Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 8
Profile of once-daily darunavir/cobicistat fixed-dose combination for the treatment of HIV/AIDS
Received 6 July 2016
Accepted for publication 6 September 2016
Published 31 October 2016 Volume 2016:8 Pages 175—182
DOI https://doi.org/10.2147/HIV.S56158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Jordi Navarro, Adrian Curran
Infectious Diseases Department, Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain
Abstract: Efficacy is the main objective of antiretroviral treatment and adherence is one of the cornerstones to achieve it. For this reason, treatment simplification is of key importance with regard to antiretroviral regimens. Rezolsta® (darunavir/cobicistat) is the first fixed-dose combination containing a protease inhibitor approved for HIV treatment. This coformulation includes darunavir, a protease inhibitor that has shown its efficacy and safety in naïve and treatment-experienced patients, and cobicistat, the new pharmacokinetic enhancer that is expected to replace ritonavir. Bioequivalence between ritonavir and cobicistat as darunavir boosters has been shown in studies involving healthy volunteers. Furthermore, efficacy and safety of darunavir/cobicistat observed in phase III studies, including naïve and pretreated patients without darunavir-associated resistance mutations, are comparable to historical data of darunavir/ritonavir 800/100 mg once-daily formulation. Adverse events with darunavir/cobicistat are scarce and mild, and basically include skin reactions and gastrointestinal disturbances. Although small increases in plasma creatinine are expected in patients receiving cobicistat due to the inhibition of creatinine transporters in kidney tubules, actual glomerular filtrate rate remains unaltered. Cobicistat does not have an inducer effect on metabolic pathways and shows much more selective inhibition than ritonavir. Therefore, isoenzyms different from CYP3A4 are supposed to be less affected by cobicistat, and thus fewer drug–drug interactions are expected.
Keywords: darunavir, cobicistat, fixed-dose combination, HIV infection, antiretroviral treatment
Introduction
Boosted darunavir is the preferred protease inhibitor (PI) for antiretroviral treatment (ART)-naïve patients that is recommended by most HIV guidelines worldwide.1,2 The efficacy of darunavir/ritonavir 800/100 mg once-daily formulation has been proved in many clinical trials in patients starting ART.3 Furthermore, darunavir/ritonavir once-daily regimen can also be used in ART-experienced patients with no darunavir resistance-associated mutations (RAMs).4
Darunavir, as most PIs, is mainly metabolized through cytochrome P450 (CYP3A4) in the liver and the gut.5 For this reason, coadministering a pharmacokinetic enhancer can increase plasma concentrations, allowing for once-daily administration and lower doses. So far, ritonavir, administered in low doses (100 mg once or twice daily), has been the only pharmacokinetic enhancer available in ART.6 Ritonavir acts as a potent CYP3A4 and P-glycoprotein (P-gp) inhibitor, increasing the oral bioavailability of coadministered drugs. This pharmacokinetic enhancement affects not only antiretroviral drugs such as PIs or elvitegravir, but also all other comedications metabolized through CYP that the patient might be receiving, with the risk of drug–drug interactions and undesirable side effects. It is worth noting that even at boosting doses, ritonavir has activity against HIV, and if the patient is not receiving a fully active ART, there might be the possibility of developing resistance mutations to PIs.7
A new pharmacokinetic enhancer has become available to boost antiretroviral drugs. Cobicistat (GS-9350) was developed from the ritonavir molecule and thus has many similarities8 (Figure 1). It has been shown to increase plasma concentrations of atazanavir, darunavir, and elvitegravir.9–13 Compared with ritonavir, cobicistat exhibits a more selective inhibition of CYP P450 system isoenzymes and does not have an inducing effect (of glucuronidation or CYP).8 In addition, cobicistat can be coformulated in fixed-dose combinations (FDCs) due to its chemical stability and great aqueous solubility.8 However, cobicistat shows no intrinsic activity against HIV replication (Table 1).
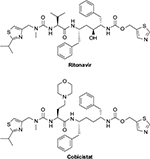  | Figure 1 Chemical structures of ritonavir and cobicistat. |
Recently, the combination of darunavir 800 mg with cobicistat 150 mg has been formulated in a FDC (Rezolsta® [darunavir/cobicistat]; Janssen-Cilag, Beerse, Belgium)14 to improve convenience and adherence without loss of virological efficacy.15 This review analyzes the efficacy and safety of once-daily darunavir/cobicistat FDC for the treatment of HIV/AIDS.
Efficacy and safety
The clinical safety and efficacy of cobicistat 150 mg as an enhancer for darunavir 800 mg once daily administered with a fully active backbone regimen of two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) was evaluated in a single-arm, open-label, phase III clinical trial (GS-US-216-130).16 This study included 313 HIV-infected patients, 295 naïve and 18 ART-experienced, without darunavir or NRTI RAMs in the baseline genotype, and the estimated glomerular filtration rate (eGFR) calculated by Cockcroft-Gault equation (eGFRCG) of ≥80 mL/min. The patients were administered darunavir 800 mg (2×400 mg tablets) plus cobicistat 150 mg (1 tablet) once daily with the NRTIs backbone selected by the investigator (tenofovir/emtricitabine in 96% of the patients). The overall virological suppression was 82% (258/313; 95% confidence interval [CI]: 78–87) at week 24 and 81% (253/313; 95% CI: 76–85) through week 48, irrespective of the baseline viral load below or above 100,000 copies/mL (81% and 80%, respectively).16 Within treatment-naïve patients, virological success was observed in 83% (244/295; 95% CI: 78–87) of the patients at week 48. These results are consistent with historical data from the ARTEMIS (naïve patients) and ODIN (experienced patients) trials with darunavir/ritonavir 800/100 mg once-daily regimen.3,4
Tolerability and safety results of darunavir/cobicistat 800/150 mg once-daily combination from this trial were in agreement with previous data reported regarding darunavir and/or cobicistat. The majority of adverse events were mild, with gastrointestinal disturbances (diarrhea, nausea, flatulence). There were 21 (7%) grade 3 or 4 adverse events, mainly rash or other skin reactions, and 16 (5%) of them led to study-drug discontinuation.16
The other clinical data come from a phase II randomized, double-blind, placebo-controlled trial (GS-US-299-102) comparing darunavir/cobicistat (as single agents) plus tenofovir disoproxil/emtricitabine versus tenofovir alafenamide/emtricitabine (in a single-tablet regimen [STR]) in 150 treatment-naïve HIV-1 infected patients.17 Virological response rate at week 48 was 84% in those patients randomized to receive darunavir/cobicistat plus tenofovir disoproxil/emtricitabine and 77% in the STR arm. As in the GS-US-216-130 study,16 drug discontinuations due to adverse events were scarce and occurred in four (2.6%) patients.
Although darunavir has been shown to exhibit in vitro activity against HIV-2 strains,18 data published in real-life setting are scarce and include patients receiving darunavir plus ritonavir containing regimens.19 Furthermore, there are no studies evaluating the efficacy of darunavir/cobicistat in HIV-2 infected patients. For this reason, this combination is only indicated for HIV-1 infection.14
Finally, there are no data regarding the use of darunavir/cobicistat combination in pediatric or adolescent populations, thus its use is only recommended in adult patients aged above 18 years.14
Cobicistat and kidneys
Cobicistat is renally excreted through glomerular filtration and secreted by the proximal tubule. In vitro data have demonstrated that cobicistat is a weak inhibitor of human renal transporters OCT2 and MATE2-K and a more potent inhibitor of OCTN1 and MATE1 than ritonavir.20 Thus, creatinine secretion through MATE1 transporter in the apical side of the tubular cell is affected. For this reason, an increase of 0.05–0.1 mg/dL in plasma creatinine concentrations and a decrease of ~10 mL/min in eGFRCG are expected at week 4 after cobicistat is started. This variation remains stable during treatment with cobicistat and reverts once it is discontinued.21 However, clearance remains unaffected20 when GFR is calculated using standardized methods to assess actual (rather than estimated) clearance, such as iohexol, a substance not secreted or reabsorbed by renal tubule.22 However, this inhibition does not affect proximal tubule secretion of tenofovir as it is excreted through different renal transporters, such as the organic anion transporter 1/3 and MRP4.21
The GS-US-216-130 study analyzed the impact of cobicistat plus darunavir in patients with normal renal function.16 A median increase of 0.1 mg/dL in baseline plasma creatinine levels was observed at week 2 and remained stable throughout week 48. Treatment discontinuation due to renal tubular disorder occurred in only one patient receiving tenofovir/emtricitabine. This renal disturbance reverted after switching to darunavir/ritonavir plus abacavir/lamivudine.
The effect of cobicistat plus darunavir in patients with mild-to-moderate renal impairment was analyzed in the GS-US-236-118 study.23 Patients with eGFR between 50 and 89 mL/min receiving ritonavir-boosted atazanavir (n=52) or darunavir (n=21) based regimens (n=21) had ritonavir switched for cobicistat. Although eGFRCG decreased to a greater extent in patients starting with eGFR >70 mL/min vs <70 mL/min (median [interquartile range], –7.6 [–15.2 to –3.6] mL/min vs –3.1 [–5.1 to 0.5] mL/min), no clinically relevant changes in cystatin C-based eGFR were seen through week 96 (–2.8 [–7.4 to 8.9] mL/min). Notwithstanding, two patients interrupted the treatment with antiretroviral drugs (one due to abnormal eGFR and one due to hematuria/proteinuria). Other reported adverse events were grade 1 hypophosphatemia (one patient), grade 2 proteinuria (one patient) and isolated increases of >0.4 mg/dL in serum creatinine levels (three patients), without any discontinuations due to proximal renal tubulopathy.
Pharmacokinetic data
After oral administration of darunavir/cobicistat 800/150 mg, darunavir is rapidly absorbed achieving maximum plasma concentrations in 3–4.5 hours. This absorption is 1.7-fold higher if darunavir is administered with food compared to when fasting, and for this reason the prescribing information recommends taking darunavir/cobicistat in fed state.14,24 Cobicistat binds in high proportion to plasma proteins (97%–98%), and a major portion of the drug is eliminated through the feces (86%) and a minimal amount through urinary excretion (8.2%).25 In healthy volunteers receiving doses of 100 and 200 mg/day of cobicistat, mean volume of distribution was 152 and 100 L, respectively.11 Cobicistat has a median plasma half-life of 3–4 hours.25
The exposure to darunavir following darunavir/cobicistat 800/150 mg once daily either as single agents or FDC formulations (candidates GS003 and GS004 and final formulation GS006) was comparable to exposure to darunavir/ritonavir 800/100 mg once daily in two phase I clinical trials with healthy volunteers (Table 2).24,26 It is worth noting that an increase in end-of-dose (C24 h) darunavir concentration was seen when coadministered with ritonavir, and this late peak implied a 30% decrease of darunavir trough concentrations when coadministered with cobicistat compared to ritonavir.26 However, when given with cobicistat, darunavir trough concentrations were still 25 times above the protein-binding-adjusted inhibitory concentration (IC50) for HIV-1 strains without darunavir RAMs (55 ng/mL),27 and probably these differences may not be clinically significant in a real-life setting.
In 60 HIV-infected patients, pharmacokinetic profile of darunavir was analyzed by giving darunavir 800 mg plus cobicistat 150 mg as single agents once daily along with an optimized background regimen selected by the investigator.16 Mean ± standard deviation darunavir AUC, Cmax and Ctrough values were 81,646 ± 26,322 ng·h/mL, 7,663 ± 1,920 ng/mL, and 1,311 ± 969 ng/mL, respectively, which were comparable to the results obtained in healthy volunteers24,26 and exhibited no association between darunavir AUC or trough concentrations and virologic response or safety.16
Drug–drug interactions with darunavir/cobicistat
As mentioned previously, cobicistat works as a pharmacokinetic enhancer mainly through CYP3A4 inhibition. However, cobicistat also has a weak inhibitory activity on CYP2D6 and on other enzymes and transporters, especially P-gp in the liver and gut, solute carrier organic anion transporters (OATP) 1B1/3 in the liver, multidrug and toxin extrusion protein 1 (MATE1) in the kidney and breast cancer resistance protein (BCRP) in the gut.14,25 Cobicistat is metabolized by CYP3A and to a minor extent by CYP2D6 and does not undergo glucuronidation.25
Despite these similarities, there are still some differences between cobicistat and ritonavir. Cobicistat is a more selective CYP3A4 inhibitor and has no clinical effect on other isoenzymes inhibited by ritonavir (eg, 2C8 and 2C9).8,28 Moreover, ritonavir shows in vivo induction activity on some CYP isoenzymes (eg, 1A2, 2C19, 2C8, 2C9, and 2B6), glucuronyl transferases (eg, UGT1A4), and P-gp.28 On the other hand, ritonavir also activates the pregnane X receptor (PXR), which regulates the expression of various drug-metabolizing enzymes, including CYP3A. Thus, although the net effect of ritonavir over CYP3A4 is inhibition, it also exhibits a potential inducer effect on this CYP isoenzyme. On the contrary, cobicistat has no inducer effect on CYP, glucoronyl transferases or PXR.8
Therefore, cobicistat has a greater selectivity for CYP3A4 and lacks an induction effect. However, due to its mechanism of action, most of the interactions seen with ritonavir are likely to be observed with cobicistat. Nowadays, there are scarce data about drug–drug interactions with cobicistat as a PI enhancer, and most of the pharmacokinetic data about cobicistat are based on cobicistat/elvitegravir/tenofovir/emtricitabine FDC studies. Indeed, no specific drug interaction trials have been published with the FDC of darunavir/cobicistat. For this reason, the potential interactions are assumed to be similar to those observed with darunavir/ritonavir. Some of the most relevant drug interactions and contraindicated drugs with darunavir/cobicistat according to its prescribing information are summarized in Table 3.14
  | Table 3 Drugs contraindicated or not recommended with darunavir/cobicistat and mechanism of interaction between drugs Notes: If CYP3A is inhibited by cobicistat, plasma concentrations of the coadministered drug increase, with the subsequent risk of greater toxicity. If CYP3A is induced by another drug, darunavir and/or cobicistat plasma concentrations decrease, with the subsequent risk of virological failure. aThe prescribing recommendations for these drugs differ between darunavir/cobicistat and darunavir/ritonavir. Reproduced from Rezolsta® [prescribing information]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002819/WC500178953.pdf. Accessed August 19, 2016.14 Abbreviations: COBI, cobicistat; CYP, cytochrome P450; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A. |
CYP3A4 is probably the most important metabolic pathway, but some drugs are metabolized through other CYP isoenzymes (CYP1A2, 2B6, 2C8, 2C9, and 2C19) or glucuronidation, which are affected by ritonavir but not by cobicistat.29 Thus, inferring ritonavir interactions to cobicistat is not always a good option. Examples include olanzapine (CYP1A2 and glucuronidation), acenocumarol (CYP2C9, 1A2, and 2C19), propofol (CYP2B6 and glucuronidation), lamotrigine and valproate (CYP2C9 and glucuronidation), gliclazide (CYP2C9 and 2C19), and mycophenolate and gemfibrozil (glucuronidation).30 Caution should be taken when administering cobicistat with P-gp substrates, such as digoxin, as plasma levels of the substrate might be increased due to a boost in intestinal absorption leading to potential severe adverse events.31 Furthermore, those patients receiving adjusted doses of concomitant drugs because of ritonavir should be closely controlled during the first 2 weeks of the switch to cobicistat to detect possible changes in drug–drug interactions.14 Therefore, it is important to know the metabolic pathways of coadministered drugs taken by patients receiving ritonavir prior to switching to cobicistat.
As a substrate of CYP3A, plasma concentrations of cobicistat are affected by inhibitors/inducers of this isoenzyme. Coadministration with other inhibitors will increase cobicistat concentrations, potentially leading to undesired effects and increased toxicity. On the contrary, CYP3A inducers could decrease cobicistat concentrations, increasing the risk of virological failure. Some non-nucleoside reverse transcriptase inhibitors (NNRTIs) such as etravirine, efavirenz or nevirapine can be potent inducers and their coadministration with cobicistat is not recommended,14 which is a significant difference between using cobicistat and ritonavir as boosters. This can be relevant for some patients currently receiving a combination of darunavir/ritonavir and an NNRTI (eg, etravirine), who cannot automatically be switched to darunavir/cobicistat. Another difference between cobicistat and ritonavir is their interaction with rifabutin. Although dose adjustments can be done to rifabutin, its coadministration with cobicistat is contraindicated.14
Cobicistat is not suitable to boost PIs different from atazanavir or darunavir due to a lack of data.25 Furthermore, the combination of darunavir/cobicistat is not potent enough to enhance the activity of other coadministered drugs that need boosting, such as elvitegravir or other PIs, as this might involve a higher risk for subtherapeutic plasma levels and subsequent virological failure.14
Taking into consideration all the potential interactions and the constant availability of new information, access to updated information is one the most important things with regard to drug–drug interactions. For this reason, clinicians should consult specific sites, such as the Liverpool HIV drug interactions website,30 before prescribing new medications to patients receiving darunavir/cobicistat.
Use of darunavir/cobicistat in the clinical practice
Ease of use is probably the strongest point of darunavir/cobicistat FDC (less not only by one pill but also by one drug bottle) without losing efficacy and maintaining almost the same flexibility as ritonavir to combine with other antiretroviral drugs. Looking in the near future, an STR with darunavir/cobicistat plus emtricitabine and tenofovir alafenamide will be the first STR based on a PI, due to the better solubility of cobicistat.8,32
Currently, integrase inhibitors have become the preferred option in most of the ART guidelines due to their higher efficacy and tolerability and better safety profile.1 NNRTIs and PIs have been relegated to alternative regimens.1 However, a PI-based treatment is still the preferred option in patients with poor adherence to treatment, in whom a high genetic-barrier regimen is needed. When adherence is an issue, there is still discussion whether it is better to use a regimen based on a small number of pills (an STR if possible) or to use a high-barrier treatment based on a PI, despite associated with a higher burden of pills. In late presenters, with low CD4 cell count and/or opportunistic infections at the diagnosis of HIV infection, some physicians would prefer to start a PI-based treatment while waiting to confirm adherence and the results of baseline resistance tests. The darunavir/cobicistat FDC simplifies the regimen in these groups of patients, reduces the risk of prescription errors and eliminates the risk of selective nonadherence.
In a time of economic constraints, darunavir/cobicistat combination could improve convenience in less expensive regimens and help in maintaining good tolerability.33 Keeping in mind that salvage regimens are mainly based on the use of PIs and that darunavir/cobicistat can be used in patients without darunavir RAMs, rescue regimens with a low burden of pills and once-daily posology are now a reality.3 Furthermore, in an attempt to avoid long-term toxicities related to the use of some NRTIs, there have been some studies that have shown a great efficacy with a dual therapy combining a boosted PI plus lamivudine as a simplification strategy,34,35 although with ritonavir as the booster. Finally, darunavir/cobicistat FDC could become a STR treatment in the selected group of patients receiving a PI monotherapy.
However, the use of a booster is associated with some disadvantages. Although cobicistat has a more selective inhibitory activity, drug interactions are similar to those observed with ritonavir given that CYP3A is the most important human metabolic pathway for the majority of the drugs. Taking into account that HIV-infected patients are getting older and polypharmacy is a reality, the lower risk of off-target interactions could be a favorable aspect for cobicistat.
Tolerability profile is similar to that observed with ritonavir and only gastrointestinal and lipid disturbances seem to be a problem in a small percentage of the patients.8,26,33 Nevertheless, cobicistat seems to have a more “metabolic-friendly” profile, as it has shown no effect on lipid accumulation in adipocytes and displays less insulin resistance than ritonavir in in vitro studies.8 However, more data and a longer follow-up period are necessary to determine whether cobicistat has better long-term toxicity profile and tolerability benefits as compared with ritonavir.
It is worth noting that cobicistat inhibits tubular creatinine secretion, which disturbs renal function estimation without affecting the real glomerular function.21 However, there are several other antiretroviral drugs with a similar effect, such as ritonavir, rilpivirine and dolutegravir, which may also increase plasma creatinine levels due to MATE1 inhibition in the renal tubular cell.36 Furthermore, there is enough data in clinical trials to consider cobicistat as a safe option in patients with impaired renal function.20,37
Conclusion
Darunavir/cobicistat FDC is an effective, well tolerated and simpler option to treat HIV-infected patients. It is expected to improve adherence and might avoid some of the drug interactions observed with ritonavir. Furthermore, the STR with darunavir/cobicistat plus emtricitabine and tenofovir alafenamide will probably be the one-pill regimen with the highest genetic barrier till date.
Author contributions
Both the authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
AC has received honoraria, speakers’ fees and/or funds for research from Bristol-Myers Squibb, Abbvie, Gilead Sciences, Janssen-Cilag, MSD, and ViiV Healthcare. JN has received honoraria, speakers’ fees and/or funds for research from Abbvie, Gilead Sciences, Janssen-Cilag, MSD, and ViiV Healthcare. The authors report no other conflicts of interest in this work.
References
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed August 19, 2016. | ||
Ryom L, Boesecke C, Gisler V, et al. Essentials from the 2015 European AIDS Clinical Society (EACS) guidelines for the treatment of adult HIV-positive persons. HIV Med. 2016;17(2):83–88. | ||
Orkin C, DeJesus E, Khanlou H, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med. 2013;14(1):49–59. | ||
Cahn P, Fourie J, Grinsztejn B, et al. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS. 2011;25(7):929–939. | ||
Rittweger M, Arasteh K. Clinical pharmacokinetics of darunavir. Clin Pharmacokinet. 2007;46(9):739–756. | ||
Hull MW, Montaner JS. Ritonavir-boosted protease inhibitors in HIV therapy. Ann Med. 2011;43(5):375–388. | ||
De Nicolo A, Simiele M, Calcagno A, et al. Intracellular antiviral activity of low-dose ritonavir in boosted protease inhibitor regimens. Antimicrob Agents Chemother. 2014;58(7):4042–4047. | ||
Xu L, Liu H, Murray BP, et al. Cobicistat (GS-9350): a potent and selective inhibitor of human CYP3A as a novel pharmacoenhancer. ACS Med Chem Lett. 2010;1(5):209–213. | ||
Ramanathan S, Warren D, Wei L, Kearney BP. Pharmacokinetic boosting of atazanavir with the pharmacoenhancer GS-9350 versus ritonavir. In: 49th ICAAC; 2009; San Francisco, CA, USA. Abstract A1-1301. | ||
Mathias A, Liu HC, Warren D, Sekar V, Kearney BP. Relative bioavailability and pharmacokinetics of darunavir when boosted with the pharmacoenhancer GS-9350 versus RTV. In: 11th International Workshop on Clinical Pharmacology of HIV Therapy; 2010; Sorrento, Italy. Abstract 28. | ||
Mathias AA, German P, Murray BP, et al. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin Pharmacol Ther. 2010;87(3):322–329. | ||
Renjifo B, van Wyk J, Salem AH, Bow D, Ng J, Norton M. Pharmacokinetic enhancement in HIV antiretroviral therapy: a comparison of ritonavir and cobicistat. AIDS Rev. 2015;17(1):37–46. | ||
German P, Warren D, West S, Hui J, Kearney BP. Pharmacokinetics and bioavailability of an integrase and novel pharmacoenhancer-containing single-tablet fixed-dose combination regimen for the treatment of HIV. J Acquir Immune Defic Syndr. 2010;55(3):323–329. | ||
Rezolsta® [prescribing information]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002819/WC500178953.pdf. Accessed August 19, 2016. | ||
Clay PG, Nag S, Graham CM, Narayanan S. Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine (Baltimore). 2015;94(42):e1677. | ||
Tashima K, Crofoot G, Tomaka FL, et al. Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a Phase IIIb, open-label single-arm trial. AIDS Res Ther. 2014;11:39. | ||
Mills A, Ortiz R, Crofoot G, et al. 48 week study of the first PI-based single tablet-regimen (STR) darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) vs. darunavir (DRV) boosted by cobicistat (COBI) and emtricitabine/tenofovir disoproxil fumarate (TVD) in HIV-infected treatmentnaïve adults. In: 54th ICAAC; 2014; Washington, USA. Abstract H-647c. | ||
Tie Y, Wang YF, Boross PI, et al. Critical differences in HIV-1 and HIV-2 protease specificity for clinical inhibitors. Protein Sci. 2012;21(3):339–350. | ||
Miranda A, Peres S, Moneti V, Azevedo T, Aldir I, Mansinho K. Clinical and laboratorial impact of antiretroviral therapy in a cohort of Portuguese patients chronically infected with HIV-2. J Int AIDS Soc. 2014;17(4 Suppl 3):19829. | ||
German P, Liu HC, Szwarcberg J, et al. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Deficienc Syndr. 2012;61(1):32–40. | ||
Stray KM, Bam RA, Birkus G, et al. Evaluation of the effect of cobicistat on the in vitro renal transport and cytotoxicity potential of tenofovir. Antimicrob Agents Chemother. 2013;57(10):4982–4989. | ||
Krutzen E, Back SE, Nilsson-Ehle I, Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984;104(6):955–961. | ||
Fisher M, McDonald C, Moyle G, et al. Switching from ritonavir to cobicistat in HIV patients with renal impairment who are virologically suppressed on a protease inhibitor. J Int AIDS Soc. 2014;17(4 Suppl 3):19824. | ||
Kakuda TN, Van De Casteele T, Petrovic R, et al. Bioequivalence of a darunavir/cobicistat fixed-dose combination tablet versus single agents and food effect in healthy volunteers. Antivir Ther. 2014;19(6):597–606. | ||
Tybost® [prescribing information]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002572/WC500153014.pdf. Accessed August 19, 2016. | ||
Kakuda TN, Opsomer M, Timmers M, et al. Pharmacokinetics of darunavir in fixed-dose combination with cobicistat compared with coadministration of darunavir and ritonavir as single agents in healthy volunteers. J Clin Pharmacol. 2014;54(8):949–957. | ||
De Meyer S, Azijn H, Surleraux D, et al. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother. 2005;49(6):2314–2321. | ||
Nathan B, Bayley J, Waters L, Post FA. Cobicistat: a novel pharmacoenhancer for co-formulation with HIV protease and integrase inhibitors. Infect Dis Ther. 2013;2(2):111–122. | ||
Marzolini C, Gibbons S, Khoo S, Back D. Cobicistat versus ritonavir boosting and differences in the drug-drug interaction profiles with co-medications. J Antimicrob Chemother. 2016;71(7):1755–1758. | ||
HIV drug interactions [homepage on the Internet]. 2016. Available from: http://www.hiv-druginteractions.org. Accessed August 19, 2016. | ||
Lepist EI, Phan TK, Roy A, et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56(10):5409–5413. | ||
Mills A, Crofoot G, Jr, McDonald C, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Deficienc Syndr. 2015;69(4):439–445. | ||
US National Institutes of Health. Study to evaluate darunavir/ritonavir + lamivudine versus continuing with darunavir/ritonavir + tenofovir/emtricitabine or abacavir/lamivudine in HIV infected subject (DUAL) NCT02159599. 2015. Available from: http://www.clinicaltrials.gov. Accessed August 19, 2016. | ||
Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15(7):785–792. | ||
Perez-Molina JA, Rubio R, Rivero A, et al. Dual treatment with atazanavir-ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15(7):775–784. | ||
Yombi JC, Pozniak A, Boffito M, et al. Antiretrovirals and the kidney in current clinical practice: renal pharmacokinetics, alterations of renal function and renal toxicity. AIDS. 2014;28(5):621–632. | ||
McDonald CK, Martorell C, Ramgopal M, et al. Cobicistat-boosted protease inhibitors in HIV-infected patients with mild to moderate renal impairment. HIV Clin Trials. 2014;15(6):269–273. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


