Back to Journals » Cancer Management and Research » Volume 12
Prognostic Significance of a Novel Score Model Based on Preoperative Indicators in Patients with Breast Cancer Spine Metastases (BCSM)
Authors Zhao C, Wang Y, Cai X, Xu W, Wang D, Wang T, Jia Q, Gong H, Sun H, Wu Z, Xiao J
Received 29 July 2020
Accepted for publication 15 October 2020
Published 10 November 2020 Volume 2020:12 Pages 11501—11513
DOI https://doi.org/10.2147/CMAR.S273785
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Chenglong Zhao,* Yao Wang,* Xiaopan Cai,* Wei Xu,* Dongsheng Wang, Ting Wang, Qi Jia, Haiyi Gong, Haitao Sun, Zhipeng Wu, Jianru Xiao
Spine Tumor Center, Department of Orthopedic Oncology, Changzheng Hospital, Navy Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianru Xiao; Zhipeng Wu
Spine Tumor Center, Department of Orthopedic Oncology, Changzheng Hospital, Navy Medical University, No. 415 Fengyang Road, Huangpu District, Shanghai, People’s Republic of China
Tel +86-21-81886843
Fax +86-21-63520020
Email [email protected]; [email protected]
Background: Surgery remains the mainstay of treatment for breast cancer spinal metastasis (BCSM) to relieve symptoms and improve the quality of life of BCSM patients. Therefore, it is important to effectively predict the prognosis of patients to determine whether they can undergo surgical operation. However, the prevalent methods for prognosis evaluation lack specificity and sensitivity for indicated malignancies like breast cancer because they are built on a relatively small number of heterogeneous types of primary tumors. The aim of the present study was to explore a novel predictive model based on the clinical, pathological and blood parameters obtained from BCSM patients before they received surgical intervention.
Methods: Altogether, 144 patients were included in this study. Univariate and multivariate analyses were performed to investigate the significance of preoperative parameters and identify independent factors for prognostic prediction of BCSM. A nomogram for survival prediction was then established and validated. Time-dependent ROC (TDROC) curves were graphed to evaluate the accuracy of the novel model vs other scoring systems including Tomita Score, revised Tokuhashi Score, modified Bauer Score and New England Spinal Metastasis Score. P values < 0.05 were considered statistically significant.
Results: Independent factors, including preoperative postmenopausal (P=0.034), visceral metastasIs (P=0.021), preoperative Frankel Score (P=0.001), estrogen receptor status (P=0.014), platelet-to-lymphocyte ratio (P=0.012), lymphocyte-monocyte ratio (P< 0.001) and albumin-globulin ratio (P=0.017), were selected into the nomogram model with the C-index of 0.834 (95% CI, 0.789– 0.890). TDROC curves showed that the Changzheng Hospital (CZ) Score system had the best performance and exhibited the largest IAUC value in comparison with the other scoring systems.
Conclusion: We constructed a nomogram model known as CZ Score based on the significant factors to predict the prognosis for BCSM patients. The result showed that CZ Score had a better value for prognostic evaluation and surgical decision-making as compared with the other scoring systems.
Keywords: breast cancer, spine metastases, nomogram, scoring system, prognosis
Introduction
Personalized management of primary breast cancer using the surgery-based multidisciplinary treatment strategy has percolated into the mainstream and gained good prognosis of patients.1 However, extension of the patients’ lifespan may in turn increase the risk of spinal metastasis of the primary cancer, which is a dilemma in front of the clinical oncologists.2 Surgery also plays a vital role in the management of spinal metastasis secondary to breast cancer in that it can alleviate intractable pain, relieve nerve compression and reconstruct the spinal stability.3–6 However, not all patients are candidates for surgical resection and therefore specific treatment options should be selected according to the general condition and life expectancy of individual patients. Unfortunately, there is no generally accepted method to evaluate the prognosis of patients with spinal metastatic lesions before surgery. Although there have been various scoring systems to help predict patients’ life expectancy for treatment decision-making, including Tomita, revised Tokuhashi and Bauer,7–9 there is no evidence-based clue to decide which one is more accurate for prognostic prediction.10,11 In addition, all these scoring systems were established two or three decades ago and need to be updated and upgraded, given the continuous progress in disease diagnosis and treatment. The New England Spinal Metastasis Score (NESMS) is just such a new observational model.12 What is more, most of these systems lack specificity and sensitivity for indicated malignancies because they are built on relatively small numbers of heterogeneous types of primary tumors.
Certain tumor types including breast cancer have specific and characteristic gene expression patterns.13–15 According to the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (Her2), breast cancer is classified into different sub-types including luminal, Her2 and triple negative.16 Numerous studies have demonstrated different sub-types of breast cancer have different clinical outcomes. The luminal sub-type is considered more prone to form bone metastasis, but it also has a relatively good prognosis.17,18 Besides, it is reported in the published literature that gene expression signatures of certain tumor types have good performance in predicting the clinical prognosis.19–21 Therefore, we considered that the gene expression patterns characteristics should be taken into account in establishing a prognostic model of breast cancer spine metastasis (BCSM).
Recently, numerous studies have focused on common blood parameters that indicate great significance of clinical evaluation of prognosis and treatment.22–24 Blood tests can reflect inflammatory response, immunity, and visceral functions. Different parts of the preoperative parameters and their combinations have been conducted to predict the prognosis in different cancers.25–27 In the process of examining the complex scoring systems currently available, we noticed that they did not contain the specific pathological indicators and normal preoperative blood parameters, although most of them are less costly and easily accessible. The aim of the present study was to explore a novel predictive model based on the clinical, pathological and blood parameters obtained from 144 BCSM patients before they received surgical intervention.
Patients and Methods
Patients
In a previous study,28 we reported a series of 87 BCSM patients and identified that visceral metastasis, multiple metastases of the spine and post-operative chemotherapy were independent clinical prognostic factors. Currently, we updated the existing data and proposed to evaluate prognosis in a new cohort of 179 patients with breast cancer in our center from January 2005 to December 2018. Of them, 144 patients were ultimately included in this study after a series of screening processes (Figure 1). All patients underwent surgical resection and subsequent chemotherapy, radiotherapy, endocrine/targeted therapy and/or bisphosphonate therapy according to the advice of the attending oncologists (Table S1). This research project was approved by the Ethics Committee of Changzheng Hospital (Shanghai, China), and informed consent was obtained from each participating patient. All procedures performed in the research involving human participants were in accordance with the Declaration of Helsinki.
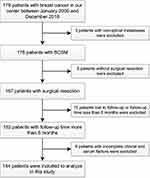 |
Figure 1 Flowchart of patients included in this study. |
The medical records of the patients, including clinical and operative reports, pathology reports and laboratory reports were retrospectively reviewed and analyzed. All patients were followed up every 3 months for the first 6 months after surgery, then every 6 months for the next 2 years and annually thereafter. The enrolled patients were all followed up for at least 6 months or until death and the final statuses (died of disease/alive with disease) were acquired through telephone interviews.
Variables
Demographic data and clinical information were collected from the medical records of the patients. The duration of spinal symptoms was from the onset of neurological symptoms to clinical treatment. Spine metastasis-free survival (SMFS) refers to time between the primary diagnosis and spinal metastasis. The Eastern Cooperative Oncology Group (ECOG) performance status, and the pre- and postoperative Frankel score were used to evaluate the neurological status (Table S2). Tomita Score, revised Tokuhashi Score and modified Bauer Score were assessed preoperatively. Postoperatively, tumor samples were subjected to pathological evaluation by two pathologists independently and ER, PR and Her2 statuses were recorded.
Four types of laboratory blood tests were retrieved for prognostic prediction. Blood cell parameters included the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR). Blood biochemistry indexes included the albumin-to-globulin ratio (AGR), calcium (Ca2+), alkaline phosphatase (ALP) and lactic dehydrogenase (LDH). Hemostatic parameters included D-dimer (D-D). Tumor markers included CA125 and CA153.
Statistical Methods
Statistical calculations were carried out using SPSS for Windows, version 22.0.0 (SPSS, IBM corp., New York, USA), GraphPad Prism for Windows, version 7 (GraphPad Software Inc., La Jolla, CA, USA) and R project version 3.4.3 (http://www.r-project.org/). Overall survival (OS) was measured as the date from spinal metastasis to cancer-related death, or to March 2019 for living patients. Age and the duration of spinal symptoms were switched into binary variables. The optimal cut-off values were according to their median values. X-tile 3.6.1 software 20 (Yale University, New Haven, CT, USA) was performed to determine the optimal cut-off values for NLR, PLR and LMR, while other blood markers were determined by their clinical standards derived by the Clinical Laboratory Department of the said hospital. All clinical factors were subjected to univariate and multivariate Cox regression analyses to identify whether they were independent prognostic factors. OS was calculated by the Kaplan–Meier method, and the difference of variables was compared using Log rank tests. A P value (two-sided) of <0.05 was considered statistically significant.
R software version 3.4.3 with related packages was then used to formulate a nomogram model known as Changzheng (CZ) Score based on the results of multivariate analysis. The risk score for each significant factor was created by β coefficient, ranging from 0 to 100 points. The corresponding predicted 6-, 12-, 24-, and 36-month OS (proportion) were also calculated. According to the points-patient distribution diagram and the OS-points scatter diagram, the cut-off values of the total points were determined to distinguish different prognostic conditions. Patients were thus divided into three groups and the survival curves were mapped by the Kaplan–Meier method. Predictive accuracy was assessed by discrimination and calibration. Harrell’s concordance index (C-index) was measured for discrimination, while calibration curves were graphed to evaluate the consistency of the nomogram-predicted OS and actual OS.
Time-dependent receiver operating characteristic (TDROC) curves were constructed to evaluate the predictive performance of CZ Score and other score systems, using R software version 3.4.3 with “survivalROC” and “timeROC” packages. ROC curves for predicting 6-, 12-, 24-, and 36-month OS in each of the four score systems were depicted and compared. An integrated area under the curve (IAUC) from TDROC curves was graphed.
Results
Demographics and Clinical Characteristics
A total of 144 women patients with a mean age of 53.08±9.53 (median 53, range 27–77) years were retrospectively reviewed (Table 1). The mean duration of spinal symptoms was 27.83±51.90 (median 12, range 4–384) weeks and the mean SMFS was 55.67±52.73 (median 44.5, range 0–240) months. The metastatic locations in these patients were recorded, including 36 in the cervical spine, 82 in the thoracic spine, 61 in the lumbar spine, and 15 in the sacrum. Of the 144 included patients, 99 (69%) suffered varying degrees of multiple spinal metastases. According to the ER, PR and Her2 status, the primary cancer was classified as the luminal sub-type in 84 patients, Her2 sub-type in 26 patients, and the triple-negative sub-type in 34 patients. During the entire follow-up period, 82 patients succumbed to the disease, and the other 62 patients were alive.
 |
Table 1 Demographics and Clinical Characteristics of 144 Patients with Breast Cancer Spine Metastases |
Univariate and Multivariate Analyses of Prognostic Factors for OS
Altogether 27 parameters were subjected to univariate analysis for prognostic prediction, and of them 18 were identified as positive (P<0.05) (Table 2). These factors were then submitted to the Cox proportional hazard model for multivariate analysis, and ultimately 7 independent factors remained highly significant, including postmenopausal [HR: 1.697 (1.040–2.770), P=0.034], visceral metastasis [HR: 1.980 (1.110–3.532), P=0.021], preoperative Frankel Score [HR: 1.730 (1.239–2.416), P=0.001], ER status [HR: 0.531 (0.321-0.880), P=0.014], PLR [HR: 2.021 (1.170–3.493), P=0.012], LMR [HR: 0.172 (0.093-0.318), P<0.001], and AGR [HR: 0.531 (0.316-0.893), P=0.017] (Table 2).
 |
Table 2 Univariate and Multivariate Analyses of the Clinical and Serum Factors Affecting Overall Survival of Patients with Breast Cancer Spine Metastases |
Construction and Evaluation of the CZ Score Model
The 7 independent factors for OS were further analyzed to establish a CZ Score nomogram model (Figure 2), with a C-index of 0.834 (95% CI, 0.789-0.890) that indicated good performance for OS prediction of patients with BCSM. In addition, the calibration curves for the probability of 6-, 12-, 24- and 36-month OS showed good consistency between nomogram prediction and actual observation (Figure 3). Thus, we calculated the CZ Score for each patient and graphed corresponding points-patient distribution diagrams and OS-points scatter diagrams (Figure 4A and B). Patients were then divided into three groups as Groups A, B and C, which represented good prognosis (≤110 points), moderate prognosis (110–240 points) and poor prognosis (>240points) respectively. Patients in Group A had significantly longer OS than those in the other two groups (Log rank test, p<0.001) (Figure 4C).
 |
Figure 2 CZ Score nomogram model for prognostic prediction of patients with Breast Cancer Spine Metastases. |
 |
Figure 3 The calibration curves for the probability of (A) 6-month, (B) 12-month, (C) 24-month and (D) 36-month overall survival. |
Comparison with Other Scoring Systems
TDROC curves were used for comparison of CZ Score with Tomita Score, revised Tokuhashi Score, modified Bauer Score and NESMS (Figure 5). The result showed that CZ Score had the best performance and possessed the largest IAUC value than the other scoring systems. At indicated time points, AUCs of CZ Score were significantly higher than those of the other scoring systems except for the revised Tokuhashi Score at 6- and 12-month (Table 3, Figure S1).
 |
Table 3 Integrated Area Under the Curve for the CZ Score, Tomita Score, Revised Tokuhashi Score, Modified Bauer Score and NESMS from Time-Dependent ROC Analysis |
 |
Figure 5 Time-dependent ROC curves of CZ Score, Tomita Score, revised Tokuhashi Score, modified Bauer Score and NESMS. |
Discussion
Preoperative prognostic prediction of patients with spinal metastatic lesions plays a vital role in surgical decision-making. However, the currently available scoring systems lack sensitivity and specificity for modern patients with complex manifestations in clinical practice because most of them were constructed about 40 years ago.10,11 Ahmed et al reviewed a series of 176 patients and compared nine scoring systems, finding that the prognostic value of the revised Tokuhashi Score was better than that of Tomita Score and modified Bauer Score.29 A study by Pollner et al11 demonstrated that Tomita Score and modified Bauer Score separated the classes of patients with good and moderate prognosis, and patients with poor conditions were easily identified with revised Tokuhashi Score.11 As most of the above-mentioned systems were constructed on a relatively small number of patients with heterogeneous types of primary tumors, they lack specificity and sensitivity for indicated malignancies. The number of patients in Tomita Score is 67 including 14 cases of BCSM.7 Similarly, of the 128 cases in the revised Tokuhashi Score, 19 had primary breast cancer, and of the 88 cases with spinal metastasis in Bauer Score, 14 were metastasized from breast cancer.30,31 In addition, even for indicated types of primary tumors, their clinical prognosis could be quite different due to specific gene expression characteristics. For example, patients with the luminal sub-type of breast cancer were considered to have better prognosis than patients with the triple-negative sub-type.17,18 The research of molecular markers of spinal metastatic tumors has also proved that such markers can alter the management/surgical paradigms utilized.32 A new score model called NESMS has attracted much attention in recent years, which adds two indexes of ambulatory function and serum albumin based on the modified Bauer Score.12 In general, the determination of the appropriate treatment of spinal metastatic tumors should be comprehensive and persuasive. The NOMS (neurologic, oncologic, mechanical, and systemic) framework just provides such a concept in treatment selection.33 In the current study, we attempted to establish a new nomogram scoring system for predicting the patient’s life expectancy based on multidimensional factors including the clinical features, molecular sub-types and blood indexes, which is named as CZ Score. Based on retrospective review and analysis of BCSM 144 patients, this CZ scoring system exhibited better performance as compared with the currently available scoring systems. The detailed data about the differences between our CZ scoring system and the other scoring systems are shown in Table 4.
 |
Table 4 Differences Between CZ Score, Tomita Score, Revised Tokuhashi Score, Modified Bauer Score and NESMS from Time-Dependent ROC Analysis |
We identified postmenopausal, visceral metastasis, preoperative Frankel Score, ER status, PLR, LMR and AGR as independent prognostic factors for patients with BCSM by using univariate and multivariate analyses. The menopause status has never been included in any previous score system. However, this index is important for patients with BCSM, because most patients are female and breast cancer is closely related to sex hormones. Another reason for the low OS of postmenopausal patients may be that they were relatively obese with cardiovascular dysfunction and low bone mineral density.34 Due to the decline of the ovarian function and the exhaustion of estrogen secretion, menopause has become a key turning point for patients’ prognosis.35 In patients with visceral metastasis, it is usually indicated that the disease has progressed to the terminal stage, when various treatment methods are often difficult to achieve the desired effect, so the survival time of patients is not satisfactory.36 Also, Frankel score is a widely accepted indicator for prognostic prediction.37 Patients in grade D and E have better muscle and nerve functions, which indicate better prognoses.
In our previous studies,28,38 we identified that patients with ER-positive breast cancer or luminal breast cancer (mainly represented by positive ER) were more prone to form bone metastasis. However, these patients also possess a relatively better prognosis than patients with other sub-types. In this study, we further demonstrated the similar conclusion in a larger series of patients and identified the ER status as a protective prognostic factor.
The activation of platelets is supposed to play a key role in tumor progression, angiogenesis, extracellular matrix degradation and release of adhesion molecules and growth factors.39 Also, an increased blood platelet count may be a reflection of systemic inflammation response to the tumor.40 A decrease in lymphocyte count is considered to be responsible for an insufficient immunologic reaction to the tumor, thus promoting tumor progression and metastasis.41,42 Monocytes are known to infiltrate tumors and differentiate into tumor-associated macrophages, such as osteoclasts in bone tumors, which are involved in tumor proliferation, invasion, metastasis, neovascularization, and recurrence.42 In this study, we demonstrated that high PLR and low LMR (which indicate a high level of platelets and monocytes, and a low level of lymphocytes) are associated with poor survival in patients with BCSM. AGR is reported as a prognostic indicator for several cancers in prior studies, which is consistent with our finding that a lower preoperative AGR level was correlated with poor prognosis. The low albumin status usually reflects poor nutrition and chronic inflammation, and the high non-albumin protein status also reveals inflammation.43
Besides, some important tumor markers in univariate analysis were excluded as independent factors by multivariate analysis. One possible reason may be that although these tumor markers were recognized to be closely related to primary tumors, they were less accurate for metastatic patients.44,45 Based on the 7 prognostic factors, we constructed the CZ Score model. Patients were classified into three groups as Group A (≤110 points, median survival 30 months), B (110–240 points, median survival 16 months), and C (>240points, median survival 6.5 months). The ROC curves and the matching AUCs revealed that the CZ Score has sufficient sensitivity and specificity for prognostic prediction and surgical decision-making of patients with BCSM.
There are several limitations in the current study. First, our study was a retrospective review of patients in a single center. Besides, as all the included patients received surgical interventions and contributed to a relatively better prognosis, potential selective bias was unavoidable. Finally, the study lacked validation to confirm whether it could be applied in other relative cases. However, our CZ Score model can still provide some clinical implications, and prospective studies with larger numbers of patients are needed for further validation.
Funding
This work was supported by National Natural Science Foundation of China (81972505, 81871470, 81902732) and the key project funding in the basic research field of the Shanghai Municipal Science and Technology Commission (17JC1400903).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. McDonald ES, Clark AS, Tchou J, et al. Clinical diagnosis and management of breast cancer. J Nucl Med. 2016;57(Suppl 1):9S–16S. doi:10.2967/jnumed.115.157834
2. Tyagi NK, Dhesy-Thind S. Clinical practice guidelines in breast cancer. Curr Oncol. 2018;25(Suppl 1):S151–S160. doi:10.3747/co.25.3729
3. Wright E, Ricciardi F, Arts M, et al. Metastatic spine tumor epidemiology: comparison of trends in surgery across two decades and three continents. World Neurosurg. 2018;114:e809–e817. doi:10.1016/j.wneu.2018.03.091
4. Lu VM, Alvi MA, Goyal A, et al. The potential of minimally invasive surgery to treat metastatic spinal disease versus open surgery: a systematic review and meta-analysis. World Neurosurg. 2018;112:e859–e868. doi:10.1016/j.wneu.2018.01.176
5. Kaloostian PE, Yurter A, Zadnik PL, et al. Current paradigms for metastatic spinal disease: an evidence-based review. Ann Surg Oncol. 2014;21(1):248–262.
6. Eleraky M, Papanastassiou I, Vrionis FD. Management of metastatic spine disease. Curr Opin Support Palliat Care. 2010;4(3):182–188. doi:10.1097/SPC.0b013e32833d2fdd
7. Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001;26(3):298–306. doi:10.1097/00007632-200102010-00016
8. Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 1990;15(11):1110–1113.
9. Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases prognostication in 241 patients. Acta Orthop Scand. 1995;66(2):143–146. doi:10.3109/17453679508995508
10. Tan KA, Tan JH, Zaw AS, et al. Evaluation of prognostic factors and proposed changes to the modified Tokuhashi score in patients with spinal metastases from breast cancer. Spine (Phila Pa 1976). 2018;43(7):512–519. doi:10.1097/BRS.0000000000002350
11. Pollner P, Horváth A, Mezei T, et al. Analysis of four scoring systems for the prognosis of patients with metastasis of the vertebral column. World Neurosurg. 2018;112:e675–e682. doi:10.1016/j.wneu.2018.01.124
12. Schoenfeld AJ, Le HV, Marjoua Y, et al. Assessing the utility of a clinical prediction score regarding 30-day morbidity and mortality following metastatic spinal surgery: the New England Spinal Metastasis Score (NESMS). Spine J. 2016;16(4):482–490.
13. Sihto H, Lundin J, Lundin M, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res. 2011;13(5):R87. doi:10.1186/bcr2944
14. Endo T, Uzawa K, Suzuki H, et al. Characteristic gene expression profiles of benign prostatic hypertrophy and prostate cancer. Int J Oncol. 2009;35(3):499–509.
15. Hotfilder M, Mallela N, Seggewiß J, et al. Defining a characteristic gene expression set responsible for cancer stem cell-like features in a sub-population of ewing sarcoma cells CADO-ES1. Int J Mol Sci. 2018;19:12. doi:10.3390/ijms19123908
16. Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–3114. doi:10.1158/0008-5472.CAN-07-5644
17. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi:10.1038/35021093
18. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi:10.1056/NEJMra1001389
19. Yang M, Xu W, Liu T, et al. Development and validation of a novel survival prediction model in patients with spinal metastasis from non-small cell lung cancer. Spine (Phila Pa 1976). 2019;44(4):246–257. doi:10.1097/BRS.0000000000002816
20. Lafuente-Sanchis A, Zúñiga Á, Estors M, et al. Association of PD-1, PD-L1, and CTLA-4 gene expression and clinicopathologic characteristics in patients with non-small-cell lung cancer. Clin Lung Cancer. 2017;18(2):e109–e116. doi:10.1016/j.cllc.2016.09.010
21. Van Laere S, Beissbarth T, Van der Auwera I, et al. Relapse-free survival in breast cancer patients is associated with a gene expression signature characteristic for inflammatory breast cancer. Clin Cancer Res. 2008;14(22):7452–7460. doi:10.1158/1078-0432.CCR-08-1077
22. Peng D, Zhang CJ, Gong YQ, et al. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. 2018;8(1):794. doi:10.1038/s41598-018-19146-y
23. Liu D, Huang Y, Li L, et al. High neutrophil-to-lymphocyte ratios confer poor prognoses in patients with small cell lung cancer. BMC Cancer. 2017;17(1):882. doi:10.1186/s12885-017-3893-1
24. Chen WZ, Shen JF, Zhou Y, et al. Clinical characteristics and risk factors for developing bone metastases in patients with breast cancer. Sci Rep. 2017;7(1):11325. doi:10.1038/s41598-017-11700-4
25. Haddad AQ, Leibovich BC, Abel EJ, et al. Preoperative multivariable prognostic models for prediction of survival and major complications following surgical resection of renal cell carcinoma with suprahepatic caval tumor thrombus. Urol Oncol. 2015;33(9):
26. Zhao WC, Zhang HB, Yang N, et al. Preoperative predictors of short-term survival after hepatectomy for multinodular hepatocellular carcinoma. World J Gastroenterol. 2012;18(25):3272–3281.
27. Li J, Li B, Zhou P, et al. Nomograms for prognostic factors of spinal giant cell tumor combining traditional clinical characteristics with inflammatory biomarkers after gross total resection. Oncotarget. 2017;8(49):86934–86946. doi:10.18632/oncotarget.21168
28. Zhao C, Zhang Z, Zhong N, et al. Outcomes and prognostic factors for surgically treated patients with breast cancer spine metastases. J Bone Oncol. 2018;12:38–43. doi:10.1016/j.jbo.2018.03.003
29. Ahmed AK, Goodwin CR, Heravi A, et al. Predicting survival for metastatic spine disease: a comparison of nine scoring systems. Spine J. 2018;18(10):1804–1814. doi:10.1016/j.spinee.2018.03.011
30. Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005;30(19):2186–2191. doi:10.1097/01.brs.0000180401.06919.a5
31. Leithner A, Radl R, Gruber G, et al. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J. 2008;17(11):1488–1495. doi:10.1007/s00586-008-0763-1
32. Goodwin CR, Abu-Bonsrah N, Rhines LD, et al. Molecular markers and targeted therapeutics in metastatic tumors of the spine: changing the treatment paradigms. Spine (Phila Pa 1976). 2016;41(Suppl 20):S218–S223. doi:10.1097/BRS.0000000000001833
33. Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18(6):744–751. doi:10.1634/theoncologist.2012-0293
34. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397.
35. Ferreira PP, Vespoli HL, Almeida-Filho BS, et al. Low bone mineral density is associated with breast cancer in postmenopausal women: a case-control study. Climacteric. 2017;20(5):491–497. doi:10.1080/13697137.2017.1329290
36. Petteys RJ, Spitz SM, Goodwin CR, et al. Factors associated with improved survival following surgery for renal cell carcinoma spinal metastases. Neurosurg Focus. 2016;41(2):E13. doi:10.3171/2016.5.FOCUS16145
37. Choi D, Fox Z, Albert T, et al. Prediction of quality of life and survival after surgery for symptomatic spinal metastases: a multicenter cohort study to determine suitability for surgical treatment. Neurosurgery. 2015;77(5):
38. Zhao C, Lou Y, Wang Y, et al. A gene expression signature-based nomogram model in prediction of breast cancer bone metastases. Cancer Med. 2019;8(1):200–208. doi:10.1002/cam4.1932
39. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.024
40. Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524–2530. doi:10.1038/bjc.2014.163
41. Goto W, Kashiwagi S, Asano Y, et al. Predictive value of lymphocyte-to-monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer. 2018;18(1):1137. doi:10.1186/s12885-018-5051-9
42. Hu RJ, Liu Q, Ma JY, et al. Preoperative lymphocyte-to-monocyte ratio predicts breast cancer outcome: a meta-analysis. Clin Chim Acta. 2018;484:1–6. doi:10.1016/j.cca.2018.05.031
43. Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol. 2016;34(11):
44. Wang W, Xu X, Tian B, et al. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–55. doi:10.1016/j.cca.2017.04.023
45. Wang G, Qin Y, Zhang J, et al. Nipple discharge of CA15-3, CA125, CEA and TSGF as a new biomarker panel for breast cancer. Int J Mol Sci. 2014;15(6):9546–9565. doi:10.3390/ijms15069546
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

