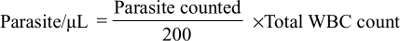Back to Journals » Infection and Drug Resistance » Volume 12
Profiles of hematological parameters in Plasmodium falciparum and Plasmodium vivax malaria patients attending Tercha General Hospital, Dawuro Zone, South Ethiopia
Received 19 August 2018
Accepted for publication 18 January 2019
Published 5 March 2019 Volume 2019:12 Pages 521—527
DOI https://doi.org/10.2147/IDR.S184489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Nefsu Awoke,1 Amsalu Arota2
1Department of Nursing, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 2School of Medicine, Department of Medical Laboratory Science, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
Background: Malaria is a major health problem in the tropics, with 300–500 million cases and 1.1–2.7 million deaths occurring annually. The hematological alterations associated with malaria infection may vary depending on: level of malaria endemicity, background hemoglobinopathy, malaria immunity, host genetic factors, and parasite strain variations.
Objective: The aim of the study was to determine the profiles of hematologic parameters in Plasmodium falciparum and Plasmodium vivax malaria infections at Tercha General Hospital, Dawuro Zone, South Ethiopia.
Methodology: A total of 340 study participants were included in the study, out of which 170 were malaria cases, and the remaining 170 were malaria negatives. An institution-based cross-sectional study was conducted. Malaria diagnosis was based on thick and thin blood films microscopy. Hematological parameters were determined by using an automated, CELL-DYN 1800 hematology analyzer. Malaria parasite density was determined by counting the asexual parasites against 200 WBCs, and then calculated by using the standard formula. The diagnostic accuracy of hematological parameters was measured by computing sensitivity, specificity, and likelihood ratios.
Results: The mean values of Hgb, Hct, platelet, WBC, RBC, and lymphocyte were significantly lower in malaria patients than malaria negatives. The prevalence of thrombocytopenia and anemia in malaria patients was 84% and 67%, respectively. There was an inverse correlation between P. falciparum and P. vivax parasite density and lymphocyte count, as well as platelet count.
Conclusion and recommendation: Thrombocytopenia and anemia were the two common hematological abnormalities observed in malaria cases. The platelet count during malaria infection was inversely correlated with the asexual stage parasite density. Patients with acute febrile illness having thrombocytopenia should alert the treating physician about the possibility of malaria infection. Malaria patients should be checked for the presence of hematological abnormalities such as anemia and have to be managed for those abnormalities.
Keywords: malaria, P. falciparum, P. vivax, hematological parameters, Dawro, anemia, thrombocytopenia, Tercha
Introduction
Malaria is a disease caused by protozoan parasites of genus Plasmodium. The five Plasmodium species well known to cause human malaria are; Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi. P. falciparum is responsible for most malaria deaths. The infection can develop suddenly and produce several life-threatening complications.1
Globally, about 3.2 billion people live in areas at risk of malaria transmission. An estimated 350–500 million clinical malaria episodes occur annually, mostly caused by infection with P. falciparum and P. vivax. Malaria causes 1.1–2.7 million deaths each year as a result of severe malaria. It is the fifth cause of death from infectious diseases worldwide, and the second leading cause of death in Africa.2 Malaria control was difficult because of drug resistance of the parasite and insecticide resistance of the vector.3
Epidemiologically, P. falciparum malaria covers 85% of all malaria cases worldwide. It occurs mainly in the hotter and more humid regions of the world and the most pathogenic species. P. vivax malaria is the second most important public health problem. It is characterized by the occurrence of malaria relapses due to the re-activation of hypnozoites in the liver cells. Although the complication of P. vivax infection is relatively less, rupture of an enlarged spleen occasionally occurs and could be life-threatening.4,5
According to WHO report, almost 90% of all malaria deaths in the world occur in Africa. An estimated one million people in Africa die from malaria each year. The important way for reducing malaria morbidity and mortality is best achieved through early diagnosis and prompt, effective treatment, which is the base for management of malaria. Microscopy remains the mainstay of parasite-based diagnosis in most health clinics and hospitals; however, the quality of microscopy-based diagnosis is inadequate for ensuring good health outcomes.6,7
Malaria is a major public health problem in Ethiopia. More than 50 million people in the country live in areas at risk of malaria transmission. It is also estimated that 9 million malaria cases occur annually throughout the country. P. falciparum and P. vivax are the two most common malarial parasites in the country.8
Hematological alterations associated with malaria infection may vary depending on the following factors: level of malaria endemicity, background hemoglobinopathy, demographic factors, and malaria immunity. The pathophysiological processes causing the hematological changes in malaria are complex, multiple, and incompletely understood.9 The immunological background of P. falciparum and P. vivax infection do have its own effect on the absolute counts of peripheral blood mononuclear cells and granulocyte subsets. The level of the cytokines TNF-α and IFN-α, which are known to induce the expression of selectins, integrins, and chemoattractant chemokines have been observed to correlate with malaria severity caused by P. falciparum and P. vivax.10
Prediction of the hematological changes in malaria enables the clinician to establish an effective and early therapeutic intervention in order to prevent the occurrence of major complications. Hematology parameters can help to provide a presumptive treatment, especially when the results of the parasitological examination are not immediately available or are uncertain to decide treatment for malaria,11–14 and help to intensively care for the patient and prevent a death that may result from such complications.15
The results of the surveillance data demonstrated that malaria continues to be a significant public health challenge in the South Ethiopian region, and the region bears a higher burden of malaria. P. falciparum is the most common cause for malaria in the region.16 The hematological parameter alteration during malaria infection was not studied previously in this study area. Therefore, this study is aimed to determine profiles of hematological parameters in P. falciparum and P. vivax malaria infection among patients attending Tercha General Hospital, Dawuro Zone.
Methods and materials
Study area and period
The study was conducted at Tercha Hospital, Dawuro Zone, SNNPR. Tercha Hospital is one of the Hospitals in SNNPR and is 512 km away from Addis Ababa. The Hospital has been serving people from Dawuro as well as the surrounding woredas (districts) of Jimma zone and Konta special woreda. The study was conducted from March 20 to May 30, 2016.
Study design
An institution-based cross-sectional study was employed.
Source population
All acute febrile patients clinically suspected of malaria attending Tercha Hospital during the study period.
Study population
Those with blood film microscopy positive malaria patients satisfying inclusion criteria attending the hospital during the study period.
Inclusion criteria: Patients with clinically suspected and blood film microscopy positive for either the asexual or sexual stages of P. falciparum and/or P. vivax and whose age is >14 years were included in the study as malaria cases.
Exclusion criteria: Those who took any anti-malarial drug in the last 2 weeks, or presenting with any other infectious disease were excluded.
Sample collection and processing
The diagnosis of malaria was based on blood film microscopy. Both thick and thin blood film was prepared with blood from a finger prick on a slide. Thin film was fixed with absolute methanol. Then, blood film was stained with 10% Giemsa stain for 10 minutes and examined with an 100× oil immersion objective by experienced microscopists. Peripheral blood smear examination was also taken as a gold standard for the diagnosis of malaria infection.
To determine the density of malaria parasite; two experienced microscopists independently counted the asexual stage Plasmodium parasite on a slide against 200 WBCs in thick blood film from each of the malaria cases. Then, the results of the two readers were averaged and used for the calculation of parasite density. Finally, parasites per μL of blood were calculated by using the formula:
|
Venous blood of 3 mL was collected by venin puncture into an EDTA tube from each study participant. A liquate of blood was taken from the whole blood sample for hematological analysis. Hematological parameters were determined by an automated hematology analyzer by using CELL-DYN 1800, which is a hematology analyzer that performs complete blood count and gives results in printouts. Hematological analysis was achieved in accordance with the standard protocol and manufacturer instructions of the hematology analyzer machine by an experienced Senior Laboratory Technologist who had special training and was certified on the automated hematology analyzer. The socio-demographic data were collected by using a questionnaire.
Data quality assurance
The SOPs were followed in all the laboratory procedures, and training was then given to laboratory assistants by the principal investigator before starting laboratory work. To ensure the accuracy of malaria diagnosis, 10% of the positive and negative samples were re-examined by an independent laboratory technologist, who was blinded to the results of the first slide-reader. For an automated hematology analyzer, a daily quality assurance check was performed in accordance with manufacturer’s recommendations. Post-analytical quality assurance was insured by using appropriate result formats and registrations.
Data analysis
First data were checked for completeness and coded. This was then entered into a computer and analyzed by using SPSS version 22.0. The appropriate descriptive statistics were applied to determine the mean, frequencies, and percentages of the study parameters. The independent-sample t-test was used to compare the mean hematological value of the malaria cases with malaria negative individuals. Pearson correlation analysis was used to test the association between two continuous variables. The diagnostic accuracy of hematological parameters was determined by computing the sensitivity, specificity, and likelihood ratio. The microscopy of peripheral blood smear was taken as a gold standard for the diagnosis of malaria. The diagnostic value of the hematological parameters was measured against non-malarious acute febrile illness. For all statistical tests, P<0.05 was considered as statistically significant. Finally, all results were described and presented using tables and figures.
Ethical statement
Ethical approval was first obtained from the Ethical Clearance Committee of Wolaita Sodo University. Then, a written letter from the Graduate Coordinator of the Department was obtained. Official permission was also obtained from Tercha Hospital and Dawro Zone Heath Department. The study was conducted according to the Declaration of Helsinki. Information on the study was explained to the participants, including the procedures, potential risks, and benefits of the study. Informed written consent was obtained from each of the study participants. For patients under 18 years of age their parents/guardian provided written informed consent. The name of the study participants was not written, rather the corresponding code was given for each of them so that the confidentiality of the result was secured. If hematological abnormalities were found, the results were referred to a physician and appropriate treatments were provided. All malaria positives cases had received antimalarial chemotherapy according to the standard guidelines. It was also assured to participants that the findings of the study were only be used for the purpose of research.
Results
Socio-demographic characteristics of the study participants
A total of 340 participants were included in the study, out of which half (170) were malaria positive cases and the other half were malaria negative. Among the positive malaria cases, the majority, (116, 68%) were males, and all (170) were in the age range of 15–50 years, with a mean age value of 27.6 years. The mean age of malaria patients was not significantly different from the age of the malaria negative groups (P>0.05).
The majority of malaria positive patients were males (116, 68%). Regarding ethnicity, about 94% of malaria cases belonged to the Dawro ethnic group. Similar to the malaria cases, the majority of malaria negatives were also male (70%).
Hematological alterations in malaria patients
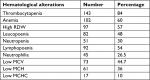
The prevalence of thrombocytopenia (platelet count <140,000/µL) in overall malaria patients studied was 84%, and the remaining 16% had normal platelet count values. There was no malaria case with a high platelet count. Thrombocytopenia was the most common hematological abnormality finding observed in malaria infection in the study.
Anemia–low Hgb concentration, defined as; Hgb <12 g/dL for females and Hgb <13 g/dL for males, was the second most frequently encountered hematological abnormality observed in malaria infection. The prevalence of occurrence of anemia in malaria patients was 60%, while the remaining 40% had normal Hgb values.
An alteration in RDW was significant hematological alteration in malaria infection, which was the presence of high RDW. High RDW was defined as an RDW value >14.5%, and it was observed in 57% of total malaria cases, with 43% of the cases having a normal RDW value. The presence of a low WBC count (leucopenia) was a frequent change detected in malaria patients, and it was found in 48% of malaria cases (Table 1).
Hematological alterations in P. falciparum and P. vivax infections
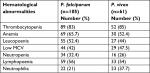
A total of 170 malaria patients participated in the study, out of which 105 (61.7%) were P. falciparum malaria cases, 61 (36%) were P. vivax cases, and the remaining 4 (2.3%) had mixed infection.
There was an almost similar high frequency of thrombocytopenia in both P. vivax and P. falciparum malaria patients, and it was found that thrombocytopenia was detected in 85% of P. vivax and 83% of P. falciparum malaria cases.
The presentation of anemia in malaria has shown species-specific differences. It was predominantly present in P. falciparum cases (65.7%) over P. vivax (52.4%) cases.
Regarding the occurrence of leucopenia in species of Plasmodium, it was observed in 52.4% of P. falciparum cases and 44% of P. vivax cases. Neutrophilia was more frequent in P. vivax infection (37.7%) than P. falciparum (21%). The frequency of lymphopoenia in the two types of malaria infection was similar, 54% in P. vivax cases and 56% in P. falciparum cases (Table 2).
Hematological values in malaria positive and negative patients
The mean hematological values of the malaria patients were compared with those of the malaria negative individuals using an independent-sample t-test, to determine the hematological impact of P. falciparum and P. vivax infections.
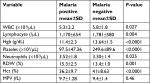
It was found that the mean values of total WBC, Hgb, platelet, Hct, RBC, and lymphocyte were significantly lower in malaria patients as compared with malaria-negative individuals (P<0.05). Conversely, the means of absolute neutrophil count and RDW value were higher in the malaria patients than the non-parasitemic groups in the study.
The mean value total WBC count was found to be significantly lower in malaria patients as compared to malaria negatives (5,300 vs 5,800, P=0.027). Similarly, the lymphocyte level was also significantly depleted in the peripheral blood of malaria cases (P=0.004). In contrast, the mean absolute neutrophil count was elevated in P. falciparum and P. vivax cases compared to malaria-negative individuals (P=0.025).
The mean Hgb value of malaria positive cases was significantly lower than that of malaria negatives (P<0.0001). The MPV was not significantly different in malaria cases or in malaria negatives (P=0.46). In contrast, the mean platelet count was found to be highly decreased in malaria patients in the study (97.5 vs 249.6; P<0.0001). The mean value of RDW was significantly higher in malaria infection than malaria negatives, P=0.001 (Table 3).
The correlations between hematological parameters and P. falciparum and P. vivax parasite densities
There was an inverse correlation between P. falciparum asexual stage parasite density and lymphocyte count (r=–0.53, P=0.002). P. vivax parasite density was also inversely correlated with the peripheral blood lymphocyte count (r=–0.47, P=0.024).
The inverse association between P. falciparum asexual stage parasitemia and Hgb level in the blood (r=–0.12; P=0.23) was not statistically significant. Similarly, in correlation analysis of the P. vivax parasitemia level and Hgb value, no significant correlation was detected (P>0.05).
Platelet counts in P. falciparum malaria infection had an inverse correlation with asexual stage parasite density (r=–0.46, P<0.0001) and also an inverse relationship between P. vivax parasitemia level and platelet counts (r=–0.48, P<0.0001).
In the correlation analysis of the peripheral blood neutrophil level with asexual stage parasitemia, a significant positive association was detected in P. falciparum infection (r=0.38, P=0.002). There was also a positive and significant correlation between P. vivax parasitemia level and neutrophil count (r=0.35, P=0.0063).
Discussion
Thrombocytopenia was the most common hematological abnormality in malaria patients. The result of this study is higher when compared with the findings from the studies by Bashawri et al,28 and Khan et al.16 This difference may be attributed to the difference in Plasmodium parasite strain, which can have different virulence and disease patterns, or the difference in the level of malaria endemicity in different geographical areas.17 The cause of thrombocytopenia in malaria is poorly understood; however, increased platelet destruction and reduced platelet lifespan are suggested to occur during malaria, which is often associated with palpable splenomegaly and circulating immune complexes.16
Anemia was the second most frequently noticed hematological abnormality in malaria infections. Anemia was predominantly presented in P. falciparum cases compared to P. vivax in this study. This indicates that anemia has shown more association with P. falciparum infection. This study is consistent with studies conducted by Jain and Kaur18 and Shah et al,19 but differs from studies conducted in Dubai which indicated that there was no significant difference in the incidence of anemia between P. falciparum (67%) and P. vivax (63%) infections,12 and there was also a nearly equal frequency of anemia in the two malaria groups, 56% in P. falciparum and 61.9% in P. vivax.20 The pathogenesis of anemia in malaria is multifactorial and incompletely understood; factors such as mechanical destruction of the parasitized red blood cells, reduced RBC production in the bone marrow, and phagocytosis of parasite infected RBC are among the mechanisms suggested to be involved.13 Tumor necrosis factor and IL-10 have also been implicated in the development of P. falciparum malarial anemia.17
Leucopenia was commonly detected in total WBC alteration and occurred in 48% of malaria patients. This is close to a previous report in which leucopenia presented in 36.5% of P. falciparum cases.20 Leucopenia was also found in 30.4% of malaria infections.21 Leucopenia was presented in 22.1%, 20.9%, and 18.4% of subjects in P. vivax, P. falciparum, and mixed infections, respectively.22
The mean values of Hgb and platelets were significantly lower in P. falciparum and P. vivax malaria patients compared to malaria-negative individuals (P<0.0001). This indicates that P. falciparum and P. vivax infections could have induced the depletion of the platelets and Hgb level in the peripheral blood of infected individuals. This agrees with the previous findings of Maina et al13 and Koltas et al.23
Neutrophilia was less frequently observed among malaria cases in this study, with the frequency of occurrence in the two types of malaria infection being P. falciparum (21%) and P. vivax (37.7%). This finding is lower than another report in which neutrophilia was observed in 48% in P. falciparum infection and 65.8% in P. vivax.21 During overwhelming P. falciparum and P. vivax infection, neutrophils will be in great demand so that their number could increase in the peripheral blood circulation.17
Lymphopoenia was also a frequent hematological alteration in malaria infection, and occurred in 54% of malaria cases, with an almost similar frequency of occurrence in two types of malaria species; 56% in P. falciparum and 54% in P. vivax. This is higher than a report from Dubai in which 24% of total malaria patients had lymphopenia.12 This suggests that the depletion of lymphocytes in the peripheral blood does not show a difference in rate of occurrence in the two types of malaria infection. There are probably two possible mechanisms, which could explain the depletion of lymphocyte sub-sets from the peripheral blood in acute P. falciparum and P. vivax malaria patients: sequestration of lymphocytes by trapping into the lymph nodes and abnormal death of the cells through apoptosis.17
The platelet count in both P. falciparum and P. vivax infection was significantly and inversely correlated with the asexual stage parasite density. This is consistent with others studies which reported an inverse relationship between malaria parasitaemia level and platelet count in the peripheral blood.13,24 The platelet level decreases as the degree of malaria parasitaemia increases, and thrombocytopenia could be used to determine the presence and severity of malaria.25 Due to an increase in TNF-α in malaria infection, more adhesion molecules and their receptors will be expressed on lymphocyte surfaces and endothelial cells of lymph nodes through the action of TNF-α; this causes more lymphocytes to be trapped in the lymph nodes, opposite to the increase in asexual stage densities.17
Thrombocytopenia has a high prevalence in this study, which is supported by a study from India,26 and Bhandary et al27 indicate that it give clues for differential diagnosis of malaria from other acute febrile illness. This suggests that thrombocytopenia can be used as a supportive diagnostic criterion for malaria, in addition to clinical indices in circumstances where microscopic diagnosis is not sufficient, as in the case of low parasite density.
Conclusion and recommendations
The common hematological parameters alterations observed in P. falciparum and P. vivax infections were; platelet count, Hgb concentration, and RDW value. Thrombocytopenia and anemia were the two most common hematological abnormality findings observed in malaria infection. An alteration in RDW was a significant hematological alteration in malaria infection, which was the presence of high RDW. There was an inverse association between P. falciparum and P. vivax asexual stage parasite density and lymphocyte count, and parasitemia level and platelet count. The neutrophil count in peripheral blood was positively correlated with parasitemia in both P. falciparum and P. vivax infection. There was no significant correlation between P. vivax parasitemia level and Hgb level.
Malaria patients should be checked for the presence of hematological abnormalities such as anemia and thrombocytopenia, and appropriate management should be undertaken for those detected abnormalities to reduce the associated complications in patients. Patients with acute febrile illness having thrombocytopenia alone or in combination anemia should alert the treating clinicians about the possibility of malaria infection. Thrombocytopenia should be used as supportive diagnostic criteria for malaria, in addition to clinical indices and other laboratory investigations.
Abbreviations
EDTA, ethylendiaminetetraacetate; Hgb, hemoglobin; Hct, hematocrit; MCHC, mean corpuscular hemoglobin concentration; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MPV, mean platelet volume; RDW, red cell distribution width; SOP, standard operating procedure; TNF-α, tumor necrosis factor alpha.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Jimma University for their financial support. Next we would like to acknowledge Tercha general Hospitals, data collectors, supervisors, study participants, and all who gave their hands in the study directly or indirectly, without whom the research would not be done. The source of funding for this research was Jimma University, as well as Tercha General Hospital.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Dronamraju K, Arese P. Emerging Infectious Diseases of the 21st Century: Malaria – Genetic and Evolutionary Aspects. New York: Springer US. 2006:125–146. | ||
World Health Organization, UNICEF. World Malaria Report. Geneva: WHO. 2005. | ||
UNICEF. Malaria African Problem: Roll Back Malaria Movement. Geneva: WHO; 2002:1–2. | ||
Gillers HM, Warell DA. Bruce-Chwati’s Essential Malariology. Great Britain: The Bath Press; 1993:140–200. | ||
Clark IA, Schofield L. Pathogenesis of malaria. Parasitol Today. 2000;16(10):451–454. | ||
World Health Organization. Malaria Microscopy Quality Assurance Manual. Geneva: WHO; 2009. | ||
World Health Organization. The World Health Report: Reducing Risks, Promoting Healthy Life. Geneva: WHO; 2002. | ||
World Malaria Report 2010 [webpage on the Internet]. World Health Organization. Available from: https://www.who.int/malaria/publications/atoz/9789241564106/en/. Accessed December 25, 2011. | ||
Price RN, Simpson JA, Nosten F, et al. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65(5):614–622. | ||
Rosenberg YJ, Anderson AO, Pabst R. HIV-induced decline in blood CD4/CD8 ratios: viral killing or altered lymphocyte trafficking? Immunol Today. 1998;19(1):10–17. | ||
D’Acremont V, Landry P, Mueller I, Pécoud A, Genton B. Clinical and laboratory predictors of imported malaria in an outpatient setting. Am J Trop Med Hyg. 2002;66(5):481–486. | ||
Abro AH, Ustadi AM, Younis NJ, Abdou AS, Hamed DA, Saleh AA. Malaria and hematological changes. Pak J Med Sci. 2008;24(2):287–291. | ||
Maina RN, Walsh D, Gaddy C, et al. Impact of Plasmodium falciparum infection on haematological parameters in children living in Western Kenya. Malar J. 2010;9(Suppl 3):S4. | ||
Murphy GS, Oldfield EC. Falciparum malaria. Infect Dis Clin North Am. 1996;10(4):747–775. | ||
Bidaki Z, Dalimi A. Biochemical and hematological alteration in vivax malaria in Kahnouj city. J Rafsanjan Univ Med Sci. 2003;3:17–24. | ||
Khan S, Khan F, Usman M, Zahid S. Malaria can lead to thrombocytopenia. Rawal Med J. 2008;33:183–185. | ||
Misgina D. Characterization of peripheral blood leucocyte subsets in acute Plasmodium falciparum and P. vivax malaria infections at Wonji sugar estate. 2005;19(2):376–379. | ||
Jain MA, Kaur MB. Comparative study of microscopic detection methods with hematological changes in malaria. Ind J Pathol Microbiol. 2005;48(4):464–467. | ||
Shah AM, Dave KK, Sakera BL, Gonsai RN. Comparative study of microscopic detection methods with hematological changes and coagulation profile in malaria. News Bulletin. 2007;2(7):37–40. | ||
Win S. Hematological changes in malaria. J Evol Med Dent Sci. 2006; 4(65),:11367–11374. | ||
Kassa D, Petros B, Messele T, Admassu A, Adugna F, Wolday D. Parasito-haematological features of acute Plasmodium falciparum and P. vivax malaria patients with and without HIV co-infection at Wonji Sugar Estate, Ethiopia. Ethiop J Health Dev. 2005;19(2):132–139. | ||
Rasheed A, Saeed S, Khan S. Clinical and laboratory findings in acute malaria caused by various Plasmodium species. JPMA. 2009; 59:220. | ||
Koltas I, Deimirhindi H, Hazar S, Ozcan K. Supportive presumptive diagnosis of P. vivax malaria; thrombocytopenia and red cell distribution width. Saudi Med J. 2007;28(4):535–539. | ||
Erhart LM, Yingyuen K, Chuanak N, et al. Hematologic and clinical indices of malaria in a semi-immune population of Western Thailand. Am J Trop Med Hyg. 2004;70(1):8–14. | ||
George I, Ewelike-Ezeani C. Hematological changes in children with malaria infection in Nigeria. J Med Med Sci. 2011;2(4):768–771. | ||
Lathia TB, Joshi R. Can hematological parameters discriminate malaria from nonmalarious acute febrile illness in the tropics? Indian J Med Sci. 2004;58(6):239–244. | ||
Bhandary N, Vikram GS, Shetty H. Thrombocytopenia in malaria. Biomed Res. 2011;22(4):489–491. | ||
Bashawri L., Mandil A., Bahnassy.A, Ahmed M., Malaria: Hematological aspects. Annals of Saudi Medicine. 2002;22:5–6. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.