Back to Journals » Clinical Interventions in Aging » Volume 18
Prognostic Value of Myocardial Function Imaging Markers in Elderly Patients Undergoing Transcatheter Aortic Valve Replacement
Authors Dadarlat-Pop A , Molnar A, Serban A , Tomoaia R, Hagiu C, Manole S , Oprea A , Mocanu L, Picos A , Mot S
Received 4 April 2023
Accepted for publication 12 September 2023
Published 25 September 2023 Volume 2023:18 Pages 1597—1606
DOI https://doi.org/10.2147/CIA.S413426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Alexandra Dadarlat-Pop,1,2 Adrian Molnar,3,4,* Adela Serban,1,2,* Raluca Tomoaia,2,5 Claudia Hagiu,6,7,* Simona Manole,8,9,* Alexandru Oprea,3,4,* Lorena Mocanu,1 Andrei Picos,10,* Stefan Mot1,2
1Cardiology Department, Heart Institute Niculae Stăncioiu, Cluj-Napoca, Romania; 2Cardiology Department, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania; 3Cardiovascular Surgery Department, Heart Institute Niculae Stăncioiu, Cluj-Napoca, Romania; 4Cardiovascular Surgery Department, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania; 5Cardiology Department, Clinical Rehabilitation Hospital, Cluj-Napoca, Romania; 6Gastroenterology Department, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania; 7“Prof. Dr. Octavian Fodor” Regional Gastroenterology-Hepatology Institute, Cluj-Napoca, Romania; 8Radiology and Medical Imaging Department, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania; 9Department of Radiology and Medical Imaging, Heart Institute Niculae Stăncioiu, Cluj-Napoca, Romania; 10Department of Prevention in Dental Medicine, ‘Iuliu Hatieganu’ University of Medicine and Pharmacy, Cluj-Napoca, Romania
*These authors contributed equally to this work
Correspondence: Raluca Tomoaia, Email [email protected]
Background: Transcatheter aortic valve replacement (TAVR) became the leading therapeutic strategy for aortic valve replacement in older patients with severe symptomatic aortic stenosis. Echocardiographic parameters that mark the left ventricle and right ventricle reverse remodeling after the TAVR are not well established. The aim of the current study is to describe the dynamics of both left ventricle (LV) and right ventricle (RV) strain derived from speckle tracking echocardiography in elderly patients at 3-months after the TAVR procedure.
Methods: We enrolled 52 consecutive patients (77 ± 4.9 years old, median STS score of 3.1) who underwent transfemoral TAVR at our tertiary care center. All patients were evaluated at baseline and 3 months following TAVR.
Results: The LV global longitudinal strain (GLS) 3-month following TAVR was significantly improved compared with baseline values (− 16 ± 4.2% vs − 16 ± 4.2%; p < 0.001) but no significant changes in the RV GLS 3 and 6 segments model following TAVR were registered. The LV ejection fraction was significantly improved 3-months after the TAVR procedure. LV-GLS at baseline demonstrated a strong positive correlation with LV-GLS at 3 months (r = 0.69) and a moderate correlation with RV strain parameters (r = 0.38 and r = 0.56), but also a negative correlation with LVEF at follow-up (r=− 0.61). Interestingly, in contrast to LVEF, none of the strain parameters correlated with age. NT-proBNP values were correlated with both LV-GLS (r = 0.37) and LVEF (r=− 0.5) at baseline. However, at follow-up, baseline NT-proBNP values remained correlated only to LV-GLS at 3-months (r = 0.24), but the correlation was weak.
Keywords: elderly population, transcatheter aortic valve replacement, aortic stenosis, global longitudinal strain, outcome
Introduction
Aortic stenosis (AS) is the most frequent valvulopathy, found especially among the elderly population.1 Aortic valve replacement is recommended in patients with symptomatic severe AS patients or in asymptomatic AS patients with reduction of the left ventricular ejection fraction.1 Transcatheter aortic valve replacement (TAVR) is preferred over surgical aortic valve replacement (SAVR) in older patients (≥75 years), patients with high risk or unsuitable for surgery.1 Also, there are other individual clinical, anatomical, and procedural characteristics which may favor TAVR over SAVR.1 Studies showed that TAVR significantly improves prognosis in high-risk patients.2 Moreover, recent trials showed TAVR benefits in low-risk patients, too.3,4 Frequently, patients undergoing TAVR present right ventricular (RV) systolic dysfunction, a condition which is often underappreciated. However, less is known about TAVR effect on global or regional myocardial mechanics, especially in the elderly population. Newer echocardiographic methods such as speckle tracking are able to detect left and right ventricle longitudinal shortening and also subtle myocardial dysfunction. The objective of this study was therefore to investigate the functional response of the left and right ventricle mechanics assessed by two-dimensional Speckle Tracking echocardiography (GLS) before and 3 months after TAVR.
Materials and Methods
Patient Population
We prospectively enrolled 54 patients with symptomatic severe AS undergoing transfemoral TAVR in our hospital between January 2021 and October 2021. Of these, 1 patient died within 3 months of follow-up and 1 patient was excluded because he refused the follow-up visit. We accordingly included 52 patients with follow-up echocardiography in our study. All patients underwent preprocedural assessment with ECG-gated computed tomographic angiography (CTA) as per standard practice. TAVR was indicated in moderate and high-risk patients according to current guideline.1
Written informed consent was obtained from each patient. The Ethics Committee of the Heart Institute Niculae Stancioiu approved the study. The study was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice. Unfortunately, the logistics of TAVR program in our tertiary center during COVID-19 pandemic were changed in order to accomplish technical and administrative restrictions imposed by national laws, and also a non-negligible number of TAVR candidates postponed the presentation to the hospital.
Echocardiography
All patients included in this study performed transthoracic echocardiograms before and at 3 months follow-up after TAVR. Patients with a poor echocardiographic imaging for endocardial tracking in at least 2 adjacent myocardial segments and any rhythm other than sinus rhythm including atrial fibrillation during the echocardiographic study were excluded. Transthoracic 2-D, Doppler, tissue Doppler imaging (TDI), strain and speckle tracking assessment were carried out using VIVID E95 GE machine with dedicated software for post-processing. Speckle-tracking echocardiography (STE) was used to assess left ventricular (LV) and right ventricular (RV) sub-endocardial mechanics, before and 3 months after TAVR. The peak of QRS complex was used by the software as a mark for end diastole. For the LV, the standard views for the longitudinal strain analysis were the four, two and three apical views by using a 16 segments model. For the RV 3 and 6 segments longitudinal strain analysis the apical four chamber view was used.
Statistical Analysis
Baseline characteristics of the patients were expressed as mean ± SD, median [IQR] or frequencies according to the type and distribution of the data. Normality was tested with the Kolmogorov–Smirnov test. All patients were evaluated at baseline and at three-months using echocardiography. Baseline and follow-up parameters were compared using t-tests for continuous variables and were represented as box-and-whisker plots. Correlations between baseline and follow-up variables were performed using the Pearson’s correlation coefficient if normal distribution and the Spearman's correlation coefficient otherwise. The magnitude of the correlations was represented with correlograms. Significant relations between echocardiographic variables were graphically expressed with scatter diagrams, using heat-maps to indicate clusters of observations. Statistical analysis was performed using MedCalc Statistical Software 19.6.1 (MedCalc Software Ltd., Ostend, Belgium; http://www.medcalc.org; 2020). A p value of <0.05 was considered significant.
Results
Baseline Characteristics and Clinical Characteristics
A total of 52 patients were included in the present analysis. The study population consisted of elderly patients (77 ± 4.9 years). The Euroscore II and the STS Mortality score were 4.3 (2.78–7.2) and 3.1 (2.1–4.7) %, respectively, and were in New York Heart Association (NYHA) class III before TAVR in most of cases (63.5%). Patients had several comorbidities not included in the risk score that substantially increased their surgical risk. Among the enrolled patients, 22 patients had coronary artery disease, 20 of them had previous or during the index hospitalization percutaneous coronary interventions and 2 of them had previous CABG. Baseline demographic data, comorbidities, functional status and laboratory findings are summarized in Table 1.
 |
Table 1 Baseline Demographic and Clinical Characteristics of TAVR-Treated Patients |
Data regarding the operative details and adverse events occurred during the index hospitalization are presented in Table 2. During the index hospitalization, patients had low rates of acute kidney injury (1.4%), 7 patients had minor vascular complications and no myocardial infarction, stroke or valve thrombosis were registered. Permanent pacemaker implantation was required in 13% of patients. The survival rate at 90 days was 98%. One patient died before the 3-month assessment.
 |
Table 2 Procedure-Related Characteristics |
Echocardiography Characteristics
2D and Doppler Echocardiography
Table 3 shows the 2 D and Doppler echocardiographic parameters before and at 3 months after TAVR. The median aortic valve area index at baseline was 0.42 cm2/m2. LVEF was significantly improved at 3 months after TAVR from 50 ± 11.5% to 57 ± 10.7%. The AVA and mean aortic pressure gradient improved significantly after the intervention, as expected. The rate of severe postprocedural paravalvular leaks (PVL) was 3.8% in our cohort. The PVLs were not associated with increased mortality.
 |
Table 3 Echocardiographic Parameters Before and After TAVR |
LV and RV Myocardial Mechanics
Both LV and RV longitudinal deformation using the global longitudinal strain (GLS) and strain rate were reduced in patients with AS before TAVR, as you can see in Table 4. At follow up, LV longitudinal strain significantly improved (p = 0.0001) – Figure 1 and Table 5. RV global longitudinal strain, by using the 6 and 3 segments model was not significantly improved after the TAVR procedure (Figure 2).
 |
Table 4 Pre versus Post TAVR LV and RV Longitudinal Strain |
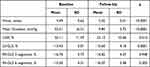 |
Table 5 Differences Regarding Echocardiographic Parameters Between Baseline and Follow-Up Examination |
 |
Figure 1 LV longitudinal strain before and 3 months after TAVR. |
 |
Figure 2 RV longitudinal strain before and 3 months following TAVR. (A) Shows the RV GLS three-segmental model and (B) the full six-segmental model. |
Correlations and Linear Regression Analyses
Correlations between analyzed parameters are presented in Figure 3.
LV-GLS at baseline demonstrated a strong positive correlation with LV-GLS at 3 months (r = 0.69) and a moderate correlation with RV strain parameters (r = 0.38 and r = 0.56), but also a negative correlation with LVEF at follow-up (r=−0.61).
In contrast to LVEF, none of the strain parameters correlated with age, as you can see in Figure 3.
NT-proBNP values were correlated with both LV-GLS (r = 0.37) and LVEF (r=−0.5) at baseline. However, at follow-up, baseline NT-proBNP values remained correlated only to LV-GLS at 3-months (r = 0.24), but the correlation was weak.
The main correlation of LV-GLS at baseline and follow-up is shown in Figure 4.
Discussion
Transcatheter aortic valve replacement (TAVR) has undoubtedly revolutionized the management of aortic stenosis (AS) and became the leading therapeutic strategy for aortic valve replacement in older patients with severe symptomatic AS. Moreover, in the last decade a reduction of interventional related complications was registered, thus leading to significantly improved associated outcomes.1 So, these advances have opened the road to the use of TAVR in lower-risk patients and the most recent ESC guidelines proposed TAVR, as the main therapeutic strategy for patients with AS aged 75 years or older.1
Also, in the last decade, advanced speckle tracking–based echocardiography has emerged as a promising tool for the evaluation of myocardial function in several cardiac diseases, including patients with aortic stenosis, but in clinical practice, left ventricular ejection fraction (LVEF) remains the most commonly used tool for assessing systolic function.1 Moreover, the classification of the types of aortic stenosis is based also on the LVEF.1 But, LVEF has relevant limitations in describing the complex myocardial three-dimensional mechanics.2
Also, the estimation of wall motion is subjective and therefore highly operator dependent and has a high interobserver and intraobserver variability. But, global longitudinal and circumferential strain measurements show very good reproducibility. Therefore, advanced speckle tracking–based echocardiography is reproducible when performed by experienced observers. The echocardiographic parameters for defining the reverse remodeling after TAVR are not well standardized, and those that could be of prognostic value are not yet established.5 Traditionally, left ventricular ejection fraction was associated with myocardial recovery after AV replacement. But, myocardial deformation analysis, including left ventricular and right ventricular strain and speckle-tracking had emerged as extremely useful parameters for the early detection of subclinical alterations of myocardial mechanics. LV and RV GLS may detect remodeling early after TAVR. Therefore, right and left ventricular strain amelioration after the TAVR procedure may represent independent predictors of longer survival and better quality of life. There are studies which showed that, even though the left ventricular ejection fraction remained unchanged, aortic valve replacement surgery improved the speckle tracking left ventricular analyzed parameters.6 But, there are limited data regarding the timing of the reverse remodeling of the left and right ventricle following the TAVR procedure. Our study demonstrated a significant beneficial impact of TAVR procedure on LV myocardial mechanics, by analyzing the global longitudinal strain, in addition to the improvement of the LVEF in elderly patients with severe AS after TAVR. Thus, shows an early recovery of LV longitudinal function after the TAVR intervention. Studies show that LV GLS directly correlates with postprocedural outcomes.6
LV-GLS at baseline presented positive correlations with both LV-GLS and RV strain parameters at 3 months follow-up, but also a negative correlation with LVEF at follow-up, thus showing that single LV-GLS measurement performed before the procedure may be associated with both LV and RV remodeling after TAVR. Interestingly, in contrast to LVEF, none of the strain parameters correlated with age, demonstrating that GLS might lead to better understanding and more clear interpretation of ventricular mechanics in patients with aortic stenosis.
NT-proBNP values were correlated with both LV-GLS and LVEF at baseline. However, at follow-up, baseline NT-proBNP values remained correlated only to LV-GLS at 3-months, but the correlation was weak. Our finding shows that GLS may be superior to LVEF in the prediction of maintained increased LV wall stress after successful TAVR. Also, other studies have shown that an improvement was found in LV-GLS at one-year post-TAVR, but no significant changes were recorded in LVEF, suggesting that strain may be a more sensitive parameter for changes in LV mechanics after TAVR.7 On the other hand, as shown in other studies, NT-proBNP values may be influenced by several comorbidities, such as obesity, renal dysfunction, etc., thus altering its prognostic and risk stratification value.8
Right ventricular dysfunction is a well-known independent adverse prognostic factor in patients with severe aortic stenosis requiring surgical replacement.9 Moreover, after the classical surgical replacement the RV function may further deteriorate. But, impairment of RV myocardial mechanics is known to be associated with a dismal prognosis despite TAVR also.10,11 The effect of TAVR on the RV function is not entirely known. There are studies showing that patients with right ventricular dysfunction at baseline may present significant RV systolic function improvement after TAVR, with important implications on the outcome.10 Therefore, the aim of the study was to evaluate the effect of TAVR on the RV function at 3-months follow-up. In our study, the RV global longitudinal strain was not significantly improved at 3 months after the TAVR procedure. The echocardiographic assessment of RV systolic function is more difficult than for LV, and the applicable parameters are less widely accepted.12 Several echocardiographic parameters are required in order to assess RV systolic dysfunction in patients undergoing TAVR. Instead, a multimodal model, including RV strain, tricuspid regurgitation and TAPSE could be better predictive parameters of outcomes.13,14 Therefore, echocardiographic follow-up, including right ventricular strain in absolutely mandatory for long-term prognostication.
Limitations
The present study has several limitations. First, the number of included patients is relatively small. Second, this is a single-centre study analysis. Even though, LV-GLS at baseline demonstrated positive correlations with LV-GLS and RV strain parameters and also a negative correlation with LVEF at follow-up, more patients are required in order to demonstrate that LV-GLS is indeed an accurate predictor after TAVR procedure. Moreover, echocardiographic findings beyond three months after TAVR should be further investigated.
Conclusions
Advanced speckle tracking–based echocardiography has emerged as a promising tool for the evaluation of myocardial function in patients undergoing TAVR and especially for the evaluation of post-TAVR myocardial recovery and prognostication. In our study, LV-GLS at baseline presented positive correlations with both LV-GLS and RV strain parameters at 3 months follow-up, but also a negative correlation with LVEF at follow-up. But, the RV global longitudinal strain was not significantly improved at 3 months after the TAVR procedure in elderly population. So, several advanced echocardiographic parameters are required in order to assess LV and RV myocardial mechanics, offering additional insights for pre-TAVR and post-TAVR evaluation, management and prognostication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Niculae Stăncioiu Heart Institute.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
1. Vahanian A, Beyersdorf F, Praz F, et al. Christophe Tribouilloy, Wojtek Wojakowski, ESC/EACTS Scientific Document Group, ESC National Cardiac Societies, 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2022;43(7):561–632. doi:10.1093/eurheartj/ehab395
2. Spethmann S, Baldenhofer G, Dreger H, et al. Recovery of left ventricular and left atrial mechanics in various entities of aortic stenosis 12 months after TAVI. Eur Heart J. 2014;15(4):389–398. doi:10.1093/ehjci/jet166
3. Mack MJ, Leon MB, Thourani VH, et al.; The PARTNER 3 Investigators. Transcatheter or surgical aortic-valve replacement in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi:10.1056/NEJMoa1814052
4. Popma JJ, Deeb GM, Yakubov SJ, et al; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi:10.1056/NEJMoa1816885
5. Al-Akchar M, Sawalha K, Mahmaljy H, et al. Echocardiographic derived parameters association with long-term outcomes after transcatheter valve replacement. Cardiovasc Revasc Med. 2020;21(8):982–985. PMID: 31948846. doi:10.1016/j.carrev.2019.12.035
6. Stangenhaus C, Vieira MLC, Fischer CH, Filho ACB, Perin MA, Caixeta AM. Myocardial deformation by echocardiogram after transcatheter aortic valve implantation. Arq Bras Cardiol. 2017;108(5):480–483. PMID: 28591325; PMCID: PMC5444896. doi:10.5935/abc.20170013
7. Wani A, Harland DR, Bajwa TK, et al. Left ventricular mechanics differ in subtypes of aortic stenosis following transcatheter aortic valve replacement. Front Cardiovasc Med. 2022;8:777206. PMID: 35111823; PMCID: PMC8803205. doi:10.3389/fcvm.2021.777206
8. Dădârlat-Pop A, Sitar-Tăut A, Zdrenghea D, et al. Profile of obesity and comorbidities in elderly patients with heart failure. Clin Interv Aging. 2020;15:547–556. PMID: 32368021; PMCID: PMC7184119. doi:10.2147/CIA.S248158
9. Cao Y, Singh V, Wang A, et al. Meta-analysis of right ventricular function in patients with aortic stenosis after transfemoral aortic valve replacement or surgical aortic valve replacement. Ther Adv Chronic Dis. 2020;11:2040622320933775. PMID: 32670537; PMCID: PMC7339069. doi:10.1177/2040622320933775
10. Al-Rashid F, Totzeck M, Saur N, et al. Global longitudinal strain is associated with better outcomes in transcatheter aortic valve replacement. BMC Cardiovasc Disord. 2020;20(1):267. doi:10.1186/s12872-020-01556-4
11. Poch F, Thalmann R, Olbrich I, et al. Changes of right ventricular function after transcatheter aortic valve replacement and association with outcomes. J Card Fail. 2021;27(12):1337–1344. PMID: 33839289. doi:10.1016/j.cardfail.2021.03.007
12. Sade LE, Katz WE. Right ventricle deserves more attention in transcutaneous aortic valve replacement patients. J Card Fail. 2021;27(12):1345–1347. PMID: 34893203. doi:10.1016/j.cardfail.2021.04.005
13. Grevious SN, Fernandes MF, Annor AK, Ibrahim M, Saint Croix GR, de Marchena E. Prognostic assessment of right ventricular systolic dysfunction on post-transcatheter aortic valve replacement short-term outcomes: systematic review and meta-analysis. J Am Heart Assoc. 2020;9(12):e014463. PMID: 32517527; PMCID: PMC7429048. doi:10.1161/JAHA.119.014463
14. Burke GM, Araujo Silva B, Marum AA, et al. Speckle tracking strain and ECG heterogeneity correlate in transcatheter aortic valve replacement-induced left bundle branch blocks and right ventricular paced rhythms. Open Heart. 2021;8(2):e001542. doi:10.1136/openhr
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


