Back to Journals » Journal of Blood Medicine » Volume 12
Prognostic Factors and Clinical Considerations for Iron Chelation Therapy in Myelodysplastic Syndrome Patients
Received 17 June 2021
Accepted for publication 15 September 2021
Published 3 December 2021 Volume 2021:12 Pages 1019—1030
DOI https://doi.org/10.2147/JBM.S287876
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Sarah Parisi,1 Carlo Finelli2
1IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli”, Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università di Bologna, Bologna, Italy; 2IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli”, Bologna, Italy
Correspondence: Sarah Parisi Email [email protected]
Abstract: Iron chelation therapy (ICT) is an important tool in the treatment of transfusion-dependent lower-risk myelodysplastic syndrome (MDS) patients. ICT is effective in decreasing iron overload and consequently in limiting its detrimental effects on several organs, such as the heart, liver, and endocrine glands. Besides this effect, ICT also proved to be effective in improving peripheral cytopenia in a significant number of MDS patients, thus further increasing the clinical interest of this therapeutic tool. In the first part of the review, we will analyze the toxic effect of iron overload and its mechanism. Subsequently, we will revise the clinical role of ICT in various subsets of MDS patients (low, intermediate, and high risk MDS, patients who are candidates for allogeneic stem cell transplantation).
Keywords: iron overload, myelodysplastic syndrome, iron chelation therapy, hepcidin
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal, acquired diseases of the hematopoietic stem cell, characterized by a variable degree of peripheral cytopenias due to ineffective erythropoiesis and by the risk of progression to acute myeloid leukemia (AML).
The risk of progression to AML is predicted at diagnosis by several scores, which allow classification of MDS patients in low, intermediate, and high-risk and to choose the more suitable therapeutic approach.1,2
The International Prognostic Scoring System (IPSS) was designed in order to classify MDS patients in different risk groups and to predict the risk of AML transformation and overall survival (OS). IPSS is applicable at diagnosis and allows the classification of MDS patients into four risk groups (low, intermediate 1 and 2, and high risk), according to the percentage of bone marrow blasts, cytogenetic features, and number of cytopenias.
Subsequently, other risk scores were proposed, in order to better characterize patients; among the other risk scores, the revised IPSS (IPSS-R) divides MDS patients into five risk groups.2
Low-risk MDS are characterized by peripheral cytopenias and most patients become red blood cell (RBC) transfusion dependent during their disease history, thus determining iron overload and consequent organ damage.
MDS patients are frequently transfusion-dependent and iron overload can represent a significant complication due to iron toxicity on the liver, heart and the endocrine system. It was also demonstrated that iron overload has a detrimental effect on the hematopoietic bone marrow, thus enhancing ineffective erythropoiesis and favoring the appearance of genomic abnormalities, with an increased risk of AML transformation.3
Several observations suggested that iron chelation therapy may have a positive effect on MDS patients outcome and on the basis of these observations a randomized trial was conducted in order to clarify the role of iron chelation therapy on low and intermediate-risk MDS patients.4
International guidelines also recommend consideration of iron chelation therapy in high-risk MDS patients who are candidates for allogeneic stem cell transplantation or in high risk patients who respond to disease-modifying therapies, such as hypomethylating agents,5,6 in order to minimize the detrimental effect of iron overload.
Iron chelation therapy in MDS patients is indicated until the patient becomes transfusion independent and/or until normalization of parameters indicating iron overload.
Here we review data about iron overload and iron chelation therapy in transfusion-dependent low and intermediate-risk MDS patients. This work is mainly focused on the clinical use of ICT in low, intermediate, and high risk MDS patients according to the available data from literature.
Iron Overload Mechanisms in MDS Patients
Iron overload is a frequent finding in MDS patients7 and several studies demonstrated its detrimental effect on overall and leukemia-free survival due to iron toxic effect on several organs, like the liver, heart and endocrine glands.8 Chronic transfusion therapy is the most important risk factor for iron overload, even if it was noticed that some patients develop iron overload at an earlier stage of the disease, even before receiving transfusions, thus suggesting a dysregulation of iron homeostasis factors as a cause.9 Chronic transfusion regimen and ineffective erythropoiesis are the main drivers of iron overload in MDS patients.
Several studies demonstrated that hepcidin, the small hepatic peptide hormone able to regulate iron absorption by binding to its receptor ferroportin, is downregulated in hematologic disorders characterized by ineffective erythropoiesis, first of all in transfusion-dependent beta-thalassemia. A similar pathway is supposed to be involved in iron overload in MDS patients.
Hepcidin action is mediated by its binding to ferroportin, that is highly expressed on duodenal enterocytes and macrophages and that is internalized and degraded after hepcidin binding, thus blocking iron absorption. Hepcidin production by the liver is enhanced by increased plasma and hepatic iron levels and by inflammation, while it is downregulated in the case of ineffective erythropoiesis. It has been postulated that hepcidin downregulation has a key role in the establishment of iron overload in diseases characterized by enhanced ineffective erythropoiesis, such as MDS. Santini et al analyzed serum hepcidin levels of 113 MDS patients and demonstrated the lowest levels in refractory anemia with ring sideroblasts (RARS) and the highest in refractory anemia with excess blasts (RAEB) and in chronic myelomonocytic anemia (CMML).10 Notably, RARS patients are known to have the highest levels of toxic non-transferrin-bound iron.
When hepcidin is downregulated, intestinal iron absorption and iron release by macrophages is enhanced, resulting in iron overload.11–21 The excess of absorbed iron is in part utilized for erythropoiesis, but the increased number of erythroid precursors typical of MDS is not able to fully utilize iron, which accumulates as non-transferrin-bound iron (NTBI) and causes organ damage. Ultimately, iron overload is maintained by hepcidin inhibition mediated by enhanced bone marrow proliferation and iron is inadequately used due to ineffective erythropoiesis and defective red blood cell (RBC) maturation.
As stated by Santini et al, a diverse degree of hepcidin downregulation has been recently described in some MDS subtypes, in particular the lowest serum levels of hepcidin were observed in patients with refractory anemia with ring sideroblasts (MDS-RS) and refractory cytopenia with multilineage dysplasia and ring sideroblasts (MDS-MLD-RS). Clinically, patients affected by MDS-MLD-RS are known to develop iron overload even before being transfusion-dependent and to have a pretransfusional hepcidin/ferritin ratio significantly decreased, with hepcidin levels inappropriately low for the degree of iron loading (evaluated by serum ferritin levels).10,22 Moreover, MDS-MLD-RS subtype (according to the WHO 2008 classification), hallmarked by the high prevalence of SF3B1 mutation and bone marrow erythroid precursors with iron deposits in their mitochondria (ring sideroblasts), are characterized by a particularly high degree of ineffective erythropoiesis, thus enhancing hepcidin inhibition.23,24 Iron incorporation within mitochondria prevents heme production, with consequent hypoxia and enhances ineffective erythropoiesis and hepcidin downregulation.25
Some genetic alterations have been associated with an increased risk of developing iron overload in MDS patients.26
SF3B1 mutation is typical of MDS-RS and MDS-MLD-RS and it is known to inhibit erythropoiesis by the dysregulation of RNA splicing of the transcription factors TAL1 and GATA1.27 SF3B1 mutation has a direct effect on iron accumulation, as it is associated with splicing alterations of some genes that regulate iron metabolism.28,29 Some patients with MDS show TET2 mutation, which has been correlated with the dysregulation of several genes involved in iron metabolism.30
The potential role of hemochromatosis gene mutations on iron regulation have been investigated in MDS patients, but no association with clinical outcomes could be established.31
Besides ineffective erythropoiesis and hepcidin regulation, transfusion therapy is the most important risk factor for iron overload in MDS patients, but it is at the same time a hallmark of supportive care in low and intermediate-1 risk MDS patients. Every unit of transfused blood contains about 200 mg of iron, so patients who require four RBC units per month will receive about 10 g iron per year, whereas natural iron losses are only 1 to 2 mg daily and the normal amount of body iron is 4 g. Therefore, given that iron overload can frequently develop in MDS patients, iron chelation therapy can be a useful tool to counteract this event and its harmful consequences.
Toxic Effects of Iron Overload
Iron overload may cause several toxic effects on different organs.
Heart disease, in particular arrhythmias and heart failure, is one of the more common causes of nonhematologic morbidity and mortality in MDS patients32,33 who are, per se, more at risk of heart failure because of age and anemia.
Pascal et al demonstrated that 17–27% of transfusion-dependent MDS patients develop heart disease with T2* magnetic resonance imaging-documented iron overload.34
Furthermore, there is some evidence that clonal hematopoiesis and the consequent inflammatory state,35–37 as well as vascular impairment induced by NTBI and ROS production predispose to atherosclerotic cardiovascular disease.38–42
The role of iron chelation therapy in delaying cardiac morbidity has been suggested by retrospective studies.43
The results of the only prospective study by Angelucci et al confirmed that iron chelation therapy with deferasirox is able to prolong significantly event free survival in patients with low- to intermediate-1-risk MDS compared to placebo.4
Iron overload first becomes evident in the liver, that is an iron storage organ, when hepatic stored iron is tenfold increased compared to normal concentration (15–20 mg/g dry weight vs normal: 2 mg/g dry weight).44,45 Liver iron overload is present in about 80% of transfusion-dependent MDS patients and correlates to fibrosis and liver dysfunction, which lead to decreased overall survival and dismal prognosis. Iron chelation therapy can reduce the rate of hepatic-related deaths in MDS patients46 and probably can prevent cirrhosis.47
MDS patients have an increased risk of infections compared to the general population because of cytopenias, in particular neutropenia and impairment of the immune function. Some studies show an increased incidence of bacterial, viral and fungal infections and infection-related mortality in transfusion-dependent MDS patients with signs of iron overlaod,48 suggesting a role of iron overload, but the exact mechanism is not fully understood (Figure 1). Two main mechanisms are thought to be related to increased risk of infections and iron overload: free unbound iron (NTBI) can be used by pathogens for their growth and excess iron is able to impair immune function.49
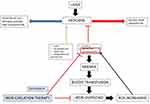 |
Figure 1 Iron overload and its effects in myelodysplastic syndrome. |
Iron can impair the function of cellular immunity, in particular it has a detrimental effect on macrophages, neutrophils and lymphocytes and decreases the production of cytokines and nitric oxide involved in the regulation of the immune response.
There is some evidence that iron chelation therapy is able to delay the occurrence of infections and to decrease the incidence of infection-related mortality, but further studies are needed to clarify this point.50–55
Iron Chelation Therapy: Indications in Low-risk MDS Patients
As previously discussed, iron overload can rapidly develop in MDS patients and it is recognized to be toxic for several organs and even for the bone marrow niche, thus favoring ineffective erythropoiesis, accumulation of genomic alterations and increased risk of AML transformation.3
So, iron chelation therapy appears to be an important tool in MDS patients, in order to prevent or minimize the detrimental effects of iron overload.
Patients who could benefit from iron chelation therapy would be studied in order to demonstrate and evaluate the degree of iron overload and organ damage prior to therapy onset and during treatment. The diagnostic work-up includes several analyses, in particular it is important to establish the exact amount of the transfusion regimen and to calculate the consequent iron intake.
Serum ferritin and transferrin saturation represent important tools to evaluate iron overload and to monitor its trend during therapy. Even in the absence of validated data, a serum ferritin level above 1000 ng/mL is considered to be suggestive for iron overload, but it is recommended to evaluate ferritin together with transferrin saturation, as ferritin levels can be inappropriately high in inflammatory states and in the presence of hepatocellular necrosis.56 Transferrin saturation levels above 60–70% are correlated with free iron in plasma as non-transferrin-bound iron (NTBI) and labile plasma iron (LPI), a subcomponent of NTBI, that is a potent redox-active form which causes the increase of intracellular radical oxygen species (ROS) with a consequent intracellular oxidative stress.57 Currently, standard tests to evaluate NTBI and LPI are not available for clinical practice.
The prospective EPIC study (evaluation of patients’ iron chelation with exjade) was conducted in order to evaluate the possibility of using plasma markers other than serum ferritin (SF) to assess iron overload in transfusion-dependent thalassemia, sickle cell disease, and MDS. The study concluded that transferrin saturation (TfSat) is not a good marker of iron overload during chelation therapy while transferrin levels or total iron binding capacity (TIBC) or LPI could represent a valid tool for monitoring patients, even if further studies are needed for conclusive data.58
The assessment of organ damage is possible by performing magnetic resonance studies of the liver, heart and pancreas and can be repeated during therapy to evaluate its efficacy.
Based on these considerations, international guidelines recommend to start iron chelation therapy in low-risk MDS patients when one or more of these conditions are present:5,59,60
- Transfusion dependency and at least 20 RBC units transfused (currently, it is under discussion whether to start chelators before the 20 transfused units based on the observation that oxidative stress may be present even earlier)61
- Serum ferritin levels above 1000 ng/dL
- Life expectancy longer than 12 months
About discontinuation of chelators, current guidelines recommend to go on with therapy as long as the transfusional need is present.62
Currently, two different iron chelators are available for the treatment of iron overload in MDS patients: deferoxamine and deferasirox. These agents differ in route and timing of administration as well as side-effect profiles. We will explain these drugs in more detail in the paragraph “Iron chelation: options”.
Iron Chelation Therapy: Indications in High-risk MDS Patients
High-risk MDS patients, according to IPSS, R-IPSS and WPSS prognostic scores, account for about 20–30% of the entire MDS population. After the introduction of disease-modifying therapies such as hypomethylating drugs the outcome among this cohort of patients improved significantly63 and the impact of iron overload and iron toxicity became evident, with a significant number of deaths due to infectious complications and cardiac diseases.64
An increasing number of patients can be considered as candidates for iron chelation therapy, with the same indications regarding starting and discontinuation as low-risk MDS, even if some patients will not receive chelation therapy because of a short life expectancy or renal and/or hepatic impairment.
Rose et al published a retrospective study on 51 intermediate to very high risk, transfusion dependent, MDS patients (according to R-IPSS prognostic score) treated with deferasirox in association (71%) or not (29%) with azacitidine. Patients who received deferasirox had a significant decrease in serum ferritin and an improvement in liver function and in one case a hematological improvement was observed.65 The authors concluded that the results of the study were comparable, in terms of safety and efficacy, with those observed in lower-risk MDS.
Several studies showed that deferasirox treatment can reduce infection incidence among high-risk MDS patients6,66 and, more intriguingly, there is some evidence of a synergistic effect of deferasirox and hypomethylating agents, since it was observed that iron overload and consequent oxidative stress is able to enhance hypermethylation of important tumor suppressor genes.67
On the basis of the abovementioned data, it could be appropriate to propose iron chelation therapy to intermediate and high-risk MDS patients if they have the following characteristics:
- Good performance status
- Age ≤65 years
- No significant comorbidities and adequate liver and renal function
- Patients candidates to receive disease-modifying therapies (hypomethylating agents and/or allogeneic stem cell transplantation).
Iron Chelation Therapy: Indications in MDS Patients Candidate for Allogeneic Stem Cell Transplantation
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative therapy for MDS patients, but only a minority of fit patients can be considered for this therapeutic option because of high risk of transplant-related mortality and morbidity due to the advanced age of most MDS patients.
Several studies confirmed that patients who have iron overload show an inferior outcome post HSCT68,69 and a prospective, multicenter study (ALLIVE) demonstrated a significant correlation between high levels of NTBI at baseline and the incidence of non-relapse mortality and between NTBI positivity and inferior overall survival (OS).70 It has been observed that conditioning regimen itself can increase NTBI levels and that NTBI/LPI levels drop after engraftment, when iron can be utilized again by restored erythropoiesis.
Given the detrimental effect of high levels of NTBI on transplant outcome, in terms of higher incidence of infections and poor engraftment, patients who are candidates for HSCT should receive iron chelation therapy pre and peritransplantation.71,72
In 2019 Cremers et al showed that the reduction of iron overload within six months after HSCT, and not prior to HSCT, can improve relapse-free survival (90% in chelated patients vs 56% in untreated patients).73
Iron Chelation Therapy: Options
Deferoxamine, the first approved iron chelator, is characterized by limited use in MDS patients because it can be administered only by intravenous, intramuscular, or subcutaneous injection and it has a very short half-life. Therefore, to be effective it requires a prolonged daily subcutaneous or intravenous infusion of 8–12 h. For this reason the use of deferoxamine in MDS patients has been limited and there are no randomized studies, that were conducted only in beta-thalassemia patients.
Side effects are injection reactions and ocular and otic toxicity.74
Another iron chelator is deferiprone, which has a very limited use in MDS patients because it can be responsible for neutropenia as a major side effect.
The most widely used iron chelator in MDS patients is deferasirox (DFX). DFX is an N-substituted bis-hydroxyphenyl-triazole, a class of tridentate iron chelators and it is able to bind ferric ions forming a soluble complex with high binding affinity for iron in its trivalent form.75–80 Preclinical and clinical studies demonstrated that DFX is two to five times more effective than deferoxamine in binding and excreting iron and that it is able to reduce liver and cardiac iron burden.81–83
Angelucci et al published the results of the only randomized, placebo-controlled, study—the TELESTO study—on the use of deferasirox in low and intermediate-1-risk MDS patients.4
The authors demonstrated that patients receiving DFX had a significantly longer median event free survival (EFS) than patients receiving placebo (approximately one year), even if they were unable to explain this result with a specific improvement in one of the main organs affected by iron overload.
Serum ferritin level monitoring seemed to be the most suitable way to evaluate clinical benefit of DFX. The study did not provide a conclusive answer about the observation that DFX can induce hematological improvement.84 On the basis of the 2006 IWG criteria, hematological improvement was observed in 27 of 121 (22.3%) deferasirox recipients vs 14 of 68 (20.6%) placebo recipients; this analysis was performed excluding patients receiving concomitant, potential erythroid-modifying treatments.
The study also confirmed the safety of DFX, increased levels of serum creatinine being the most frequent adverse event.
Some concerns about the TELESTO study have to be reported. Even if the TELESTO trial had been designed as a Phase 3 randomized study, slow enrollment and high dropout forced investigators to downgrade it to Phase 2 trial providing only exploratory value of evidence regarding outcomes. Also the issue of EFS benefit was put in question,85 since significant separation of EFS curves occurred only after three years of therapy when the study was profoundly affected by dropout and crossover between the two groups.
Several studies demonstrated that DFX can induce additional effects other than the decrease of serum ferritin levels and tissue deposition of iron. These additional effects are about overall survival, the risk of leukemia transformation, and hematological improvement.
The effect of iron chelation therapy in MDS patients on survival was shown by a number of retrospective studies and by a meta-analysis that reported the results of eight different studies involving 1562 patients: this meta-analysis demonstrated a significant advantage in terms of overall survival in chelated MDS patients vs nonchelated patients, with a mean difference in median OS of 61.2 months.62 The results coming from a meta-analysis can be controversial because authors could only utilize observational studies (no randomized trials were available) and because some selection bias could have conditioned the results (better prognosis in patients receiving ICT, different drugs for chelation).
Leitch et al published a prospective trial which showed a better survival in MDS patients receiving ICT independently from age, R-IPSS score, comorbidities, and disease-modifying therapies.86
The IRON2 retrospective trial reported an increase in OS (133 vs 105 months; p=0.009) and an increase in cardiac event free survival (137 vs 90 months; p=0.004) in MDS patients receiving ICT (mostly DFX, 72%) than patients not treated with ICT.87
The results of the previously mentioned TELESTO study, further supported the significant effect of DFX on EFS, reporting a median EFS of 1440 days in the chelated patients vs 1091 days in patients not treated with DFX (p=0.015).4
Preclinical studies suggested that ICT can reduce the risk of leukemic evolution by decreasing the ROS production and consequent genetic instability.88 A prospective study published by Lyons et al confirmed the positive effect of ICT, mostly with DFX, in reducing leukemic evolution and increasing leukemia free survival (LFS) in transfusion-dependent MDS patients.89 In a cohort of 263 patients who received ICT the LFS was 40.6 months, compared to 27.3 months in nonchelated patients.
Several studies showed that a significant percentage of MDS patients treated only with DFX obtained an improvement of peripheral cytopenia that could be defined as a hematological improvement according to the international working group response criteria.90
In the EPIC study (evaluation of patients’ iron chelation with exjade) 10 to 20% of patients receiving DFX obtained a significant erythroid response.91 Angelucci et al published the results of the study conducted by the Gruppo Italiano Malattie Ematologiche dell’adulto (GIMEMA) about the use of deferasirox in transfusion-dependent, lower-risk MDS patients: besides the reduction in serum ferritin of more than 36% following 12 months of therapy, a significant proportion of patients (11%) obtained transfusion independence.92
The multicenter trial by Nolte et al showed an hematological improvement in 18% of lower risk MDS patients receiving deferasirox, platelet response being the more frequent one (30%) and erythroid response the less frequent (6%).93
In the study by List et al 51 (28%) of 173 patients obtained hematological improvement according to international working group 2006 criteria.94 Similar results came from two Italian registry data, the Roman Myelodysplasia Group (GROM) and the Basilicata Registry: among 118 transfusion-dependent MDS patients receiving DFX, 17.6% obtained an hematological improvement in the erythroid serie, 5.9% in platelets and 7.1 in neutrophils.95
Breccia et al published their data about 40 MDS patients who received deferasirox outside of clinical trials: efficacy in serum ferritin reduction and safety were confirmed even in association with other therapies (such as azacitidine); moreover, four patients had a reduction of transfusion requirement and a mean increase in Hb levels of 2 g/dL.96
The abovementioned data were consistent with results from Extend and EXjange studies, published by Gattermann et al. Treatment with deferasirox proved to be effective in serum ferritin levels reduction in chelation-naïve (p=0.0002) and prechelated patients (p=0.06).97
The main studies on the use of deferasirox in low-risk MDS patients are summarized in Table 1.
 |
Table 1 Principal Studies on Deferasirox in Low-risk MDS Patients |
The biologic reason for this result is controversial, but the two main mechanisms that can explain the possibility of obtaining a hematological improvement with ICT are the reduction in ROS production and NFk-B pathway inhibition.
ICT protects from iron overload and from its detrimental effects, first of all oxidative stress that may damage nucleic acids, proteins and lipids. Rachmilewitz et al showed a decrease of ROS in red blood cells (RBC) after three months of chelation therapy with deferasirox in a cohort of 15 low-risk MDS patients.98 Chan et al established a correlation between serum ferritin levels and ROS in CD34+ cells in MDS patients.99 These observations suggest a role of ICT in reducing oxidative stress by decreasing ferritin levels and a possible positive effect on hematopoiesis.
In the US03 trial 9.4% of MDS patients treated with deferasirox obtained an hematological improvement and in this cohort of patients a normalization of LPI values was seen after chelation therapy, this further suggesting a link between oxidative stress and peripheral cytopenias.100
The second pathway that could explain the role of ICT in the improvement of cytopenias is the reduction of NF-kB activity. NF-kB is a transcription factor involved in myelopoiesis and its role in the pathogenesis of MDS is fully described101,102 as well as the efficacy of NF-kB inhibitors on leukemic cells. NF-kB pathway is activated by ROS, which are increased in transfusion-dependent MDS patients with iron overload.
Messa et al demonstrated that deferasirox, but not the other commercially available iron chelators, acts as a selective inhibitor of NF-kB in leukemic cells in vitro. The study suggests that deferasirox is able to inhibit NF-kB action independently from iron sequestering, but the exact cellular target is still unknown.103
These data pave the way to the use of iron chelation therapy in MDS patients not only for the treatment of secondary hemosiderosis, but also as a target therapy against malignant clones, in association with other therapies.
Conclusions
In the last few years multiple reviews have been published on the topic, so it is worth giving a perspective about the latest findings in the current review.
It is well known that the prognosis of patients with MDS is related both to the biological characteristics of the disease and, especially for lower-risk MDS, to age and comorbidities. It is also important to note that prognosis can be negatively affected by several factors, such as the severity of cytopenias and iron overload induced by red blood cell transfusions. The excess of iron not only is responsible for organ damage, but it is able to induce genomic instability and to modify the hematopoietic niche, favoring progression to acute leukemia.
Iron overload can be effectively treated with iron chelation therapy and, in particular with deferasirox, the use of which is recommended by international guidelines in lower-risk MDS patients, after the demonstration that it can significantly prolong event free survival and most likely also overall survival.
The observation that a number of MDS patients can obtain an improvement of peripheral cytopenias during therapy with deferasirox put increasing interest on this treatment and probably paved the way to new studies, in order to confirm this data. Further studies are also needed to evaluate and clarify the biological basis of hematological improvement induced by ICT in MDS patients.
Disclosure
Sarah Parisi: no conflict of interest to declare.
Carlo Finelli: Celgene BMS: research funding, advisory committees, speaker fees; Novartis: advisory committees, speaker fees; Takeda: consultancy.
References
1. Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes [erratum appears in Blood 91:1100, 1998]. Blood. 1997;89:2079–2088. doi:10.1182/blood.V89.6.2079
2. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2456. doi:10.1182/blood-2012-03-420489
3. Leitch HA, Buckstein R, Zhu N, et al. Iron overload in myelodysplastic syndromes: evidence based guidelines from the Canadian consortium on MDS. Leuk Res. 2018;74:21–41. doi:10.1016/j.leukres.2018.09.005
4. Angelucci E, Li J, Greenberg P, et al. Iron chelation in transfusion-dependent patients with low- to intermediate-1–risk myelodysplastic syndromes. Ann Intern Med. 2020;172(8):513–522. doi:10.7326/M19-0916
5. Santini V, Alessandrino PE, Angelucci E, et al. Clinical management of myelodysplastic syndromes: update of SIE, SIES, GITMO practice guidelines. Leuk Res. 2010;34:1576–1588. doi:10.1016/j.leukres.2010.01.018
6. Musto P, Maurillo L, Simeon V, et al. Iron-chelating therapy with deferasirox in transfusion-dependent, higher risk myelodysplastic syndromes: a retrospective, multicentre study. Br J Haematol. 2017;177:741–750. doi:10.1111/bjh.14621
7. Fenaux P, Rose C. Impact of iron overload in myelodysplastic syndromes. Blood Rev. 2009;23(Suppl 1):S15–19. doi:10.1016/S0268-960X(09)70005-0
8. Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. doi:10.1200/JCO.2005.01.7038
9. Cortelezzi A, Cattaneo C, Cristiani S, et al. Nontransferrin-bound iron in myelodysplastic syndromes: a marker of ineffective erythropoiesis? Hematol J. 2000;1:153–158. doi:10.1038/sj.thj.6200028
10. Santini V, Girelli D, Sanna A, et al. Hepcidin levels and their determinants in different types of myelodysplastic syndromes. PLoS One. 2011;6:e23109. doi:10.1371/journal.pone.0023109
11. Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360. doi:10.1146/annurev-med-050109-142444
12. Pullarkat V. Objectives of iron chelation therapy in myelodysplastic syndromes: more than meets the eye? Blood. 2009;114:5251–5255. doi:10.1182/blood-2009-07-234062
13. Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi:10.1016/j.cmet.2005.01.003
14. Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi:10.1126/science.1104742
15. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi:10.1016/j.cell.2010.06.028
16. Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi:10.1038/nm1629
17. Tanno T, Noel P, Miller JL. Growth differentiation factor 15 in erythroid health and disease. Curr Opin Hematol. 2010;17:184–190. doi:10.1097/MOH.0b013e328337b52f
18. Kroot JJ, Kemna EH, Bansal SS, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94:1748–1752. doi:10.3324/haematol.2009.010322
19. Castagna A, Campostrini N, Zaninotto F, Girelli D. Hepcidin assay in serum by SELDI-TOF-MS and other approaches. J Proteomics. 2010;73:527–536. doi:10.1016/j.jprot.2009.08.003
20. Winder A, Lefkowitz R, Ghoti H, et al. Urinary hepcidin excretion in patients with myelodysplastic syndrome and myelofibrosis. Br J Haematol. 2008;142:669–671. doi:10.1111/j.1365-2141.2008.07225.x
21. Murphy PT, Mitra S, Gleeson M, Desmond R, Swinkels DW. Urinary hepcidin excretion in patients with low grade myelodysplastic syndrome. Br J Haematol. 2009;144:451–452. doi:10.1111/j.1365-2141.2008.07455.x
22. Cui R, Gale RP, Zhu G, et al. Serum iron metabolism and erythropoiesis in patients with myelodysplastic syndrome not receiving RBC transfusions. Leuk Res. 2014;38:545–550. doi:10.1016/j.leukres.2014.01.016
23. Bondu S, Alary A-S, Lefèvre C, et al. A variant erythroferrone disrupts iron homeostasis in SF3B1-mutated myelodysplastic syndrome. Sci Transl Med. 2019;11:eaav5467. doi:10.1126/scitranslmed.aav5467
24. Zhu Y, Li X, Chang C, et al. SF3B1-mutated myelodysplastic syndrome with ring sideroblasts harbors more severe iron overload and corresponding over-erythropoiesis. Leuk Res. 2016;44:8–16. doi:10.1016/j.leukres.2016.02.011
25. Shenoy N, Vallumsetla N, Rachmilewitz E, et al. Impact of iron overload and potential benefit from iron chelation in low-risk myelodysplastic syndrome. Blood. 2014;124:873–881. doi:10.1182/blood-2014-03-563221
26. Vinchi F, Hell S, Platzbecker U. Controversies on the consequences of iron overload and chelation in MDS. Hemasphere. 2020;4(3):e357. doi:10.1097/HS90000000000000357
27. Jin S, Su H, Tran NT, et al. Splicing factor SF3B1K700E mutant dysregulates erythroid differentiation via aberrant alternative splicing of transcription factor TAL1. PLoS One. 2017;12:e0175523. doi:10.1371/journal.pone.0175523
28. Dolatshad H, Pellagatti A, Fernandez-Mercado M, et al. Disruption of SF3B1 results in deregulated expression and splicing of key genes and pathways in myelodysplastic syndrome hematopoietic stem and progenitor cells. Leukemia. 2015;29:1092–1103. doi:10.1038/leu.2014.331
29. Visconte V, Avishai N, Mahfouz R, et al. Distinct iron architecture in SF3B1-mutant myelodysplastic syndrome patients is linked to an SLC25A37 splice variant with a retained intron. Leukemia. 2015;29:188–195. doi:10.1038/leu.2014.170
30. Inokura K, Fujiwara T, Saito K, et al. Impact of TET2 deficiency on iron metabolism in erythroblasts. Exp Hematol. 2017;49:56–67 e5. doi:10.1016/j.exphem.2017.01.002
31. Fabiani E, Valentini L, Cilloni D, Voso MT. Could haemochromatosis (HFE) gene mutations affect response to iron chelation in myelodysplastic syndrome? - Response to Lucijanic and Kusec. Br J Haematol. 2019;186(4):639–640. doi:10.1111/bjh.15938
32. Della Porta MG, Kuendgen A, Malcovati L, et al. Myelodysplastic syndrome (MDS)-specific comorbidity index for predicting the impact of extra-hematological comorbidities on survival of patients with MDS. Blood. 2008;112:11. doi:10.1182/blood-2008-02-078170
33. Brunner AM, Blonquist TM, Hobbs GS, et al. Risk and timing of cardiovascular death among patients with myelodysplastic syndromes. Blood Adv. 2017;1:2032–2040. doi:10.1182/bloodadvances.2017010165
34. Pascal L, Beyne-Rauzy O, Brechignac S, et al. Cardiac iron overload assessed by T2* magnetic resonance imaging and cardiac function in regularly transfused myelodysplastic syndrome patients. Br J Haematol. 2013;162:413–415. doi:10.1111/bjh.12368
35. Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi:10.1126/science.aag1381
36. Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi:10.1056/NEJMoa1408617
37. Swirski FK. From clonal haematopoiesis to the CANTOS trial. Nat Rev Cardiol. 2018;15:79–80. doi:10.1038/nrcardio.2017.208
38. Vinchi F, Porto G, Simmelbauer A, et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J. 2019:1–16. doi:10.1093/eurheartj/ehy883
39. Xu S. Iron and atherosclerosis: the link revisited. Trends Mol Med. 2019;25:659–661. doi:10.1016/j.molmed.2019.05.012
40. Vinchi F, Muckenthaler MU, Da Silva MC, et al. Atherogenesis and iron: from epidemiology to cellular level. Front Pharmacol. 2014;5:1–20.3. doi:10.3389/fphar.2014.00094
41. Vinchi F, Simmelbauer A, Altamura S, et al. Iron accelerates cardiovascular disease: the importance of maintaining iron balance. Atherosclerosis. 2016;252:E241–E242. doi:10.1016/j.atherosclerosis.2016.07.030
42. Vinchi F. Non-transferrin-bound iron in the spotlight: novel mechanistic insights into the vasculotoxic and atherosclerotic effect of iron. Antioxid Redox Signal. 2021;35(6):387–414. doi:10.1089/ars.2020.8167
43. Wong CAC, Leitch HA. Delayed time from RBC transfusion dependence to first cardiac event in lower IPSS risk MDS patients receiving iron chelation therapy. Leuk Res. 2019;83:106170. doi:10.1016/j.leukres.2019.106170
44. Telfer PT, Prestcott E, Holden S, et al. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110:971–977. doi:10.1046/j.1365-2141.2000.02298.x
45. Milic S, Mikolasevic I, Orlic L, et al. The role of iron and iron overload in chronic liver disease. Med Sci Monit. 2016;22:2144–2151. doi:10.12659/MSM.896494
46. Neukirchen J, Fox F, Kündgen A, et al. Improved survival in MDS patients receiving iron chelation therapy - a matched pair analysis of188 patients from the Düsseldorf MDS registry. Leuk Res. 2012;36:1067–1070. doi:10.1016/j.leukres.2012.04.006
47. Metzgeroth G, Dinter D, Schultheis B, et al. Deferasirox in MDS patients with transfusion-caused iron overload—a phase-II study. Ann Hematol. 2009;88:301–310. doi:10.1007/s00277-008-0588-3
48. Girmenia C, Candoni A, Delia M, et al. Infection control in patients with myelodysplastic syndromes who are candidates for active treatment: expert panel consensus-based recommendations. Blood Rev. 2019;34:16–25. doi:10.1016/j.blre.2018.10.002
49. Ganz T. Iron and infection. Int J Hematol. 2018;107:7–15. doi:10.1007/s12185-017-2366-2
50. Wong CAC, Wong SAY, Leitch HA. Iron overload in lower international prognostic scoring system risk patients with myelodysplastic syndrome receiving red blood cell transfusions: relation to infections and possible benefit of iron chelation therapy. Leuk Res. 2018;67:75–81. doi:10.1016/j.leukres.2018.02.005
51. Kontoghiorghes GJ, Kolnagou A, Skiada A, et al. The role of iron and chelators on infections in iron overload and non iron loaded conditions: prospects for the design of new antimicrobial therapies. Hemoglobin. 2010;34:227–239. doi:10.3109/03630269.2010.483662
52. Thompson MG, Corey BW, Si Y, et al. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob Agents Chemother. 2012;56:5419–5421. doi:10.1128/AAC.01197-12
53. Neupane GP, Kim DM. In vitro time-kill activities of ciprofloxacinalone and in combination with the iron chelator deferasirox against Vibrio vulnificus. Eur J Clin Microbiol Infect Dis. 2010;29:407–410. doi:10.1007/s10096-010-0875-5
54. Lyons RM, Marek BJ, Paley C, et al. Relation between chelation and clinical outcomes in lower-risk patients with myelodysplastic syndromes: registry analysis at 5 years. Leuk Res. 2017;56:88–95. doi:10.1016/j.leukres.2017.01.033
55. Leitch HA. Optimizing therapy for iron overload in the myelodysplastic syndromes. Drugs. 2011;71:155–177. doi:10.2165/11585280-000000000-00000
56. Angelucci E, Pilo F. Iron chelation therapy in MDS – the final answer. Clin Lymphoma Myeloma. 2019;19(S1):S75–S76. doi:10.1016/j.clml.2019.07.425
57. Pilo F, Angelucci E. A storm in the niche: iron, oxidative stress and haemopoiesis. Blood Rev. 2018;32:29–35. doi:10.1016/j.blre.2017.08.005
58. Porter JB, El-Alfy M, Viprakasit V, et al. Utility of labile plasma iron and transferrin saturation in addition to serum ferritin as iron overload markers different underlying anemias before and after deferasirox treatment. Eur J Haematol. 2016;96(1):19–26. doi:10.1111/ejh.12540
59. Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:60–87. doi:10.6004/jnccn.2017.0007
60. Malcovati L, Hellstrom-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122:2943–2964. doi:10.1182/blood-2013-03-492884
61. Bennett JM; Working Group on Transfusional Iron Overload. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol. 2008;83:858–861. doi:10.1002/ajh.21269
62. Mainous AG, Tanner RJ, Hulihan MM, et al. The impact of chelation therapy on survival in transfusional iron overload: a meta-analysis of myelodysplastic syndrome. Br J Haematol. 2014;167:720–723. doi:10.1111/bjh.13053
63. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: that is the question! Blood Rev. 2018;32:368–377. doi:10.1016/j.blre.2018.03.002
64. Goldberg SL, Chen E, Corral M, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol. 2010;28:2847–2852. doi:10.1200/JCO.2009.25.2395
65. Rose C, Brechignac S, Vassilief D, et al. Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myelodysplasies). Leuk Res. 2010;34:864–870. doi:10.1016/j.leukres.2009.12.004
66. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, Phase III study. Lancet Oncol. 2009;10:223–232. doi:10.1016/S1470-2045(09)70003-8
67. Goncalves AC, Cortesao E, Oliveiros B, et al. Oxidative stress levels are correlated with P15 and P16 gene promoter methylation in myelodysplastic syndrome patients. Clin Exp Med. 2016;16:333–343. doi:10.1007/s10238-015-0357-2
68. Platzbecker U, Bornhäuser M, Germing U, et al. Red blood cell transfusion-dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndromes (MDS). Biol Blood Marrow Transplant. 2008;14:1217–1225. doi:10.1016/j.bbmt.2008.08.006
69. Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing. allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95:476–484. doi:10.3324/haematol.2009.011429
70. Wermke M, Eckoldt J, Götze KS, et al. Enhanced labile plasma iron and outcome in acute myeloid leukaemia and myelodysplastic syndrome after allogeneic haemopoietic cell transplantation (ALLIVE): a prospective, multicentre, observational trial. Lancet Haematol. 2018;5:e201–e210. doi:10.1016/S2352-3026(18)30036-X
71. Petzer V, Wermke M, Tymoszuk P, et al. Enhanced labile plasma iron in hematopoietic stem cell transplanted patients promotes Aspergillus outgrowth. Blood Adv. 2019;3(11):1695–1700. doi:10.1182/bloodadvances.2019000043
72. Isidori A, Loscocco F, Visani G, et al. Iron toxicity and chelation therapy in hematopoietic stem cell transplant. Transpl Cell Ther. 2021;27:371379. doi:10.1016/j.jtct.2020.11.007
73. Cremers EMP, de Witte T, de Wreede L, et al. A prospective non interventional study on the impact of transfusion burden and related iron toxicity on outcome in myelodysplastic syndromes undergoing allogeneic hematopoietic cell transplantation. Leuk Lymphoma. 2019;60:2404–2414. doi:10.1080/10428194.2019.1594215
74. Mahesh S, Yelena Ginzburg AV. Iron overload in myelodysplastic syndromes. Leuk Lymphoma. 2008;49:427–438. doi:10.1080/10428190701843221
75. Stumpf JL. Deferasirox. Am J Health Syst Pharm. 2007;64(6):606–616. doi:10.2146/ajhp060405
76. Vanorden HE, Hagemann TM. Deferasirox – an oral agent for chronic iron overload. Ann Pharmacother. 2006;40(6):1110–1117. doi:10.1345/aph.1G566
77. Shashaty G, Frankewich R, Chakraborti T, et al. Deferasirox for the treatment of chronic iron overload in transfusional hemosiderosis. Oncology. 2006;20(14):1799–1806.
78. Barton JC. Chelation therapy for iron overload. Curr Gastroenterol Rep. 2007;9(1):74–82. doi:10.1007/s11894-008-0024-9
79. Piga A, Galanello R, Forni GL, et al. Randomized Phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91(7):873–880.
80. Nisbet-Brown E, Olivieri NF, Giardina PJ, et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2003;361(9369):1597–1602. doi:10.1016/S0140-6736(03)13309-0
81. Cappellini MD, Cohen A, Piga A, et al. A Phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood. 2006;107(9):3455–3462. doi:10.1182/blood-2005-08-3430
82. Pennell DJ, Porter JB, Cappellini MD, et al. Deferasirox for up to 3 years leads to continued improvement of myocardial T2* in patients with β-thalassemia major. Haematologica. 2012;97(6):842–848. doi:10.3324/haematol.2011.049957
83. Porter JB. Deferasirox: an effective once-daily orally active iron chelator. Drugs Today. 2006;42(10):623–637. doi:10.1358/dot.2006.42.10.1009901
84. Killick SB. Iron chelation therapy in low risk myelodysplastic syndrome. Br J Haematol. 2017;177:375–387. PMID: 28300275. doi:10.1111/bjh.14602
85. Lucijanic M, Lovrinov M, Skelin M. Iron chelation in transfusion-dependent patients with low- to intermediate-1-risk myelodysplastic syndromes. Ann Intern Med. 2020;173(7):595. doi:10.7326/L20-1055
86. Leitch HA, Parmar A, Wells RA, et al. Overall survival in lower IPSS risk MDS by receipt of iron chelation therapy, adjusting for patient-related factors and measuring from time of first red blood cell transfusion dependence: an MDS-CAN analysis. Br J Haematol. 2017;179:83–97. doi:10.1111/bjh.14825
87. Remacha AF, Arrizabalaga B, Villegas A, et al. Evolution of iron overload in patients with low-risk myelodysplastic syndrome: iron chelation therapy and organ complications. Ann Hematol. 2015;94:779–787. doi:10.1007/s00277-014-2274-y
88. Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica. 2006;91:1588–1590.
89. Lyons RM, Marek BJ, Paley C, et al. Comparison of 24-month outcomes in chelated and non-chelated lower-risk patients with myelodysplastic syndromes in a prospective registry. Leuk Res. 2014;38:149–154. doi:10.1016/j.leukres.2013.11.004
90. Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425.
91. Gattermann N, Finelli C, Porta MD, et al. Deferasirox in iron overloaded patients with transfusion-dependent myelodysplastic syndromes: results from the large 1-year EPIC study. Leuk Res. 2010;34:1143–1150. doi:10.1016/j.leukres.2010.03.009
92. Angelucci E, Santini V, Di Tucci AA, et al. Deferasirox for transfusion-dependent patients with myelodysplastic syndromes: safety, efficacy, and beyond (GIMEMA MDS0306 Trial). Eur J Haematol. 2014;92:527–536. doi:10.1111/ejh.12300
93. Nolte F, Hochsmann B, Giagounidis A, et al. Results from a 1-year, open-label, single arm, multi-center trial evaluating the efficacy and safety of oral Deferasirox in patients diagnosed with low and int-1 risk myelodysplastic syndrome (MDS) and transfusion-dependent iron overload. Ann Hematol. 2013;92:191–198. doi:10.1007/s00277-012-1594-z
94. List AF, Baer MR, Steensma DP, et al. Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J Clin Oncol. 2012;30:2134–2139. doi:10.1200/JCO.2010.34.1222
95. Maurillo L, Breccia M, Buccisano F, et al. Deferasirox chelation therapy in patients with transfusion-dependent MDS: a ‘realworld’ report from two regional Italian registries: gruppo Romano Mielodisplasie and Registro Basilicata. Eur J Haematol. 2015;95:52–56. doi:10.1111/ejh.12476
96. Breccia M, Fisinger P, Loglisci G, et al. Deferasirox treatment for myelodysplastic syndromes: “real-life” efficacy and safety in a single-institution patient population. Ann Haematol. 2012;91:1345–1349. doi:10.1007/s00277-012-1481-7
97. Gattermann N, Jarish A, Shlag R, et al. Deferasirox treatment of iron-overloaded chelation-naïve and prechelated patients with myelodysplastic syndromes in medical practice: results from the observational studies Extend and EXJjange. Eur J Haematol. 2012;88(3):260–268. doi:10.1111/j.1600-0609.2011.01726.x
98. Rachmilewitz E, Merkel D, Ghoti H, et al. Improvement of oxidative stress parameters in MDS patients with iron overload treated with deferasirox. Blood. 2008;112(11):A924–A925. doi:10.1182/blood.V112.11.2675.2675
99. Chan LSA, Buckstein R, Reis MD, et al. Iron overload and haematopoiesis in MDS: does blood transfusion promote progression to AML? Blood. 2008;112(11):928a. doi:10.1182/blood.V112.11.2685.2685
100. List AF, Baer MR, Steensma D, et al. Deferasirox (ICL670); Exjade) reduces serum ferritin (SF) and labile plasma iron (LPI) in patients with myelodysplastic syndromes (MDS). Blood. 2008;112(11):523a.
101. Guzman M, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98(8):2301–2307. doi:10.1182/blood.V98.8.2301
102. Carvalho G, Coquelle A, Vozenin MC, et al. NF-kappaB constitutes a potential therapeutic target in high-risk myelodysplastic syndrome. Blood. 2006;107(3):1156–1165. doi:10.1182/blood-2005-05-1989
103. Messa E, Carturan S, Maffè C, et al. Deferasirox is a powerful NF-κB inhibitor in myelodysplastic cells and in leukemia cell lines acting independently from cell iron deprivation by chelation and reactive oxygen species scavenging. Haematologica. 2010;95(8):1308–1316. doi:10.3324/haematol.2009.016824
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
