Back to Journals » OncoTargets and Therapy » Volume 13
Prognostic Value of Ki67 in Patients with Stage 1–2 Endometrial Cancer: Validation of the Cut-off Value of Ki67 as a Predictive Factor
Authors Jiang P , Jia M, Hu J, Huang Z, Deng Y, Lai L, Ding S, Hu Z
Received 28 July 2020
Accepted for publication 25 September 2020
Published 27 October 2020 Volume 2020:13 Pages 10841—10850
DOI https://doi.org/10.2147/OTT.S274420
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Peng Jiang, Mingzhu Jia, Jing Hu, Zhen Huang, Ying Deng, Li Lai, Shanshan Ding, Zhuoying Hu
Department of Gynecology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Zhuoying Hu
Department of Gynecology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Tel +86 23 89011092
Fax +86 23 89011082
Email [email protected]
Objective: The purpose of this study was to find a cut-off value of the immunohistochemical parameter Ki67 for stage I–II endometrial cancer.
Materials and Methods: The clinicopathological data of 318 patients with stages I–II endometrial cancer who received primary surgical treatment were retrospectively analyzed. A cut-off value of Ki67 for predicting recurrence of endometrial cancer was determined by using the receiver operating characteristic curve and the Youden index. The Cox regression was performed to screen factors associated with recurrence of endometrial cancer. Based on the cut-off value of Ki67, the patients were divided into two groups, and the differences of clinicopathological parameters between the two groups were compared.
Results: The receiver operating characteristic curve showed that the optimal cut-off value of Ki67 for predicting recurrence of patients with stages I–II endometrial cancer was 38%. The multivariate Cox regression analysis demonstrated that the histotypes (P=0.012), myometrial invasion (P=0.014), cervical stromal invasion (P=0.001), Ki67 (P=0.002), estrogen receptor (ER) (P=0.045) and P53 (P=0.032) were significant prognostic predictors for recurrence of endometrial cancer. The recurrence-free survival and the disease-specific survival of patients in the high-Ki67 group (Ki67 ≥ 38%) were much lower than those in the low-Ki67 group (Ki67 < 38%) (P=0.000, P=0.001, respectively). Among the 118 patients with early low-risk endometrial cancer who did not receive adjuvant treatment after surgery, the recurrence-free survival of patients in the high-Ki67 group was also lower than those in the low-Ki67 group (P=0.000).
Conclusion: The Ki67 was demonstrated to be a useful prognostic factor in patients with stages I–II endometrial cancer, and the Ki67 labeling index 38.0% was optimal cut-off value for predicting recurrence.
Keywords: Ki67, cut-off value, endometrial cancer, recurrence
Introduction
Ki67 expression is commonly used as a marker of cell proliferation,1 whose expression can be easily visualized by immunohistochemistry. This is why it is widely used to reflect the prognosis of several malignancies.2 In breast cancer, Ki67 expression has been widely used as a prognostic indicator to guide clinical work, at the St. Gallen Consensus Conference, the majority of the expert panel voted that a threshold of ≥ 20% should be defined as high Ki67 status in breast cancer.3 In many other related studies, various cut-off values of Ki67 labeling index have also been determined for postoperative management of breast cancer.2,4 In endometrial cancer, the risk of recurrence is usually determined by the patient’s pathological diagnosis, including staging, histological subtype, tumor grade, lymph node status, and depth of muscular invasion.5–7 However, more and more studies show that Ki67 can be used as an important prognostic predictor of endometrial cancer,1,8,9 but currently there is still no recognized acceptable cut-off value of Ki67 in endometrial cancer. The present study aimed to determine the clinical value of Ki67 as a prognostic marker in patients with stages I–II endometrial cancer and find an optimal cut-off value of Ki67 in endometrial cancer.
Materials and Methods
Study Population
The study was prospectively approved by the Ethics Committee of Chongqing Medical University and conducted in accordance with the Declaration of Helsinki. The archived data of the patients with endometrial cancer who had undergone primary surgical treatment between October 2013 and May 2017 were retrieved from the First Affiliated Hospital of Chongqing Medical University. Inclusion criteria were as follows: 1) Patients were diagnosed with stages I–II endometrial cancer according to the staging system of International Federation of Gynecology and Obstetrics (FIGO) 2009 guidelines;10 2) Patients had a complete case record, including age, body mass index, comorbidities (hypertension or diabetes), surgical procedures, pathological results (histotype, depth of myometrial invasion, cervical stromal invasion), four immunohistochemical makers (Ki67, ER, PR, and P53), and postoperative adjuvant therapy. The exclusion criteria were as follows: 1) The patient did not follow the standard surgical treatment;11 2) Patients with other malignancies; 3) Patients who had received chemotherapy or radiotherapy before surgery; and 4) Patients without regular follow-ups.12
Treatment and Follow-Up
Patients received standard surgical treatment, including at least total hysterectomy with bilateral salpingo-oophorectomy, with or without nodal staging (sentinel lymph node±pelvic±para-aortic lymphadenectomy),11 followed by adjuvant treatment (chemotherapy and/or radiotherapy) stipulated by multidisciplinary discussions and international guidelines.13 Follow-up visits were performed every 3 months for the first 2 years, every 6 months for the following 3 years, and once a year thereafter. All the patients were followed up from the day of surgery onwards, follow-up care plans included regular physical examinations and necessary auxiliary checks.14 The follow-up deadline was June 2019. Since most relapsed patients were concentrated within 2 years after surgery, apart from those who died during follow-up, the remaining patients had follow-up >2 years.
Histology and Immunohistochemistry
All patients’ postoperative specimens were fixed by formalin tissue fluid within the prescribed time and then sent to the Pathological Laboratory Center of Chongqing Medical University for further processing. All specimens were processed with the same standards according to the instructions of pathological section staining and immunohistochemistry in the department of pathology.15 Briefly, samples were made into formalin-fixed, paraffin-embedded specimens. H&E staining was used to confirm which parts were cancerous, then a representatively cancerous part was chosen and a dot was set with a diameter of 2 mm to stand for the whole sample. The histotypes, lesion size, lesion and infiltration range were initially judged by the primary pathologist, and reviewed by the superior physician. The low risk histotypes were defined as G1 or G2 endometrioid adenocarcinoma, while the high risk histotypes were defined as G3 endometrioid adenocarcinoma or non-endometrioid adenocarcinoma including serous carcinoma, clear cell carcinoma and other histotypes.16
Immunohistochemistry (IHC) of ER, PR, Ki67 and P53 was performed with an automated immunostainer (Leica Bond-Max, Milton Keynes, UK) according to the manufacturer’s instruction.9 Tissue slides were dried overnight at 70°C, deparaffinized with xylene, and then gradually hydrated. The sections were subjected to heat-induced epitope retrieval at 100°C for 20 minutes, cooled to 20°C, and then treated with 0.3% H2O2 in methanol for 5 minutes to block endogenous peroxidase activity. The following primary mouse monoclonal antibodies were used in IHC: ER (clone 1D5, 1:50), PR (clone PgR636, 1:500), Ki67 (clone MIB1, 1:300) and P53 (clone DO7, 1:200). The samples were incubated for 30 minutes at room temperature by using an anti-mouse secondary antibody (Leica). Diaminobenzidine was used as a chromogen (DAB Substrate System, DAKO). PBS (phosphate-buffered saline) were used as wash buffer for three times between each two procedures. Immunohistochemical results of ER, PR, Ki67 and P53 were independently evaluated by two experienced pathologists and recorded as the percentage of positively stained tumor cells (0–100%). Pathologists’ assessment for the proportion of positive tumor cells were considered to be consistent if the proportion differed ≤10%; If the initial assessment was considered to be inconsistent (the proportion differed >10%), then the results were re-evaluated (unblinded) and a consensus reached. The two pathologists’ results for proportion were finally averaged to represent the final result of the proportion of positive tumor cells.17
ER and PR were considered positive when expression was seen in >5% of the tumor cells, which is the cut-off used in daily clinical practice for the characterization of breast cancer and which was shown to be valuable in endometrial carcinomas as well.8,18 According to the 3-tier system for P53 immunohistochemistry interpretation,19 the overexpression (the proportion of positive tumor cells ≥75%) and complete absence (the proportion of positive tumor cells was 0%) both considered as abnormal (aberrant/mutation-type), in contrast to the normal (wild-type) pattern with p53 expression levels in between these extremes (0–75%). Immunohistochemical parameters Ki67 was recorded as continuous variables based on the proportion of positive tumor cells (0–100%) (Supplementary Figure 1).
Recurrence
Recurrence was considered if lesions were confirmed by physical examination, histological examination, or images, including computed tomography, magnetic resonance imaging, ultrasonography, bone scintigraphy, positron emission tomography or specific X-rays.14 Recurrence was divided into vaginal stump recurrence, central pelvic region recurrence, lymph nodes (upper para-aortic) recurrence, peritoneal metastases, and metastasis to other organs.16 Recurrence-free survival was defined as time between date of complete surgical removal of the malignancy and either date of (histologically or radiologically confirmed) recurrence.20 Disease-specific survival was defined as time from primary surgery to death as a result of recurrence.21
Statistical Analysis
Student’s t-tests were used for continuous variables and χ2 tests were used for categorical variables. The optimal cut-off value of the Ki67 labeling index was determined by receiver operating characteristic curve and Youden Index (Youden index = sensitivity + specificity −1).22 Multivariate Cox regression model was used to screen the independent prognostic predictors of recurrence of endometrial cancer, which included variables of P<0.05 in the univariate Cox regression model. Then, all the patients were divided into two groups (a high-Ki67 group and a low-Ki67 group) according to the cut-off value of Ki67, and Kaplan-Meier analysis was used to determine the duration of recurrence-free survival and disease-specific survival. Log rank test was used to assess the difference of recurrence-free survival and disease-specific survival between two groups. Statistical Product and Service Solutions (SPSS, version 25.0, IBM Statistics, Chicago, IL, USA) software was used for statistical analysis of the data. P-<0.05 was considered to indicate statistically significant difference.
Results
Characteristics of Patients and Tumors
A total of 349 patients with stages I–II endometrial cancer underwent primary surgical treatment at the First Affiliated Hospital of Chongqing Medical University from October 2013 to May 2017, among which 318 patients meeting the criteria were recruited. While 31 were excluded due to missing data, lost to follow-up, or underwent a non-standard surgical approach (Supplementary Figure 2). The clinicopathological characteristics of 318 patients are summarized in Table 1. The majority of the patients had FIGO stage I tumors (87.1%) and the tumors were mainly endometrioid adenocarcinoma (87.4%). Adjuvant treatment had been administered to 172 (54.1%) patients. The median follow-up of all patients were 46 (range 12–67) months. There were 46 (14.5%) recurrence in total, of which 25 died due to recurrence of endometrial cancer, while five were due to other causes. The median follow-up and recurrence-free survival for patients with recurrence were 36 (range 12–60) and 22.5 (range 6–48) months (Supplementary Table 1). The distribution of immunohistochemical results are summarized in Table 2, among which the Ki67 labeling index ranged from 0 to 90% (median 30%) (Supplementary Figure 3).
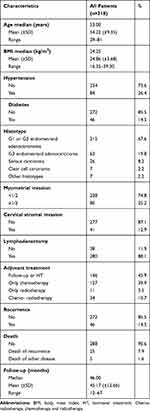 |
Table 1 Patient Characteristics (n=318) |
 |
Table 2 Distribution of Immunohistochemical Results of ER, PR, P53, and Ki67 |
The Optimal Cut-off Value of the Ki67 Labeling Index
The univariate Cox regression analysis confirmed that a high Ki67 labeling index was significantly associated with a short recurrence-free survival (P=0.000). Then, the receiver operating characteristic curve and Youden index (Youden index = sensitivity + specificity −1) revealed that the optimal cut-off value of Ki67 labeling index for predicting recurrence of endometrial cancer was 38.0% (area under the curve = 0.708; sensitivity, 65.2%; specificity, 70.6%) (Figure 1).
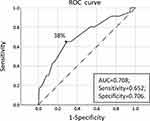 |
Figure 1 The receiver operating characteristic curve of Ki67. |
Univariate and Multivariate Analysis of Recurrence-Free Survival
The univariate Cox regression analysis was used to analyze the clinicopathological factors and four immunohistochemical markers (Ki67, ER, PR, and P53) that might affect the recurrence of endometrial cancer, and the factors with P>0.05 were excluded from the multivariate analysis, including body mass index, P=0.301, hypertension, P=0.952, diabetes, P=0.496, and lymphadenectomy, P=0.847. The factors with P<0.05, including age, histotype, myometrial invasion, cervical stromal invasion, adjuvant treatment, and all four immunohistochemical markers, were further included in the multivariate Cox regression. Finally, six factors, including histotype (P=0.012), myometrial invasion (P=0.014), cervical stromal invasion (P=0.001), Ki67 (P=0.002), ER (P=0.045) and P53 (P=0.032), were identified as independent prognostic factors for recurrence of endometrial cancer (Table 3).
 |
Table 3 Univariate and Multivariate Analysis of Factors Predicting Endometrial Cancer Recurrence |
Comparison of Clinicopathological Parameters Between the Low-Ki67 Group and the High-Ki67 Group
When the cut-off value of Ki67 was set as 38.0%, the high Ki67 labeling index was not only significantly associated with relapse of endometrial cancer, but also related to the following factors: age (P=0.003), histotype (P=0.000), ER (P=0.000), progesterone receptor (P=0.000) (Table 4). According to the cut-off value of Ki67 labeling index, patients with a Ki67 labeling index ≥38% were defined as the high-Ki67 group, and <38% were defined as the low-Ki67 group. Median follow-up time of the low-Ki67 group was 47 (range 19–67) months while for the high-Ki67 group was 45 (range 12–67) months, the 3-year recurrence-free survival rates for the low-Ki67 group and the high-Ki67 group were 92.3% (95% CI, 88.6–96.0%) and 76.7% (95% CI, 68.7–84.7%) and the 5-year recurrence-free survival rates were 91.7% (95% CI, 87.8–95.6%) and 69.2% (95% CI, 59.6–78.8%), respectively (P=0.000; Figure 2A). The 3-year disease-specific survival rates for the low-Ki67 group and the high-Ki67 group were 96.9% (95% CI, 94.5–99.3%) and 89.1% (95% CI, 83.0–95.2%), and the 5-year overall survival rates were 94.9% (95% CI, 91.6–98.2%) and 81.7% (95% CI, 73.3–90.1%), respectively (P = 0.001; Figure 2B);
 |
Table 4 Comparison of Clinicopathological Parameters Between the Low-Ki67 Group and the High-Ki67 Group (n=318) |
 |
Figure 2 Kaplan-Meier Survival Curve of the low-Ki67 group and the high-Ki67 group. |
The Prognostic Significance of the Cut-off Value of Ki67 in the Patients with Early Low-Risk Endometrial Cancer
In all patients, a total of 118 patients had early low-risk endometrial cancer (these patients were with FIGO stage Ia, with low risk histotypes and without other obvious high risk factors for recurrence of endometrial cancer). They only received endocrine therapy or follow-up after surgery instead of adjuvant therapy. There were 91 early low-risk endometrial cancer patients in the low-Ki67 group and only 1 (1.1%) patient relapsed, while 27 early low-risk endometrial cancer patients in the high-Ki67 group and 7 (25.9%) patients relapsed (Supplementary Table 2). The 3-year recurrence-free survival rates for the patients with early low-risk endometrial cancer in the low-Ki67 group and the high-Ki67 group were 98.7% (96.2–100%) and 72.6% (55.2–90.0%), respectively (P = 0.000; Figure 2C).
Discussion
Uncontrolled proliferation is a hallmark of malignant tumors. Ki67 is currently a recognized marker of cell proliferation, which reflects the proportion of malignant cells and is related to tumor progression, metastasis and prognosis.23 In esophageal cancer, colorectal cancer, pancreatic cancer and other malignant tumors, high Ki67 labeling index has been proved to be associated with poor prognosis.24–26 In female malignant tumors, the cut-off value of Ki67 has been used as an important prognostic indicator of breast cancer.4 While in endometrial cancer, Ki67 has also shown its potential value, but there is no acceptable cut-off value currently. In this study, we found an optimal cut-off value (38%) of Ki67 for predicting the recurrence of endometrial cancer and multivariate Cox regression analysis suggested that high Ki67 labeling index (≥38%) was an independent risk factor for stages I–II endometrial cancer recurrence. According to the optimal cut-off value, the recurrence-free survival rate and disease-specific survival rate of patients in the high-Ki67 group were much lower than those in the low-Ki67 group, which indicated that we should pay more attention to the prognostic management of patients in the high-Ki67 group, including appropriately extending the time of postoperative adjuvant therapy, increasing the number of cycles of adjuvant therapy, conducting closer follow-up after surgery, etc. Most importantly, among the 146 patients who did not receive adjuvant therapy (chemotherapy and/or radiotherapy) after surgery, except for some patients who refused to receive adjuvant therapy due to personal factors, there were still 118 patients who only received the postoperative endocrine treatment or follow-up instead of the adjuvant therapy due to early FIGO stage (FIGO stage Ia), low risk histotype, and without other obvious high risk factors of recurrence. However, some of them still relapsed. The survival analysis results showed that patients in the high-Ki67 group also had a lower recurrence-free survival rate than patients in the low-Ki67 group, which indicated that the endometrial cancer patients in the high-Ki67 group should not be treated solely with endocrine therapy or follow-up after surgery, even if they were with early FIGO stages and low risk histotypes, they might also need appropriate supplementary chemoradiotherapy.4 Certainly, when making the decision regarding adjuvant treatment, we should not consider Ki67 only. At present, the selection of patients for adjuvant therapy still mainly depends on traditional clinicopathological methods. For example, recent European guidelines and National Comprehensive Cancer Network guidelines indicated that there was no clear rules for post-operational radiotherapy and chemotherapy of patients with endometrial cancer, but suggest that supplemental radiotherapy or even combination chemotherapy may be considered when patients have the following risk factors: late FIGO stage, poor pathological classification, older age (especially ≥60 years), extensive lymphovascular space invasion, and deeper myoinvasion (>50%), etc.6,7 Despite all this, the immunohistochemical markers such as Ki67 could still become a contributor for individualized adjuvant treatment, especially for patients with early FIGO stages and low risk histotypes.
It is worth mentioning that, besides the classic clinicopathological parameters such as FIGO stage, histotype, myometrial invasion, cervical stromal invasion, which have long been proved to be independent prognostic indicators in other studies, the immunohistochemical markers ER and P53 were also found to be significantly associated with the recurrence of endometrial cancer in the multivariate analysis in this study, which was consistent with many similar studies.19,23 The loss of ER and PR expression has been widely proven to be an independent predictor of poor prognosis, even for low-grade endometrial cancer, which may predict lymph node metastasis and the risk of recurrence. The loss of PR expression also indicates a poor prognosis for women with high-grade cancers, including histotypes that were previously considered to be unaffected by hormones, such as serous carcinomas,17 even though PR showed no obvious correlation with recurrence of endometrial cancer due to the “collinearity” between factors in the multivariate analysis in this study. Similarly, abnormal expression of P53 (overexpression and complete deletion) usually means mutation of the TP53 gene. Among the four subgroups of the Cancer Genome Atlas (TCGA) molecular typing of endometrial cancer, the prognosis of P53 mutation group is consistently the worst one, and is prone to further deterioration due to unfavorable clinicopathological factors.27 Therefore, the above immunohistochemical markers should also be paid attention to when evaluating the prognosis of patients.
What needs to be explained is that the univariate analysis found that age, adjuvant treatment and PR were significantly associated with recurrence, while the multivariate analysis did not find significant correlations in this study, which might be explained by the fact that this was a single-center study, the other independent prognostic factors including myometrial invasion, cervical stromal invasion, histotype, Ki67, ER and P53 were more strongly associated with recurrence of endometrial cancer. And it did not deny the importance of the three factors (age, adjuvant treatment and PR) in predicting the prognosis of endometrial cancer. In fact, many articles have reported that the three factors are important predictors for endometrial cancer recurrence.11,18,20,28 Especially, contrary to our impression, it seemed that patients who received adjuvant treatment had more recurrences than those who received none according to the hazard ratio in the univariate analysis. This might be explained by the fact that most of these patients who received adjuvant treatment had the later FIGO stages, with the high risk histotypes, which led to a strong “collinearity” between the adjuvant treatments and these high risk factors,29 and the protective effect of adjuvant treatments was not enough to offset the high risk of recurrence incurred by these high risk factors. Therefore, in the univariate analysis, adjuvant treatments were suggested as a “risk factor for recurrence.” However, the “collinearity” among factors was minimized after the multivariate analysis, and the adjuvant treatments were proved to be the “protective factors” (the hazard ratio of adjuvant treatments in multivariate analysis<1). Although the analysis results showed that adjuvant treatments had no significant correlation with relapse, other reports state that they have a positive impact on improving recurrence-free survival of patients, especially those with high-risk relapse factors.30,31
Of course, this study also has its limitations, including its retrospective design at a single institution, which requires multi-center research and further prospective studies to participate in joint verification. Secondly, there is a lack of consensus regarding the use of the Ki67 labeling index, and the “hottest spot” assessment method of Ki67 is currently commonly used.32,33 In our study, the hot-spot scoring of Ki67 was used, and it demonstrated the prognostic significance of the Ki67 labeling index. However, It is still necessary to establish an international standard methodology for using Ki67.
In summary, we have found an optimal cut-off value of Ki67 and confirmed that it can be a reliable prognostic indicator for stages I–II endometrial cancer. Especially for patients with early low-risk endometrial cancer who would not receive adjuvant treatment after surgery, the cut-off value of Ki67 may be instructive for postoperative treatment of these patients.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of Chongqing Medical University (Ethics approval number:2020-166) and conducted in accordance with the Declaration of Helsinki. All patients provided their informed consent before starting the treatment. As it was a retrospective clinical study, all the patients were contacted by telephone to obtain verbal informed consent and it was approved by the ethics committee. All data about the patients was anonymized or maintained with confidentiality.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ikeda Y, Oda K, Ishihara H, et al. Prognostic importance of CDK4/6-specific activity as a predictive marker for recurrence in patients with endometrial cancer, with or without adjuvant chemotherapy. Br J Cancer. 2015;113(10):1477–1483. doi:10.1038/bjc.2015.369
2. Cserni G, Voros A, Liepniece-Karele I, et al. Distribution pattern of the Ki67 labelling index in breast cancer and its implications for choosing cut-off values. Breast. 2014;23(3):259–263. doi:10.1016/j.breast.2014.02.003
3. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi:10.1093/annonc/mdt303
4. Ohara M, Matsuura K, Akimoto E, et al. Prognostic value of Ki67 and p53 in patients with estrogen receptor-positive and human epidermal growth factor receptor 2-negative breast cancer: validation of the cut-off value of the Ki67 labeling index as a predictive factor. Mol Clin Oncol. 2016;4(4):648–654. doi:10.3892/mco.2016.776
5. Wright JDBMN, Medel NIB, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379(9823):1352–1360. doi:10.1016/S0140-6736(12)60442-5
6. Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16–41. doi:10.1093/annonc/mdv484
7. Wui-Jin Koh MC, Research FHC, Alliance CSCC, et al. NCCN guidelines version 2.2019 endometrial carcinoma. NCCN Guidelines for Patients available at www.nccn.org 2018.
8. Ferrandina G, Ranelletti FO, Gallotta V, et al. Expression of cyclooxygenase-2 (COX-2), receptors for estrogen (ER), and progesterone (PR), p53, ki67, and neu protein in endometrial cancer. Gynecol Oncol. 2005;98(3):383–389. doi:10.1016/j.ygyno.2005.04.024
9. Yang B, Shan B, Xue X, et al. Predicting lymph node metastasis in endometrial cancer using serum CA125 combined with immunohistochemical markers PR and Ki67, and a comparison with other prediction models. PLoS One. 2016;11(5):e0155145. doi:10.1371/journal.pone.0155145
10. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–104. doi:10.1016/j.ijgo.2009.02.012
11. Ouldamer L, Bendifallah S, Body G, et al. Predicting poor prognosis recurrence in women with endometrial cancer: a nomogram developed by the FRANCOGYN study group. Br J Cancer. 2016;115(11):1296–1303. doi:10.1038/bjc.2016.337
12. Marcos-Sanmartin J, Lopez Fernandez JA, Sanchez-Paya J, et al. Does the type of surgical approach and the use of uterine manipulators influence the disease-free survival and recurrence rates in early-stage endometrial cancer? Int J Gynecol Cancer. 2016;26(9):1722–1726. doi:10.1097/IGC.0000000000000808
13. Colombo NPE, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, ESMO Guidelines Working Group. Endometrial cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl6):vi33–vi38. 2013. doi:10.1093/annonc/mdt353
14. Ouldamer L, Bendifallah S, Body G, et al. Change in hazard rates of recurrence over time following diagnosis of endometrial cancer: an age stratified multicentre study from the FRANCOGYN group. Eur J Surg Oncol. 2018;44(12):1914–1920. doi:10.1016/j.ejso.2018.07.053
15. Yu X, Guo S, Song W, et al. Estrogen receptor α (ERα) status evaluation using RNAscope in situ hybridization: a reliable and complementary method for IHC in breast cancer tissues. Hum Pathol. 2017;61:121–129. doi:10.1016/j.humpath.2016.12.005
16. Takahashi K, Yunokawa M, Sasada S, et al. A novel prediction score for predicting the baseline risk of recurrence of stage I–II endometrial carcinoma. J Gynecol Oncol. 2019;30(1):e8. doi:10.3802/jgo.2019.30.e8
17. Smith D, Stewart CJR, Clarke EM, et al. ER and PR expression and survival after endometrial cancer. Gynecol Oncol. 2018;148(2):258–266. doi:10.1016/j.ygyno.2017.11.027
18. van der Putten LJM, Visser NCM, van de Vijver K, et al. Added value of estrogen receptor, progesterone receptor, and L1 cell adhesion molecule expression to histology-based endometrial carcinoma recurrence prediction models: an ENITEC Collaboration Study. Int J Gynecol Cancer. 2018;28(3):514–523. doi:10.1097/IGC.0000000000001187
19. Köbel MRB, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol. 2019;38(Suppl 1):S123–S31. doi:10.1097/PGP.0000000000000488
20. Huijgens ANJ. Factors predicting recurrent endometrial cancer. FVV in ObGyn. 2013;5(3):179–186.
21. Seebacher V, Hofstetter G, Polterauer S, et al. Does thyroid-stimulating hormone influence the prognosis of patients with endometrial cancer? A multicentre trial. Br J Cancer. 2013;109(1):215–218. doi:10.1038/bjc.2013.282
22. Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding youden index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16(1):73–81. doi:10.1097/01.ede.0000147512.81966.ba
23. Gulseren V, Kocaer M, Ozdemir IA, Cakir I, Sanci M, Gungorduk K. Do estrogen, progesterone, P53 and Ki67 receptor ratios determined from curettage materials in endometrioid-type endometrial carcinoma predict lymph node metastasis? Curr Probl Cancer. 2020;44(1):100498. doi:10.1016/j.currproblcancer.2019.07.003
24. Frank J, Jacob K, Florian G, et al. Loss of p16 and high Ki67 labeling index is associated with poor outcome in esophageal carcinoma. Oncotarget. 2020;11(12):1007–1016. doi:10.18632/oncotarget.27507
25. Li J, Liu Z-Y, Yu H-B, Xue Q, He W-J, Yu H-T. Clinicopathological significance of Ki67 expression in colorectal cancer: a protocol of systematic review and meta-analysis. Medicine. 2020;99(20):e20136. doi:10.1097/MD.0000000000020136
26. Temraz S, Shamseddine A, Mukherji D, et al. Ki67 and P53 in relation to disease progression in metastatic pancreatic cancer: a single institution analysis. Pathol Oncol Res. 2019;25(3):1059–1066. doi:10.1007/s12253-018-0464-y
27. Raffone A, Travaglino A, Mascolo M, et al. TCGA molecular groups of endometrial cancer: pooled data about prognosis. Gynecol Oncol. 2019;155(2):374–383. doi:10.1016/j.ygyno.2019.08.019
28. Versluis MA, de Jong RA, Plat A, et al. Prediction model for regional or distant recurrence in endometrial cancer based on classical pathological and immunological parameters. Br J Cancer. 2015;113(5):786–793. doi:10.1038/bjc.2015.268
29. Pierre-Graud Claret P-G, Bobbia X, de La Coussaye JE. Collinearity and multivariable analysis. Intensive Care Med. 2016;42(11):1834. doi:10.1007/s00134-016-4528-8
30. Alessia Aloisi FP, Scaletta G, Capriglione S, et al. Chemotherapy as adjuvant treatment for intermediate-high risk early-stage endometrial cancer: a Pilot Study. Int J Gynecol Cancer. 2015;25(8):1418–1423. doi:10.1097/IGC.0000000000000505
31. Emons G, Vordermark D. Adjuvant treatment for endometrial cancer. Curr Opin Oncol. 2019;31(5):404–410. doi:10.1097/CCO.0000000000000558
32. Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst. 2011;103(22):1656–1664. doi:10.1093/jnci/djr393
33. Honma N, Horii R, Iwase T, et al. Ki-67 evaluation at the hottest spot predicts clinical outcome of patients with hormone receptor-positive/HER2-negative breast cancer treated with adjuvant tamoxifen monotherapy. Breast Cancer. 2015;22(1):71–78.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
