cut-off values were calculated by ROC curves based on OS, the optimal values were inconsistent with results of previous research, which might be a result of different population and survival end-point. Finally, the relevant mechanism of inflammatory reaction to tumor was not investigated in the present study, thus, further basic research needs to be performed to identify the detailed mechanism of how inflammatory cells and their progeny are involved in the pathogenesis and progression of GBC.
Conclusion
In conclusion, preoperative NLR, dNLR, PLR, and LMR were significant and powerful prognostic indicators for OS in GBC patients who have undergone radical operation. Furthermore, NLR and LMR were independent prognostic markers for OS. The nomogram based on NLR, LMR, and conventional clinicopathological indicators could accurately predict individualized survival probability in GBC and might support clinicians in treatment optimization and clinical decision-making.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Written informed consent was obtained from every eligible patient or their family members. This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Shuai Wang and Zhen-Gang Sun are co-corresponding authors.
Disclosure
The authors declare that they have no conflicts of interest regarding this study.
References
1. Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15(2):168–181. doi:10.1634/theoncologist.2009-0302
2. Baiu I, Visser B. Gallbladder cancer. JAMA. 2018;320(12):1294. doi:10.1001/jama.2018.11815
3. Hemminki K, Hemminki A, Forsti A, Sundquist K, Li X. Genetics of gallbladder cancer. Lancet Oncol. 2017;18(6):e296. doi:10.1016/S1470-2045(17)30072-4
4. Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol. 2015;33(16):1845–1848. doi:10.1200/JCO.2014.59.7591
5. Boutros C, Gary M, Baldwin K, Somasundar P. Gallbladder cancer: past, present and an uncertain future. Surg Oncol. 2012;21(4):e183–e191. doi:10.1016/j.suronc.2012.08.002
6. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi:10.2147/CLEP.S37357
7. Barreto SG, Dutt A, Sirohi B, Shrikhande SV. Gallbladder cancer: a journey of a thousand steps. Future Oncol. 2018;14(13):1299–1306. doi:10.2217/fon-2017-0576
8. Cronin KA, Ries LA, Edwards BK, Surveillance T. Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer. 2014;120 Suppl 23:3755–3757. doi:10.1002/cncr.29049
9. SJ H, HK W, MA J, Watson M, Richardson LC. Gallbladder Cancer Incidence and Mortality, United States 1999–2011. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1319–1326. doi:10.1158/1055-9965.EPI-15-0199
10. Sun Y, Song W, Hou Q, Li J, Guo H. Gallbladder perforation: a rare complication of postoperative chemotherapy of gastric cancer. World J Surg Oncol. 2015;13:245. doi:10.1186/s12957-015-0659-6
11. Kakaei F, Beheshtirouy S, Nejatollahi SM, Zarrintan S, Mafi MR. Surgical treatment of gallbladder carcinoma: a critical review. Updates Surg. 2015;67(4):339–351. doi:10.1007/s13304-015-0328-x
12. Bal MM, Ramadwar M, Deodhar K, Shrikhande S. Pathology of gallbladder carcinoma: current understanding and new perspectives. Pathol Oncol Res. 2015;21(3):509–525. doi:10.1007/s12253-014-9886-3
13. Rakic M, Patrlj L, Kopljar M, et al. Gallbladder cancer. Hepatobiliary Surg Nutr. 2014;3(5):221–226. doi:10.3978/j.issn.2304-3881.2014.09.03
14. Bai Y, Liu ZS, Xiong JP, et al. Nomogram to predict overall survival after gallbladder cancer resection in China. World J Gastroenterol. 2018;24(45):5167–5178. doi:10.3748/wjg.v24.i45.5167
15. Yifan T, Zheyong L, Miaoqin C, Liang S, Xiujun C. A predictive model for survival of gallbladder adenocarcinoma. Surg Oncol. 2018;27(3):365–372. doi:10.1016/j.suronc.2018.05.007
16. Abe T, Amano H, Hanada K, et al. Preoperative systemic inflammation and complications affect long-term gallbladder carcinoma outcomes following surgery with curative intent. Anticancer Res. 2016;36(9):4887–4894. doi:10.21873/anticanres.11053
17. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi:10.1016/S1470-2045(14)70263-3
18. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
19. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi:10.1016/j.critrevonc.2013.03.010
20. Zhang L, Wang R, Chen W, et al. Prognostic significance of neutrophil to lymphocyte ratio in patients with gallbladder carcinoma. HPB (Oxford). 2016;18(7):600–607. doi:10.1016/j.hpb.2016.03.608
21. Duan J, Pan L, Yang M. Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: a meta-analysis. Medicine (Baltimore). 2018;97(49):e13340. doi:10.1097/MD.0000000000013340
22. Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204–1212. doi:10.1158/1055-9965.EPI-14-0146
23. Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971–978. doi:10.1016/j.ctrv.2015.10.003
24. Chen C, Geng Z, Shen H, et al. Long-term outcomes and prognostic factors in advanced gallbladder cancer: focus on the advanced t stage. PLoS One. 2016;11(11):e0166361. doi:10.1371/journal.pone.0166361
25. Nigam J, Chandra A, Kazmi HR, Parmar D, Singh D, Gupta V. Prognostic significance of survivin in resected gallbladder cancer. The Journal of Surgical Research. 2015;194(1):57–62. doi:10.1016/j.jss.2014.07.054
26. Sherman M. Hepatocellular carcinoma: screening and staging. Clin Liver Dis. 2011;15(2):
27. Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42(3):161–170. doi:10.3109/07853890903405753
28. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi:10.1093/carcin/bgp127
29. Locatelli M, Ferrero S, Martinelli Boneschi F, et al. The long pentraxin PTX3 as a correlate of cancer-related inflammation and prognosis of malignancy in gliomas. J Neuroimmunol. 2013;260(1–2):99–106. doi:10.1016/j.jneuroim.2013.04.009
30. Pinato DJ. Cancer-related inflammation: an emerging prognostic domain in metastatic castration-resistant prostate carcinoma. Cancer. 2014;120(21):3272–3274. doi:10.1002/cncr.28889
31. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi:10.1016/j.immuni.2004.07.017
32. Adams S, Goldstein LJ, Sparano JA, Demaria S, Badve SS. Tumor infiltrating lymphocytes (TILs) improve prognosis in patients with triple negative breast cancer (TNBC). Oncoimmunology. 2015;4(9):e985930. doi:10.1080/2162402X.2015.1008371
33. Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32(1):22–28. doi:10.1097/01.mpa.0000188305.90290.50
34. De Giorgi U, Mego M, Scarpi E, et al. Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer. Clin Breast Cancer. 2012;12(4):264–269. doi:10.1016/j.clbc.2012.04.004
35. Pattison CW, Woods KL, Morrison JM. Lymphocytopenia as an independent predictor of early recurrence in breast cancer. Br J Cancer. 1987;55(1):75–76. doi:10.1038/bjc.1987.15
36. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi:10.1038/bjc.2011.189
37. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi:10.1038/nrc1256
38. Hagemann T, Lawrence T. Investigating macrophage and malignant cell interactions in vitro. Methods Mol Biol. 2009;512:325–332. doi:10.1007/978-1-60327-530-9_18
39. Mano Y, Aishima S, Fujita N, et al. Tumor-associated macrophage promotes tumor progression via STAT3 signaling in hepatocellular carcinoma. Pathobiology. 2013;80(3):146–154. doi:10.1159/000346196
40. Evani SJ, Prabhu RG, Gnanaruban V, Finol EA, Ramasubramanian AK. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. Faseb J. 2013;27(8):3017–3029. doi:10.1096/fj.12-224824
41. Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70(5):325–330. doi:10.1016/j.humimm.2009.02.008
42. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–446. doi:10.1038/nrc.2016.52
43. Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120(4):1151–1164. doi:10.1172/JCI37223
44. Schiffmann LM, Fritsch M, Gebauer F, et al. Tumour-infiltrating neutrophils counteract anti-VEGF therapy in metastatic colorectal cancer. Br J Cancer. 2019;120(1):69–78. doi:10.1038/s41416-018-0198-3
45. Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71(7):2411–2416. doi:10.1158/0008-5472.CAN-10-2583
46. Parikh K, Kumar A, Ahmed J, et al. Peripheral monocytes and neutrophils predict response to immune checkpoint inhibitors in patients with metastatic non-small cell lung cancer. Cancer Immunol Immunother. 2018;67(9):1365–1370. doi:10.1007/s00262-018-2192-2
47. Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32(3):201–215.
48. Suzana S, Boon PC, Chan PP, Normah CD. Malnutrition risk and its association with appetite, functional and psychosocial status among elderly Malays in an agricultural settlement. Malays J Nutr. 2013;19(1):65–75.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

