Back to Journals » Clinical Interventions in Aging » Volume 18
Prognosis of Older Adult Patients Suffering from Atrial Fibrillation and Hypokalemia
Authors Wang XD, Wang Y, Liu J, Yao JW, Zhang J, Zhang YN
Received 24 May 2023
Accepted for publication 8 August 2023
Published 17 August 2023 Volume 2023:18 Pages 1363—1371
DOI https://doi.org/10.2147/CIA.S422801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Xue-Dong Wang, Yu Wang, Jing Liu, Ji-Wen Yao, Jing Zhang, Yi-Nan Zhang
Department of Cardiology, Beijing Hepingli Hospital, Beijing, People’s Republic of China
Correspondence: Xue-Dong Wang, Department of Cardiology, Beijing Hepingli Hospital, No. 18 of Hepingli North Street, Dongcheng District, Beijing, People’s Republic of China, Tel +86 10 5804 3212, Fax +86 10 6429 5714, Email [email protected]
Objective: To examine the effects of hypokalemia on the prognosis of older adult patients with atrial fibrillation (AF).
Methods: We enrolled 794 older adult patients ≥ 75 years suffering from AF, and divided them into two groups according to the inclusion and exclusion criteria: Group 1, (hypokalemia group), 246 cases, serum K+< 3.5 mmol/L; Group 2, (normal blood potassium group), 548 cases, 3.5mmol/L≤serum K+< 5.5 mmol/L. The two groups of patients were followed for 70 months to observe the occurrence of clinical events. The primary endpoint was cardiovascular death and the secondary endpoint was all-cause death.
Results: The median follow-up time was 15.00 months. In terms of baseline profile characteristics, serum creatinine levels were significantly lower in Group 1 than in Group 2 patients (P=0.002). In terms of the relationship between hypokalemia and clinical outcomes, Kaplan-Meier survival analysis revealed that the incidence of clinical primary endpoint in Group 1 was significantly higher than that in Group 2 (P < 0.001), and the incidence of the secondary endpoint did not differ significantly between the two groups (P> 005). Based on multivariate Cox regression risk model analysis, coronary heart disease, hemoglobin content, serum uric acid and usage of anticoagulant drugs were the independent variables related to the primary endpoint of cardiovascular death (all P< 0.01).
Conclusion: The incidence of hypokalemia in older adult patients with AF was 30.98%. Hypokalemia was closely related to the cardiovascular death, and coronary heart disease, hemoglobin content, serum uric acid level, and usage of anticoagulant drugs were the independent risk factors for the primary endpoint event.
Keywords: atrial fibrillation, older adult, hypokalemia, prognosis
Background
Arrhythmia is the most prevalent type of atrial fibrillation seen clinically, and its prevalence rises with age. With the development of society and economy and the aging of the population, the number of older adult patients in China suffering from atrial fibrillation has increased significantly. According to the latest epidemiological report, the prevalence of atrial fibrillation in China in men and women over the age of 75 is as high as 5.4% and 4.9%, respectively.1
The characteristics of disease in the elderly are unique, and there are three major aspects to consider: Weakness in elderly people due to decreased activity tolerance and weakened nutrition absorption; older adult patients frequently suffer from multiple illnesses and take multiple drugs at the same time (diseases such as cardiovascular disease and cancer and drugs such as diuretics, non-steroidal anti-inflammatory drugs, antiplatelet drugs, etc.); elderly people frequently suffer from renal dysfunction. Based on the aforementioned, elderly people often suffer from electrolyte imbalance, and hypokalemia is highly prevalent.2
Hypokalemia is a clinically common electrolyte disease characterized as a blood potassium level of < 3.5 mmol/L. Hypokalemia is linked to a variety of arrhythmias.3 According to recent research, low serum potassium levels increase the risk of atrial fibrillation;4 however, the prognosis of older adult patients with atrial fibrillation combined with hypokalemia has not yet been reported. This retrospective cohort study was designed to elucidate the prognostic outcomes of older adult patients with atrial fibrillation combined with hypokalemia.
Information and Methods
Research Design
We conducted this retrospective cohort study at a single center. The Beijing Hepingli Hospital ethics review committee approved the research protocol (No. BJSHPLYY-IRB-LW-2023-01). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Study Population
The population of the study consisted of older adult patients hospitalized with atrial fibrillation in Beijing Hepingli Hospital from January 2016 to December 2020. The inclusion and exclusion criteria are shown in Figure 1. Based on the inclusion and exclusion criteria, 922 patients were enrolled and 128 patients were excluded, and patients lost to follow-up were also excluded. Finally, 794 patients were included—336 males (42.3%) and 458 females (57.7%) with an average age of 83.64±5.65 years (range 75 to 107 years).
 |
Figure 1 Research technology roadmap. |
Patients were divided into two groups based on their serum potassium level at admission,: Group 1, (hypokalemia group), 246 cases, serum K+<3.5 mmol/L; Group 2, (normal blood potassium group), 548 cases, 3.5mmol/L≤serum K+<5.5mmol/L (Figure 1).
Data Collection
Study data were obtained from the electronic medical records of the hospitalized patients. The data included past medical history, baseline clinical features, laboratory tests during admission, echocardiography results, discharge diagnosis, and follow-up clinical results. The blood samples were all fasting venous blood collected in the morning after admission, and the automatic biochemical analyzer (LX20 biochemical analyzer, Beckman Coulter, USA) was used to determine the serum potassium ions, uric acid, blood creatinine, and other biochemical indicators. All data were collected by six trained independent investigators after the patients signed an informed consent form.
Follow-Up Methods and Endpoints
All selected patients were followed-up by telephone. If there was a record of re-hospitalization, the hospitalization records and outpatient data were obtained, and the event was recorded. The primary endpoint of the study was death from cardiovascular causes and the secondary endpoint was all-cause death.
Statistical Analysis
The count data are expressed as constituent ratio (%) and the measurement data are expressed by the mean ± standard deviation (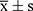 ). The mean of two samples was compared using t-test, and the constituent ratio of two samples was compared using the chi-squared test. The relationship between blood potassium level and clinical endpoint was examined using Kaplan-Meier survival analysis, and multivariate Cox stepwise regression risk model was used to analyze the independent factors related to clinical endpoint events; the factors included in the regression equation were determined based on whether the clinical significance was related and p value <0.1 in univariate analysis. The adjusted confounding factors included age, drinking history, smoking history, hypertension, type 2 diabetes, dyslipidemia, chronic obstructive pulmonary disease, hypothyroidism, blood creatinine level, high-sensitivity C-reactive protein, diuretic use, spironolactone use, ACEI/ARB/ARNI use, beta blocker use, digoxin and insulin use.
). The mean of two samples was compared using t-test, and the constituent ratio of two samples was compared using the chi-squared test. The relationship between blood potassium level and clinical endpoint was examined using Kaplan-Meier survival analysis, and multivariate Cox stepwise regression risk model was used to analyze the independent factors related to clinical endpoint events; the factors included in the regression equation were determined based on whether the clinical significance was related and p value <0.1 in univariate analysis. The adjusted confounding factors included age, drinking history, smoking history, hypertension, type 2 diabetes, dyslipidemia, chronic obstructive pulmonary disease, hypothyroidism, blood creatinine level, high-sensitivity C-reactive protein, diuretic use, spironolactone use, ACEI/ARB/ARNI use, beta blocker use, digoxin and insulin use.
Reverse Kaplan-Meier survival analysis was used to calculate the median follow-up time. SPSS 23.0 (IBM Inc., Armonk, NY, USA) and R language (R Project for Statistical Computing) were used for statistical analysis. The difference was statistically significant if P< 0.05.
Results
Baseline Characteristics of Patients in the Two Groups
The serum creatinine level of Group 1 was significantly lower than that of Group 2 (P=0.002), but there were no statistically significant differences in other aspects, such as baseline characteristics of patients between the two groups, concomitant diseases, basic treatment drugs, and some laboratory results (P> 0.05). There was a trend of increasing differences in hsCRP levels between the two groups (P = 0.057), but no statistically significant difference was observed (Table 1).
 |
Table 1 Demographic and Clinical Characteristics of the Patients |
Relationship Between Blood Potassium Levels and Clinical Endpoint in the Two Groups
Based on the median follow-up time, there were 56 cases (7.05%) of cardiovascular death between the two groups, including 32 cases (13.01%) in Group 1 and 24 cases (4.38%) in Group 2, the former was significantly higher than the latter (HR 0.347; 95% CI 0.205 to 0.590, P< 0.001) (Figure 2A); regarding the secondary endpoint, there were 87 cases (10.96%), including 33 cases (13.41%) in Group 1 and 54 cases (9.85%) in Group 2. There was no significant difference between the two groups (HR 0.750; 95% CI 0.486 to 1.157, P=0.193) (Figure 2B).
 |
Figure 2 Kaplan-Meier analysis of clinical endpoint events. (A) Major endpoints (cardiogenic death). (B) Secondary endpoint: all-cause death. |
Analysis of Multivariate Cox Survival Regression Model for Clinical Endpoint Events
In multivariate Cox survival regression analysis, Coronary Heart Disease (CHD) (HR2.917, 95% CI: 1.227 to 6.938, P=0.015), Hb content (HR 0.985, 95% CI: 0.974 to 0.996, P = 0.007), administration of anticoagulants (HR 2.041, 95% CI: 1.126 to 3.700, P = 0.019), and serum uric acid levels (HR 1.002, 95% CI: 1.000 to 1.004, P = 0.016) were independently associated with cardiovascular death as the primary endpoint after adjusting for confounding factors such as age, chronic kidney disease, hypothyroidism, serum creatinine level, high-sensitivity c-reactive protein, and serum magnesium level (Table 2 and 3).
 |
Table 2 Univariate Cox Regression Analysis of Clinical Endpoints |
 |
Table 3 Multivariate Cox Regression Analysis of Clinical Endpoints |
Discussion
This study showed that there was a close relation between hypokalemia and cardiovascular death. In addition, the independent risk factors for the primary endpoint event included coronary heart disease, hemoglobin content, serum uric acid level, and usage of anticoagulant drugs. These findings suggested that prevention and treatment of hypokalemia were of great significance in improving the prognosis of patients with atrial fibrillation.
In the present study, the incidence of hypokalemia in older adult patients with atrial fibrillation was 30.98%, which was higher than the incidence of hypokalemia in general hospitalized patients (20%) reported in previous studies,5 indicating that as age increased, the incidence of hypokalemia showed an increasing trend.6 In this study, serum creatinine levels in the hypokalemia group were significantly different from those in the normal blood potassium group, as determined by the statistical analysis of general data (the level of serum creatinine in the hypokalemia group was significantly lower than that in the normal blood potassium group), suggesting that the cause of hypokalemia in older adult patients with atrial fibrillation was not primarily associated with renal function, and that renal hypofunction frequently resulted in hyperkalemia.7 Therefore, in older adult patients with atrial fibrillation, the occurrence of hypokalemia is more likely to be related to poor gastrointestinal absorption function and reduced potassium ion intake.
In this study, both groups had a median follow-up of 15.00 ±1.45 months (95% CI: 12.166 to 17.834, Reverse Kaplan-Meier method). Hypokalemia was significantly associated with cardiovascular death as the primary endpoint, but not with all-cause death as the secondary endpoint (Figure 2A and B). The reason for the difference in results is associated with hypokalemia specifically affecting the myocardium. As stated previously, extracellular low potassium affects the depolarization and repolarization of ventricular myocardium from a variety of aspects, resulting in a considerably increased probability of arrhythmia and consequently increased incidence of cardiovascular death. In this study, the all-cause death of older adult patients was related to the progression of multiple diseases, with hypokalemia not playing a significant role.
CHD-Hb, administration of anticoagulants, and serum uric acid levels were independently linked with cardiovascular death as the primary endpoint, irrespective of the results of univariate analysis and Cox regression multivariate analysis. Considered an independent variable for the primary endpoint in univariate analysis, serum magnesium was not an independent related variable in multivariate analysis, however it is close to being an independent related variable (P = 0.052). This indicates that low potassium and magnesium levels often coexist in older adult patients with atrial fibrillation and create the electrophysiological basis for the susceptibility to malignant arrhythmia.8
In older adult patients with atrial fibrillation combined with CHD, coronary artery stenosis is often severe.9 In addition, when atrial fibrillation occurs, the atrial muscles lose their ability to contract effectively and the diastolic filling volume of the left ventricle is reduced by at least a quarter.10 Therefore, the ventricular muscle experiences severe ischemia. At this time, hypokalemia can result in a significant increase in the late Na+ current (INa-L),11 as well as an increase in the early after-depolarization, thereby inducing various arrhythmias.
Similar to the mechanism of CHD, Hb is an independent variable associated with the primary endpoint. When Hb is low, the absolute amount of Hb-bound oxygen per unit volume of blood decreases, and oxygen delivery is reduced. During atrial fibrillation, ventricular muscles are in a condition of severe hypoxia due to the reduction in fraction. Thus, hypokalemia can also result in various arrhythmias.12
Anticoagulation is one of the primary treatments for older adult patients with atrial fibrillation.13,14 Many older adult patients with atrial fibrillation fail to receive standardized anticoagulant therapy due to the fact that older adult patients often suffer from multiple basic diseases, and due to their physical weakness, low-weight status, and simultaneous use of multiple drugs. Additionally, older adult patients are often bedridden or less active. These factors lead to a significantly increased risk of arterial thromboembolism and the possibility of cardiovascular death in older adult patients with atrial fibrillation.
Serum uric acid levels were also an independent variable associated with cardiovascular death as the primary endpoint in univariate and multivariate analyses. Serum uric acid is a recognized cardiovascular disease risk factor,15 and it is associated with the occurrence of a variety of cardiovascular diseases, including atrial fibrillation.16 The increase in serum uric acid levels are predictive of an increase in inflammation and oxidative stress.17 Inflammation, oxidative stress, and hypokalemia together are the basis for various arrhythmia, resulting in an increase in cardiovascular death.18
This study has certain limitations. It is a single-centre retrospective cohort study and the patients were not consecutively included, which may have led to selection bias. In addition, older adult patients are often weak after admission and some auxiliary examinations cannot be performed, resulting in an increase in cases with incomplete sample data and more patients being excluded. Future prospective cohort studies with larger samples are needed to validate the conclusions of this study.
Conclusion
Older adult patients usually suffer from multiple diseases and have different prognosis, which deserves attention. In this study, we explored the prognosis of older adult patients with atrial fibrillation and hypokalemia. In case of older adult patients with atrial fibrillation and hypokalemia, the incidence of cardiovascular death increased significantly and the prognosis of these patients worsened.
Abbreviations
ACEI, Angiotensin-Converting Enzyme Inhibitors; ARB, Angiotensin Receptor Blocker; ARNI, Angiotensin Receptor Neprilysin inhibitors; HsCRP, High-sensitivity C-reactive protein; CHD, Coronary heart disease; Hb, Hemoglobin.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Beijing Hepingli Hospital (No: BJSHPLYY-IRB-LW-2023-01). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
No external funding received to conduct this study.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Du X, Guo L, Xia S, et al. Atrial fibrillation prevalence, awareness and management in a nationwide survey of adults in China. Heart. 2021;107(7):535–541. PMID: 33509976; PMCID: PMC7958113. doi:10.1136/heartjnl-2020-317915
2. Chu T, Wu Z, Xu A. Association between preoperative hypokalemia and postoperative complications in older adult patients: a retrospective study. BMC Geriatr. 2022;22(1):743. PMID: 36096723; PMCID: PMC9469624. doi:10.1186/s12877-022-03445-1
3. Thu Kyaw M, Maung ZM. Hypokalemia-induced arrhythmia: a case series and literature review. Cureus. 2022;14(3):e22940. PMID: 35411269; PMCID: PMC8989702. doi:10.7759/cureus.22940
4. Farah R, Nassar M, Aboraya B, Nseir W. Low serum potassium levels are associated with the risk of atrial fibrillation. Acta Cardiol. 2021;76(8):887–890. PMID: 32723154. doi:10.1080/00015385.2020.1799573
5. Kardalas E, Paschou SA, Anagnostis P, Muscogiuri G, Siasos G, Vryonidou A. Hypokalemia: a clinical update. Endocr Connect. 2018;7(4):R135–R146. PMID: 29540487; PMCID: PMC5881435. doi:10.1530/EC-18-0109
6. Guzel T, Aktan A, Kılıç R, et al. OralAnticoagulant use and long-term follow-up results in patients with non-valvular atrial fibrillation in Turkey AFTER-2 study. AnatolJ Cardiol. 2022;26(7):567–576. doi:10.5152/AnatolJCardiol.2022
7. Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377–384. PMID: 21181208; PMCID: PMC3061004. doi:10.1007/s00467-010-1699-3
8. Krämer BK, Endemann D. Kardiale Risiken der Hypokaliämie und Hypomagnesiämie [Cardiac risks of hypokalemia and hypomagnesemia]. Ther Umsch. 2000. 57(6):398–399. doi:10.1024/0040-5930.57.6.398. German. PMID: 10894026.
9. Guzel T, Kış M, Şenöz O. The correlation between the left atrial volume index and atrial fibrillation development in heart failure with mildly reduced ejection fraction and long-term follow-up results. Acta Cardiol. 2022;77(7):647–654. doi:10.1080/00015385.20222.2067674
10. Kim TH, Shim CY, Park JH, et al. Left ventricular diastolic dysfunction is associated with atrial remodeling and risk or presence of stroke in patients with paroxysmal atrial fibrillation. J Cardiol. 2016;68(2):104–109. PMID: 26603328. doi:10.1016/j.jjcc.2015.10.008
11. Pezhouman A, Singh N, Song Z, et al. Molecular basis of hypokalemia-induced ventricular fibrillation. Circulation. 2015;132(16):1528–1537. PMID: 26269574; PMCID: PMC4618042. doi:10.1161/CIRCULATIONAHA.115.016217
12. Williams AJ, Weiner C, Reiff D, Swenson ER, Fuller RW, Hughes JM. Comparison of the effect of inhaled selective and non-selective adrenergic agonists on cardiorespiratory parameters in chronic stable asthma. Pulm Pharmacol. 1994;7(4):235–241. PMID: 7620239. doi:10.1006/pulp.1994.1026
13. Chung MK, Refaat M, Shen WK, et al.; ACC Electrophysiology Section Leadership Council. Atrial fibrillation: JACC Council Perspectives. J Am Coll Cardiol. 2020;75(14):1689–1713. PMID: 32273035. doi:10.1016/j.jacc.2020.02.025
14. Aktan A, Güzel T, Aslan B, et al. Comparison of the real-life clinical outcomes of warfarin with effective time in therapeutic range and non-vitamin K antagonist oral anticoagulants: insight from theAFTER-2 trial. Cardiol Pol. 2023;81(2):132–140. doi:10.33963/KP.a2022.0287
15. Saito Y, Tanaka A, Node K, Kobayashi Y. Uric acid and cardiovascular disease: a clinical review. J Cardiol. 2021;78(1):51–57. PMID: 33388217. doi:10.1016/j.jjcc.2020.12.013
16. Pak S, Yatsynovich Y, Valencia D, Chen T. Serum uric acid and atrial fibrillation: meta-analysis. Crit Pathw Cardiol. 2018;17(3):161–166. PMID: 30044258. doi:10.1097/HPC.0000000000000150
17. Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis. 2007;17(6):409–414. PMID: 17643880. doi:10.1016/j.numecd.2007.02.011
18. Welcome MO, Dogo D, Nikos E. Cellular mechanisms and molecular pathways linking bitter taste receptor signalling to cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction in heart diseases. Inflammopharmacology. 2022;6:1–29. PMID: 36471190; PMCID: PMC9734786. doi:10.1007/s10787-022-01086-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
