Back to Journals » Cancer Management and Research » Volume 12
Procyanidin Compound (PC) Suppresses Lipopolysaccharide-Induced Cervical Cancer Cell Proliferation Through Blocking the TLR4/NF-κB Pathway
Authors Yang H, Fang Z, Qu X, Zhang X, Wang Y
Received 8 August 2019
Accepted for publication 20 November 2019
Published 22 January 2020 Volume 2020:12 Pages 497—509
DOI https://doi.org/10.2147/CMAR.S226547
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
This paper has been retracted.
Haiyan Yang,* Ziyu Fang,* Xiaoli Qu, Xiaoli Zhang, Yifeng Wang
Department of Obstetrics and Gynecology, Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, Guangxi, 545005, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoli Qu
Department of Obstetrics and Gynecology, Fourth Affiliated Hospital of Guangxi Medical University, No. 1 Liushi Road, Yufeng District, Liuzhou, Guangxi 545005, People’s Republic of China
Tel +86 772-3817070
Email [email protected]
Purpose: Evidence suggested that procyanidin compound (PC) could inhibit the progression of cervical cancer (CC); however, the mechanism still remains unclear. We aimed to study the potential mechanism of PC acting on CC cells.
Patients and Methods: After a 24 hr incubation of lipopolysaccharide (LPS) (1 μg/mL), human CC SiHa and HeLa cells were cultured with various concentrations (20, 40, and 80 μg/mL) of PC for 24 hrs, then the cell viability was detected using Cell Counting Kit-8 (CCK-8). The migration and invasion abilities were assessed by scratch and Transwell assays. Apoptosis and cell cycle were detected using flow cytometry. Real-time quantitative PCR (RT-qPCR) and Western blot were used for expression analysis of the inflammatory cytokines. The pathway components were measured to evaluate the involvement of toll-like receptor 4/nuclear factor kappa-light-chain-enhancer of activated B cells (TLR4/NF-κB) pathway.
Results: PC inhibited the LPS-primed cell viability in a dose-dependent manner. After PC treatment, cell migration and invasion were inhibited, cell number at the G2/M phase was increased. The CC cell apoptosis was triggered through upregulating levels of cleaved caspase-3 and Bax and downregulating the level of B-cell lymphoma 2 protein. A significant reduction was shown in the levels of interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α. Furthermore, a remarkable reduction in the ratio of TLR4 and the p-P65/t-P65 and in the progression of P65 translocation into the nucleus was observed.
Conclusion: Our results revealed that the inhibitory effect of PC on CC cell proliferation relies on the induction of apoptosis and inhibition of inflammatory cytokines.
Keywords: apoptosis, cell cycle, inflammatory cytokines, P65-NF-κB translocation
Introduction
Cervical cancer (CC), which is one of three most common malignancies, has been the fourth leading cause to cancer-associated death among women in the world.1,2 The average age of patients diagnosed with CC was mainly between 30 and 55 years old, however, the incidence of the disease among young women increases in the recent year, with estimated 527,600 cases and 265,700 deaths worldwide in 2012.3,4 According to the statistics in 2012, the number of confirmed cases and CC-related deaths were roughly 528,000 and 266,000, respectively. In addition, approximately 85% of CC cases came from developing countries, and nearly half of the patients faced death threats due to the poor medical system and the lack of appropriate screening and therapeutic facilities and drugs.5 Currently, the molecular mechanism of CC pathogenesis has attracted much attention. Recently, a high association between the progression of CC and toll-like receptor 4 (TLR4) has been proved.6 TLR4 belongs to the toll-like receptor family and is well known for recognizing exogenous ligands such as lipopolysaccharide (LPS).7 TLR4 was also reported to participate in shaping tumor microenvironment and in promoting carcinogenesis and tumor progression.8 A previous study has demonstrated a remarkably high level of TLR4 in human CCHeLa cells.9 LPS can promote the activation of TLR4/NF-κB, which connects inflammation with cancer progression in CC cells.10 To be more specific, after the stimulation of LPS, activated TLR4 triggers the myeloid differentiation primary response gene 88 (MyD88), which induces the IκB and IKK phosphorylation. Then, IKK-mediated signal pathway could promote nuclear factor kappa-light-chain-enhancer of activated B cells (P65-NF-κB) translocate into nuclei, thus promoting the production of pro-inflammatory cytokines to increase the inflammatory response.11 In addition, accumulating evidence suggested that the activation of TLR4/NF-κB pathway could also enhance the resistance to chemotherapy in CC cells.12–14 These findings indicated that the TLR4/NF-κBcould served as a considerable therapeutic target for CC treatment.
Natural products play a critical role in the discovery and the development of numerous drugs for the treatment of various types of cancers via different mechanisms{Muhammad, 2017 #35}. Procyanidin compound (PC) is a type of flavonoid that exists extensively in the skin and seed of many plants such as grapes, pear, and apple.15 Currently, PC has generated considerable research interest due to its potent antioxidative activity,16 antiviral,17 anticarcinogenic,18 anti-inflammatory19,20 and neuroprotective activities.21,22 Recent research has revealed that the treatment of PC could induce cell death via activating autophagy and apoptosis, and subsequently suppressing effectively the progression of human gastric cancer cells.18 Meanwhile, Chen et al provided evidence about the treatment effects of PC on the CC, which was related to the activation of mitochondria apoptosis pathway.23 However, the role of TLR4 in positive effects of PC is still obscure. Hence, this study aimed to further investigate the underlying mechanism of the inhibitory effects of PC on the CC progression.
Materials and Methods
Cell Culture
Human CC cell lines SiHa and HeLa were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Thermo Scientific Hyclone, Logan, Utah, USA) containing 10% FBS (Thermo Scientific Hyclone) at 37°C in the presence of 5% CO2.
Cell Viability Detection
The SiHa and HeLa cell viabilities were examined by Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan), respectively. The cells were embedded in 96-well plates (5 × 103cells per well) for a 24 hrs incubation and then cultured with different concentrations of LPS (0, 0.1, 1, 10, and 100 μg/mL) for 24 hrs to select an appropriate experimental dosage range. Then, SiHa cells were pretreated with different concentrations of PC (0, 20, 40, 60, 80, and 100 μg/mL) for 12, 24, and 48 hrs. It might be a limitation not using positive control, which could be done in the future study. After being detected by CCK-8 kit, three different concentrations of PC were determined as low, middle and high experimental doses. The protective effect of PC was studied, and the SiHa and HeLa cells induced by LPS were incubated with culture medium containing three different doses of PC.
In brief, SiHa cells were plated onto 96-well plates (5 × 103 cells per well) for 24 hrs at 37°C in 5% CO2. After 24 hrs of LPS induction, the cells were then cultured with PC at three concentrations. Then, the cells were harvested at 12, 24, and 48 hrs and 10 μL CCK-8 reagents were supplemented into each well for another 1 hr incubation. Absorbance at 450 nm was examined using a microplate reader (Bio-Rad Laboratories, Inc. USA).
Cell Migration Detection
The treated HeLaor SiHa cells were embedded into 6-cm culture dishes and maintained in 5% CO2 at 37°C. After cell reached 90% confluence, a cell-free line was created by a sterile pipette tip. Twenty-four hours after scratch, the condition of wound healing was photographed on a microscope and the migration rates were calculated based on the changes of the width of wound closure.
Cell Invasion Detection
The cell invasion of HeLa and SiHa was assessed using Transwell chamber. Briefly, 3 × 104 treated cells were embedded into the 8-μm pore size of the 6-well Matrigel invasion chambers (BD Biosciences, San Jose, USA). The top chamber contained 200 μL serum-free DMEM, and the bottom chamber was supplemented with 600 μL DMEM with 20% FBS. After incubation at 37°C in 5% CO2 for 24 hrs, the cells on the upper surface of the membrane were wiped out, while the invaded cells onto the bottom chamber were fixed with 4% paraformaldehyde for 15 mins and then stained with 0.1% crystal violet solution for 20 mins. Five views were randomly chosen for counting the number of cells attached to the lower side of the membranes and calculating relative invasion rates.
Apoptosis Detection
The effects of PC on the apoptosis of HeLa and SiHa cells were analyzed by flow cytometry using the Annexin V-FITC/propidium iodide (PI) Apoptosis Detection Kit (KeyGEN, Nanjing, China). After induction by LPS for 24 hrs, the SiHa and HeLa cells were cultured with different concentrations of PC for another 24 hrs and were then harvested, washed with PBS and incubated with binding buffer (10 mM HEPES pH 7.4, 140 mMNaCl, 2.5 mM CaCl2). Next, the cells were cultured with PI and annexin V-FITC in the dark for 10 min at 37°C. Then, the stained cells were analyzed by a FACSCalibur flow cytometer (BD biosciences).
Cell Cycle Analysis
Cell cycle distributions were determined by PI staining as previously described.24 After induction by LPS for 24 hrs, the SiHa and HeLa cells were treated with indicated concentrations of PC for another 24 hrs. After being washed with cold PBS, the cells were fixed with ice-cold 70% ethanol overnight at 4°C and then washed twice with PBS. Subsequently, the cells were stained with fluorescent probe solution (50 mg/mL PI and 10 μg/mLRNaseA) and kept on ice in the dark for half an hour. The cell cycle was analyzed by FACSCalibur flow cytometer (BD biosciences).
Preparation of Cytosolic and Nuclear Extracts
The SiHa and HeLa cell nuclear and cytoplasmic protein isolations were prepared using a nuclear/cytosol fractionation kit (Moutain View, CA). Briefly, the cells were seeded into tubes containing PBS by scraping with a cell scraper and centrifuged at 600 × g for 5 mins at 4°C. The cell pellet was gently resuspended with cytosolic extraction buffer and maintained on ice for 15 mins and centrifuged at 14,000 × g for 15 mins at 4°C. Then, the supernatant (cytoplasmic fraction) was stored at 80°C for later use. Next, the nuclear pellet was kept on ice with nuclear extraction buffer for 30 mins and then centrifuged at 12,000 × g for 15 mins at 4°C. The supernatant extracted from nuclear was also gently transferred to clean and pre-chilled tubes and stored at 80°C.
Western Blot
The cell lysates of SiHa and HeLa cells were prepared using RIPA lysis buffer (Beyotime, China) and then measured by Bradford Protein Assay kit (Bio-Rad, USA). After denaturation at 95°C, protein samples penetrated through SDS-polyacrylamide gel and were then electrotransferred to polyvinylidenedifluoride membranes (Bio-Rad). Afterwards, the membranes were blocked with Tris-buffered saline containing 5% non-fat dry milk for 1 hr at room temperature before being mixed with the primary antibodies overnight at 4°C. After being washed by PBS, HRP-conjugated secondary antibodies (1:5000, #ab205718, Abcam, Cambridge, UK) were cultured with membranes at 4°C for 1 hr. Protein bands were detected with an enhanced chemiluminescence detection system (Millipore, Billerica, MA, USA). GAPDH served as the internal control and nucleoproteins were normalized by HDAC1 gene. Antibodies used in this study were as follows: cleavedcaspase-3 (1:500, #ab13847, Abcam), B-cell lymphoma 2 (Bcl-2, 1:1000, #ab32124, Abcam), Bax (1:1000, #ab32503, Abcam), TLR4 (1:500, #ab13556, Abcam), p-P65 (1:1000, #ab86299, Abcam), t-P65 (1:1000, #ab237591,Abcam) and Histone deacetylase 1 (HDAC1,1:300, #ab53091, Abcam).
Real-Time Quantitative PCR (RT-qPCR)
Total RNA from SiHa or HeLa cells was isolated byTRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA 1 μg was used for cDNA synthesisthroughiScript™ reverse transcription (Bio-Rad), the reaction was performed in a three-step program (65°C for 5 min, 30°C for 6 min and 50°C for 50 min). The relative mRNA was analyzed using the SYBR Green real-time PCR Master mix (Toyobo Co., Ltd., Osaka, Japan) on the ABI7900HT machine (Applied Biosystems, Carlsbad, CA, USA). The 20 µL reaction contained 10 μM of primers, 10μL SYBR fluorescent dye, 2 μL cDNA and RNase Free dH2O. The cycling programs were as follows: an initial denaturation at 95°C for 10 min, followed by 45 cycles of 95°C for 15 s, 56°C for 30 s. The mRNA expression levels were calculated followed the 2−ΔΔCtmethod25 and normalized against that of GAPDH. All primers were listed in Table 1.
 |
Table 1 Primers for RT-qPCR |
Statistical Analysis
Data were expressed as mean ± S.E.M, and one-way analysis of variance or Student’s t-test was used for statistical analysis. p<0.05 was considered as statistically significant.
Results
PC Treatment Could Inhibit LPS-Induced SiHa and HeLaCell Proliferation
The SiHa and HeLa cells were cultured with LPS at the concentrations of 0, 0.1, 1, 10, and 100 μg/mL for 24 hrs to determine an appropriate experimental dosage range. As the positive effects of LPS (1 μg/mL) on cell viability were closer to the saturation point compared to the 0 μg/mL of LPS (p<0.01, Figure 1A); thus, 1 μg/mL was selected as the experimental concentration. In a separate experiment, the cells were pretreated with PC (0, 20, 40, 60, 80, and 100 μg/mL) for 12, 24 and 48 hrs. After CCK-8 detection, we found that both SiHa and HeLa cell viability were significantly increased under the treatment of 20 μg/mL of PC (p<0.01) and the cell viability reduced from 20 to 40 μg/mL of PC; however, the cell survival was close to zero under the 100 μg/mL PC treatment for 48 hrs. Therefore, 20 μg/mL PC was set as low-dose group, 40 and 80 μg/mL PC was determined as the middle- and high-dose in order to study the protective effect of PC (Figure 1B and C). The positive effects of LPS on cell viabilities of SiHa and HeLa cells were realized in a dose-dependent manner, and PC inhibited cell viability in dose-and time-dependent manners.
After induction with 1 μg/mL LPS for 24 hrs, SiHa and HeLa cells were then subjected with low, middle and high doses of PC (20, 40 and 80 μg/mL). After incubation for 12, 24, and 48 hrs, the cells were collected for cell viability detection (Figure 2A and B). We found that LPS pretreatment could significantly increase the cell viabilities of SiHa and HeLa cells (p<0.01). However, the increased cell viabilities were all notably reduced (p<0.01) when the cells were co-treated with 20 μg/mL in all groups. More importantly, the inhibitory effects of PC on SiHa and HeLa cell viability were increasingly obvious as the treatment time prolonged. Taken together, the inhibitory effects of PC treatment on the LPS-induced SiHa and HeLa cell proliferation were realized in dose- and time-dependent manners.
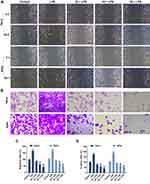
PC Treatment Significantly Suppressed the Migration and Invasion in LPS-Induced SiHa and HeLa Cells
Cell migration and invasion played important roles in the progression of CC metastasis. Our study found that LPS pretreatment could notably enhance the migratory capacity of SiHa and HeLa cells and accelerate the wound-healing progress (p<0.01, Figure 3A and C). When LPS-induced cells were treated with the low concentration of PC, the migration rates in both two types of cells were decreased in the control group (p<0.01). The PC at 40 and 80 μg/mL showed a stronger inhibition to the migration ability, and the migration rate in 40μg/mL PC group was much lower than that in low-dose PC group, while the rate in high-dose PC group was lower than middle-dose PC group (p<0.01). Furthermore, the ability of PC in affecting cell invasion was similar to the migratory capacity (Figure 3B and D). After 24 hrs of pretreatment of LPS, a significant increase in the number of invaded cells was observed, compared with the control group (p<0.01). PC treatment could also remarkably decrease the number of invaded cells, and the number has continued to decline as the dose of PC (p<0.01) increased. Thus, PC treatment could effectively inhibit the LPS-induced cell migration and invasion in SiHa and HeLa cells.
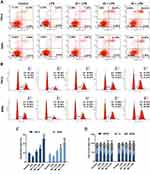
PC Treatment Induced Cell Apoptosis and Promoted Cell Cycle G2/M Phase Arrest in SiHa and HeLa Cells
To investigate whether the inhibition of PC on CC cell viability was mediated through regulating cell apoptosis and cell cycle progression, the apoptosis and cell cycle distribution were analyzed by flow cytometry. As shown in Figure 4A and C, after pretreatment with LPS, the apoptosis rate of SiHa and HeLa cells were decreased significantly and synchronously, compared with the control (p<0.01), indicating that LPS could further enhance the resistance of CC cells to apoptosis. After treatment with PC, we observed that the low concentration of PC was effective enough to abolish the LPS-induced apoptosis resistance, and that the 40 μg/mL of PC further enhanced the apoptosis at the basis of 20 μg/mL treatment (p<0.01). When SiHa and HeLa cells were cultured with a high concentration of PC, the apoptosis rates were increased markedly, which were more than two times of the rate in control groups (p<0.01). In Figure 4B and D, the results of cell cycle distribution analysis showed that after the induction of LPS, the number of G0/G1 phase cell was obviously increased (p<0.05) and decreased at the G2/M phase (p<0.01), indicating that LPS treatment promoted the cell cycle progression. However, we also observed that PC concentration was negatively associated with the cell cycle progression. The 20 μg/mL of PC could basically abolish the positive effects of LPS on cell cycle progression. More importantly, the number of G2/M phase had a rapid increase as the concentration of PC increased (p<0.01); however, the number of G0/G1 phase cell was reduced, which may indicate that PC treatment had the ability to inhibit the cell cycle progression through inducing cell cycle G2/M phase arrest. Thus, our data implied that the inhibitory effects of PC on the CC cell proliferation were attributed to the induction of apoptosis and cell cycle G2/M phase arrest.
PC Treatment Participated in the Regulation of Apoptosis-Associated Proteins
To further verify the effects of PC on the CC cell apoptosis, the expressions of apoptosis-associated proteins (cleaved caspase-3, Bcl-2, and Bax) were measured by Western blot. As shown in Figure 5A and B, the pretreatment of LPS could significantly enhance the level of Bcl-2 and down-regulated cleaved caspase-3 and Bax levels in HeLa cells in comparison to the control (p<0.01). After PC treatment, we also observed that low-dose PC was enough to counteract the effects of LPS on the apoptosis-associated proteins. The 40 and 80 μg/mL of PC could further upregulate the levels of cleaved caspase-3 and Bax proteins and inhibit the expression of Bcl-2 (p<0.01). The effects of LPS and PC on the apoptotic proteins in SiHa were basically consistent with those in HeLa cells (Figure 5C and D). Therefore, LPS could induce the upregulation of Bcl-2 expression, and downregulation of Cleaved caspase-3 and Bax protein levels in CC cells, while PC could effectively reverse the regulatory effects of LPS on these apoptosis-related proteins. These results further verified that the promoting effects of PC on CC cell apoptosis.
PC Treatment Could Abolish LPS-Induced Inflammatory Cytokines Production in SiHa and HeLa Cells
The effects of PC on the production of inflammatory cytokines from LPS-simulated SiHa and HeLa cells were assessed. In Figure 6A and B, we found that LPS stimulation could remarkably promote the expressions of interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α, compared with the control group. Meanwhile, the elevated IL-6, IL-1β, and TNF-α expressions were obviously suppressed by PC in a dose-dependent manner. Schematic diagram of proposed mechanism was proposed (Figure 6C). LPS may regulate inflammation response by promoting the expression of TLR4 and p65 activation. Taken together, PC could effectively inhibit LPS-induced inflammatory cytokine expressions in CC.
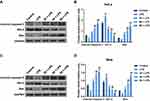
PC Inhibited the Activation TLR4/NF-κB Pathway in LPS-Stimulated SiHa and HeLa Cells
As mentioned earlier, TLR4 played a key role in the LPS-mediated inflammatory cytokine expressions via regulating NF-κB pathway. In our study, the protein level of TLR4 in the LPS-simulated HeLa and SiHa cells went up significantly in comparison to the control group (p<0.01, Figure 7A–D). The components of the NF-κB pathway were detected to further explore whether the regulation of NF-κB pathways was involved in the mechanism of PC acting on the LPS-simulated SiHa and HeLa cells. As shown in Figure 7A–D, LPS stimulation could significantly enhance the p-P65 protein level (p<0.01); however, no apparent change of t-P65 protein level was observed among control, LPS stimulation and PC treatment groups. The p-P65/t-P65 ratio was notably inhibited by PC treatment in a dose-dependent manner (p<0.01). Additionally, we also found that LPS stimulation contributed to the translocation of P65 from the cytoplasm to nuclear, according to the phenomena of the significant increase occurred to cytoplasm P65 and decrease occurred to nuclear P65 (p<0.01, Figure 7E–H). However, PC treatment could effectively inhibit the LPS-induced P65 translocation in a dose-dependent manner. Collectively, these findings indicated that the anti-inflammatory capacity of PC was attributed to the involvement of TLR4/NF-κB pathway in LPS-simulated SiHa and HeLa cells.
Discussion
Although chemotherapy is still a fundamental therapeutic schedule for most malignancies, drug side effects and a rapid development of drug resistance significantly hinder the efficacy of chemotherapy.26,27 Hence, it is still necessary to identify safer and more effective anti-cancer drugs. The inhibitory effects of PC on the progression of CC have been reported; however, the underlying mechanism remains unclear. In this current study, we observed that the activation of TLR4/NF-κB pathway could be effectively suppressed by PC treatment in the LPS-stimulated SiHa and HeLa cells.
Firstly, previous study has shown that grape seed proanthocyanidins (GSPs) could effectively reduce the cell viability of SiHa and HeLa cells in a dose-dependent manner.23 In our results, we also observed that the PC treatment led to a dose-and time-dependent reduction in cell viability. Secondly, it is well known that elevated migratory and invasive capacities are essential for the metastasis of tumor cells.28 GSPs were reported to significantly suppress the migratory and invasive ability of tongue squamous cell carcinoma (TSCC) cells through blocking the secretion of matrix metalloproteinases.29 Similarly, the experiment of in vivo bioluminescence imaging showed that dietary administration of GSPs could suppress the migration of intravenously injected melanoma cells through the activation of β-catenin and its downstream targets in the lungs of immune-compromised nude mice.30 In our results, the reduction of SiHa and HeLa cells migration and invasion in our study indicated that the treatment of PC could also markedly inhibit the metastasis progression of CC by controlling tumor cell migration and invasion. In addition, the results of cell cycle distribution assay observed that the treatment of PC resulted in a remarkable cell cycle arrest at the G2/M phase, and such a cell cycle G2/M phase arrest provided an opportunity for the induction of apoptosis cell death in CC cells.23 In the recent year, Chen et al found that PCs extracted from Uncaria species could inhibit breast cancer progression via regulating the components, including Bax, Bcl-2 and cleaved caspase-3, of mitochondrial pathway, to induce tumor cells to go through apoptosis,31 and such a result was consistent with our data. Not studying the expression of cell cycle regulatory protein expression might be a limitation. Although LPS stimulation further strengthened the resistance of CC cells to apoptosis, PC treatment could still abolish the functional effects of LPS and promote CC cell apoptosis by modulating associated protein levels via the mitochondrial pathway. The pro-apoptotic effects of PC have also been revealed in many other cancers, such as breast cancer,32 nasopharyngeal carcinoma33, and ovarian cancer.34 Collectively, these results indicated that PC could effectively restrain the LPS-induced CC cell proliferation and development through promoting cell cycle arrest and activating mitochondrial apoptosis pathway. Certainly, it would be perfect to perform the animal study, which would be done in the future study.
In this present study, we found that the pretreatment of SiHa and HeLa cells with LPS induced a significant increase of TLR4 protein level, while PC inhibited CC cell progression that was accompanied by the down-regulation of TLR4. TLR4 played an essential role in the LPS-mediated inflammatory response in CC cells.2 After LPS stimulation, TLR4 triggered the myeloid differentiation primary response gene 88 (MyD88), which has been proved to participate in the activation of IL-1 receptor-associated kinases (IRAKs) and the adaptor molecules TNF Receptor-Associated Factor 6 (TRAF6). Subsequently, TRAF6 activation stimulated the autophosphorylation of TAK1 to activate the IκB Kinase (IKK). The activated IKK complex phosphorylated IκB and induced its ubiquitylation and degradation, which could allow P65-NF-κB translocate into the nucleus, therefore promoting the production of pro-inflammatory cytokines.11 Meanwhile, the results of clinical studies also found the overexpression of TLR4 and NF-κB in the CC in comparison to the surrounding tissues.10 Combined with these studies, we further detected the protein levels of NF-κB signal and found that PC could significantly inhibit the LPS-induced phosphorylation of P65 and block the P65-NF-κB translocation into nuclei. Therefore, we speculated that the anti-inflammatory effect of PC on CC cells was attributed to the inhibition of the TLR4/NF-κB activation.
Conclusion
In summary, our results revealed that the inhibition of the CC cell proliferation by PC was mediated through the induction of tumor cell apoptosis and inhibition of inflammatory cytokine secretion in CC. In this study, LPS stimulation enhanced CC cell migratory and invasive capacity and promoted the cell cycle progression and resistance to apoptosis. Moreover, TLR4 activation by LPS could induce the inflammatory response via the NF-κB pathway. However, PC could not only trigger the mitochondrial apoptosis pathway and induce the apoptosis of CC cells, but also block the TLR4/NF-κB inflammation pathway.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Pimple S, Mishra G, Shastri S. Global strategies for cervical cancer prevention. Curr Opin Obstet Gynecol. 2016;28(1):4–10. doi:10.1097/GCO.0000000000000241
2. He A, Ji R, Shao J, He C, Jin M, Xu Y. TLR4-MyD88-TRAF6-TAK1 complex-mediated NF-kappaB activation contribute to the anti-inflammatory effect of V8 in LPS-induced human cervical cancer SiHa cells. Inflammation. 2016;39(1):172–181. doi:10.1007/s10753-015-0236-8
3. Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev. 2017;26(4):444–457. doi:10.1158/1055-9965.EPI-16-0858
4. Guardado-Estrada M, Juarez-Torres E, Roman-Bassaure E, et al. The distribution of high-risk human papillomaviruses is different in young and old patients with cervical cancer. PLoS One. 2014;9(10):e109406. doi:10.1371/journal.pone.0109406
5. Mathew A, George PS. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix–worldwide. Asian Pac J Cancer Prev. 2009;10(4):645–650.
6. Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28(5):886–892. doi:10.1248/bpb.28.886
7. Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. doi:10.3389/fimmu.2014.00316
8. Oblak A, Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin Dev Immunol. 2011;2011:609579. doi:10.1155/2011/609579
9. Wang Y, Weng Y, Shi Y, Xia X, Wang S, Duan H. Expression and functional analysis of Toll-like receptor 4 in human cervical carcinoma. J Membr Biol. 2014;247(7):591–599. doi:10.1007/s00232-014-9675-7
10. Cheng YX, Qi XY, Huang JL, et al. Toll-like receptor 4 signaling promotes the immunosuppressive cytokine production of human cervical cancer. Eur J Gynaecol Oncol. 2012;33(3):291–294.
11. Shrimali D, Shanmugam MK, Kumar AP, et al. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013;341(2):139–149. doi:10.1016/j.canlet.2013.08.023
12. Lee HL, Park MH, Hong JE, et al. Inhibitory effect of snake venom toxin on NF-kappaB activity prevents human cervical cancer cell growth via increase of death receptor 3 and 5 expression. Arch Toxicol. 2016;90(2):463–477. doi:10.1007/s00204-014-1393-5
13. Wang J, Lin D, Peng H, Shao J, Gu J. Cancer-derived immunoglobulin G promotes LPS-induced proinflammatory cytokine production via binding to TLR4 in cervical cancer cells. Oncotarget. 2014;5(20):9727–9743. doi:10.18632/oncotarget.2359
14. Dijkgraaf EM, Heusinkveld M, Tummers B, et al. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73(8):2480–2492. doi:10.1158/0008-5472.CAN-12-3542
15. Piratvisuth T, Marcellin P, Popescu M, Kapprell HP, Rothe V, Lu ZM. Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int. 2013;7(2):429–436. doi:10.1007/s12072-011-9280-0
16. Wang H, Zhang C, Lu D, et al. Oligomeric proanthocyanidin protects retinal ganglion cells against oxidative stress-induced apoptosis. Neural Regen Res. 2013;8(25):2317–2326. doi:10.3969/j.issn.1673-5374.2013.25.002
17. Sharma SD, Meeran SM, Katiyar SK. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E(2) and prostaglandin E(2) receptors. Mol Cancer Ther. 2010;9(3):569–580. doi:10.1158/1535-7163.MCT-09-0638
18. Nie C, Zhou J, Qin X, et al. Reduction of apoptosis by proanthocyanidin-induced autophagy in the human gastric cancer cell line MGC-803. Oncol Rep. 2016;35(2):649–658. doi:10.3892/or.2015.4419
19. Mansoor KA, Matalka KZ, Qa’dan FS, Awad R, Schmidt M. Two new proanthocyanidin trimers isolated from Cistus incanus L. demonstrate potent anti-inflammatory activity and selectivity to cyclooxygenase isoenzymes inhibition. Nat Prod Res. 2016;30(17):1919–1926. doi:10.1080/14786419.2015.1089242
20. Salinas-Sanchez DO, Jimenez-Ferrer E, Sanchez-Sanchez V, et al. Anti-inflammatory activity of a polymeric proanthocyanidin from Serjania schiedeana. Molecules. 2017;22:6. doi:10.3390/molecules22060863
21. Assis LC, Hort MA, de Souza GV, et al. Neuroprotective effect of the proanthocyanidin-rich fraction in experimental model of spinal cord injury. J Pharm Pharmacol. 2014;66(5):694–704. doi:10.1111/jphp.2014.66.issue-5
22. Strathearn KE, Yousef GG, Grace MH, et al. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinsons disease. Brain Res. 2014;1555:60–77. doi:10.1016/j.brainres.2014.01.047
23. Chen Q, Liu XF, Zheng PS. Grape seed proanthocyanidins (GSPs) inhibit the growth of cervical cancer by inducing apoptosis mediated by the mitochondrial pathway. PLoS One. 2014;9(9):e107045. doi:10.1371/journal.pone.0107045
24. Bailon-Moscoso N, González-Arévalo G, Velásquez-Rojas G, et al. Phytometabolite dehydroleucodine induces cell cycle arrest, apoptosis, and DNA damage in human astrocytoma cells through p73/p53 regulation. PLoS One. 2015;10:8. doi:10.1371/journal.pone.0136527
25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif). 2001;25(4):402–408. doi:10.1006/meth.2001.1262
26. Pearce A, Haas M, Viney R, et al. Incidence and severity of self-reported chemotherapy side effects in routine care: a prospective cohort study. PLoS One. 2017;12(10):e0184360. doi:10.1371/journal.pone.0184360
27. Fonia A, Cota C, Setterfield JF, Goldberg LJ, Fenton DA, Stefanato CM. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76(5):948–957. doi:10.1016/j.jaad.2016.12.027
28. Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19(11):1450–1464. doi:10.1038/nm.3391
29. Yang N, Gao J, Cheng X, et al. Grape seed proanthocyanidins inhibit the proliferation, migration and invasion of tongue squamous cell carcinoma cells through suppressing the protein kinase B/nuclear factor-kappaB signaling pathway. Int J Mol Med. 2017;40(6):1881–1888. doi:10.3892/ijmm.2017.3162
30. Vaid M, Singh T, Prasad R, Kappes JC, Katiyar SK. Therapeutic intervention of proanthocyanidins on the migration capacity of melanoma cells is mediated through PGE2 receptors and beta-catenin signaling molecules. Am J Cancer Res. 2015;5(11):3325–3338.
31. Chen XX, Leung GP, Zhang ZJ, et al. Proanthocyanidins from Uncaria rhynchophylla induced apoptosis in MDA-MB-231 breast cancer cells while enhancing cytotoxic effects of 5-fluorouracil. Food Chem Toxicol. 2017;107(Pt A):248–260. doi:10.1016/j.fct.2017.07.012
32. Chen XX, Lam KH, Chen QX, et al. Ficus virens proanthocyanidins induced apoptosis in breast cancer cells concomitantly ameliorated 5-fluorouracil induced intestinal mucositis in rats. Food Chem Toxicol. 2017;110:49–61. doi:10.1371/journal.pone.0142157
33. Yao K, Shao J, Zhou K, et al. Grape seed proanthocyanidins induce apoptosis through the mitochondrial pathway in nasopharyngeal carcinoma CNE-2 cells. Oncol Rep. 2016;36(2):771–778. doi:10.3892/or.2016.4855
34. Liu J, Bai J, Jiang G, et al. Anti-tumor effect of pinus massoniana bark proanthocyanidins on ovarian cancer through induction of cell apoptosis and inhibition of cell migration. PLoS One. 2015;10(11):e0142157.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.