Back to Journals » Infection and Drug Resistance » Volume 15
Procoagulant Microvesicles in COVID-19 Patients: Possible Modulators of Inflammation and Prothrombotic Tendency
Authors Hamali HA , Saboor M , Dobie G , Madkhali AM, Akhter MS , Hakamy A, Al-Mekhlafi HM, Jackson DE , Matari YH, Mobarki AA
Received 22 December 2021
Accepted for publication 20 April 2022
Published 29 April 2022 Volume 2022:15 Pages 2359—2368
DOI https://doi.org/10.2147/IDR.S355395
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Hassan A Hamali,1 Muhammad Saboor,1,2 Gasim Dobie,1 Aymen M Madkhali,1 Mohammad S Akhter,1 Ali Hakamy,3 Hesham M Al-Mekhlafi,2 Denise E Jackson,4 Yahya H Matari,5 Abdullah A Mobarki1
1Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, Jazan University, Gizan, Saudi Arabia; 2Medical Research Center, Jazan University, Gizan, Saudi Arabia; 3Department of Respiratory Therapy, Faculty of Applied Medical Sciences, Jazan University, Gizan, Saudi Arabia; 4Thrombosis and Vascular Diseases Laboratory, School of Health and Biomedical Sciences, Royal Melbourne Institute of Technology (RMIT) University, Bundoora, VIC, Australia; 5Laboratory Department, Baish General Hospital, Gizan, Saudi Arabia
Correspondence: Hassan A Hamali, Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, Jazan University, P.O. Box 1906, Gizan, 45142, Saudi Arabia, Tel +966173295000, Email [email protected]
Background: The hypercoagulability and thrombotic tendency in coronavirus disease 2019 (COVID-19) is multifactorial, driven mainly by inflammation, and endothelial dysfunction. Elevated levels of procoagulant microvesicles (MVs) and tissue factor–bearing microvesicles (TF-bearing MVs) have been observed in many diseases with thrombotic tendency. The current study aimed to measure the levels of procoagulant MVs and TF-bearing MVs in patients with COVID-19 and healthy controls and to correlate their levels with platelet counts, D-Dimer levels, and other proposed calculated inflammatory markers.
Materials and Methods: Forty ICU-admitted patients with COVID-19 and 37 healthy controls were recruited in the study. Levels of procoagulant MVs and TF-bearing MVs in the plasma of the study population were measured using enzyme linked immunosorbent assay.
Results: COVID-19 patients had significantly elevated levels of procoagulant MVs and TF-bearing MVs as compared with healthy controls (P< 0.001). Procoagulant MVs significantly correlated with TF-bearing MVs, D-dimer levels, and platelet count, but not with calculated inflammatory markers (neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and platelet/neutrophil ratio).
Conclusion: Elevated levels of procoagulant MVs and TF-bearing MVs in patients with COVID-19 are suggested to be (i) early potential markers to predict the severity of COVID-19 (ii) a novel circulatory biomarker to evaluate the procoagulant activity and severity of COVID-19.
Keywords: microvesicles, tissue factor, procoagulant, D-dimer, NLR, PLR, PNR, COVID-19
Introduction
At the end of 2019, novel coronavirus disease 2019 (COVID-19) emerged, causing severe acute respiratory syndrome, and affecting the whole world till date, as well as paralyzing all life activities and placing devastating burdens on the economies and healthcare systems of many countries. COVID-19 is one of the most contagious viral infection, associated with a high percentage of morbidity and mortality in a short time.1,2 Apart from the severe acute respiratory syndrome, COVID-19 also involves other pathophysiological processes, including inflammatory response, endothelial damage, and coagulopathy associated with systemic organ involvement, disseminated intravascular coagulopathy (DIC),2 and cardio- and cerebrovascular complications.3 COVID-19ʹs pathogenesis and endothelial dysfunction drive hypercoagulability and thrombotic tendency, which are hallmarks of this disease.3,4 In addition, inflammatory responses, vascular damage, and oxidative stress play key roles in driving the microthrombogenic status, in which microvesicles (MVs) have a crucial role. MVs are involved in microthrombi in cerebrovascular compilations.3
Microvesicles are small membrane fragments that can be released from activated platelets or cells undergoing apoptosis. Moreover, they are found in blood, saliva, semen, urine, and tissues,5–9 and are released from almost all types of cells, including megakaryocytes, endothelial cells, leukocytes, erythrocytes, stem cells, vascular smooth muscle cells, mast cells, and even in vitro in culture cell lines. MVs are submicron in size (from 10 nm to 1000 nm) and heterogeneous in contents.5,6 MVs are a key player in driving the thrombotic tendency in many diseases. Elevated levels of procoagulant MVs have been reported in physiological states10–12 and pathological13–16 conditions, including viral infection including dengue infection17 and COVID-19 infection.18–24 Furthermore, MVs have been shown to be associated with thrombosis.15,16,18,19 MVs are procoagulant in nature because of the expression of phosphatidylserine (PS) on their surfaces. PS is a negatively charged phospholipid that is generated through the flip-flop mechanism from activated platelets or cells undergoing apoptosis.25 The procoagulant activity of MVs increases in the presence of tissue factor (TF), and PS on the surface of the procoagulant MVs enhances TF activity.26 MVs have the ability to support the binding of coagulation complexes and the acceleration of thrombin formation have been shown in vivo and in vitro.27,28 The aims of the current study were to evaluate the levels of procoagulant MVs and TF-bearing MVs in patients with COVID-19 and to correlates their levels with D-Dimer levels and other inflammatory markers such as neutrophils-lymphocytes ratio (NLR), mean platelet ratio to mean platelet count (MPR) and mean platelet count to mean neutrophil count ratio (PNR).
Materials and Methods
This case-control study involved a total of 77 adult participants, including intensive care unit (ICU)-admitted patients with COVID-19 (n=40; 22 males and 18 females) and healthy controls (n=37; 23 males and 14 females). The patient group consisted of diagnosed COVID-19 patients ICU-admitted patients to Baish General Hospital, Baish, Jazan region, Saudi Arabia. The patient groups were diagnosed with RT-polymerase chain reaction (RT-PCR). Healthy controls were blood donors, who donated blood in the blood center in Baish General Hospital with normal hematological parameters and negative status of transfusion transmitted infections.
Sample Collection
Venous blood samples were collected in vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) and sodium citrate anticoagulants from patients and controls. The EDTA anticoagulated blood samples were used for complete blood count (CBC) analysis, whereas the sodium citrated samples were used for the evaluation of coagulation profiles, D-dimer levels and procoagulant MVs, and TF-bearing MV analysis.
Plasma Preparation and Microvesicles Isolation
Citrated whole blood was centrifuged at 3000 rpm for 30 minutes at 22 °C, followed by a second centrifugation step at 13,000 rpm for 2 minutes to obtain platelet-free plasma (PFP). The supernatant (PFP) was separated and distributed into two aliquots; one was used freshly for the evaluation of the coagulation profile and D-dimer test, whereas the other was stored at −80 °C for MVs and TF-bearing MVs analysis.
Complete Blood Count
CBC from EDTA anticoagulant tubes was performed on a Sysmex XN-550 Hematology Analyzer (Sysmex, Kobe, Japan).
Coagulation Profile and D-Dimer Testing
A Stago compact analyzer (Diagnostica Stago, Asnieres sur Seine, France) was used to measure the coagulation profile including prothrombin time; PT and activated partial thromboplastin; aPTT and D-Dimer levels. The D-dimer levels was evaluated in patients with COVID-19 using immune-turbidimetric Assay (Diagnostica Stago, Asnieres sur Seine, France).
Complete Blood Count–Derived Parameters
The complete blood count-derived parameters were neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and platelet/neutrophil ratio (PNR). NLR obtained by dividing neutrophil count/lymphocyte count), PLR obtained by dividing platelet count/lymphocyte count), and PNR obtained by platelet by neutrophil count).21
Detection of Microvesicles and Tissue Factor–Bearing Microvesicles
The analyses of MVs and TF-bearing MVs in the plasma were conducted according to the manufacturer procedure. Procoagulant MV and TF-bearing MV levels were measured using a Zymuphen MP-Activity Kit and a Zymuphen MP-TF ELISA Kit (Aniara Diagnostica, OH, USA), respectively.
Statistical Analysis
GraphPad Prism (version 8.0) was used for statistical analysis. Unless otherwise stated, the data in the current study are shown as mean ± standard deviation (SD). Independent unpaired t-test was used to test for the differences between patients and controls, and Chi-square test was used for the demographic data analysis. Single-sample t-test was used for the evaluation of correlation coefficients. Significant statistical differences were considered when P-values were less than 0.05.
Results
Demographic Data
Forty COVID-19 patients (22 males and 18 females; patient group) and 37 healthy controls (23 males and 14 females; control group) were evaluated in both groups. No significant differences were seen between the genders of the study groups. The age range of the patient group was 56–65 years (60.2±2.5), and that of the control group was 40–63 years (51.1±3.9) (Table 1).
Hematological Parameters
The hematological values of both groups are shown in Table 1. The results showed statistically significant differences among all CBC parameters except mean cell hemoglobin, red cell distribution width, and absolute monocyte count. A significant higher white blood cell (WBC) count including neutrophils was observed in the patient group as compared to the control group (P < 0.0001). Conversaly, significantly low platelet count (P < 0.0001), absolute lymphocyte count (P < 0.0001), absolute eosinophil count (P < 0.0001) and absolute basophil count (P < 0.05) were observed in the patient group as compared with the control group, whereas a higher absolute monocyte count was observed in patients as compared with controls, however, the difference was not statistically significant.
Complete Blood Count–Derived Parameters
The NLR, and PLR, were higher and lower PNR in the patient group as compared with the control group (P < 0.0.5; Table 2).
Coagulation and D-Dimer Tests
The PT and aPTT results were significantly higher in the patient group as compared with the control group (P < 0.0001). The mean values of D-dimers, in the patient group, were 3.2±3.5 ng/mL (Table 3) which were considered high as compared with the preestablished interlaboratory reference range).
Procoagulant Microvesicles and Tissue Factor-Bearing Microvesicles
Table 4 and Figure 1A show that the level of MVs in the patient group was ~5.6-folds higher than the control group (21.73±12.5 nM vs 3.9±1.8 nM; P < 0.0001). Similarly, increased levels of TF-bearing MVs (~3.6-folds) were also observed in the patient group as compared to control group (1.9±0.7 pg/mL vs 0.5±0.3 pg/mL; P < 0.0001) (Table 4 and Figure 1B).
Correlation Studies
Statistically significant positive correlation was observed between MVs and TF-bearing MVs levels among the COVID-19 patient group (r = 0.4703, R squared = 0.2212; P = 0.0022), and D-dimer levels (r = 0.516, R squared = 0.2663; P =0.0011) (Figure 2; Table 5). In addition, statistically significant negative correlation was observed between MVs and platelets (r= - 0.3347, R squared = 0.1120; P = 0.0429). On the other hand, no significant correlation was observed between MVs levels and NLR, PLR, or PNR (Table 5).
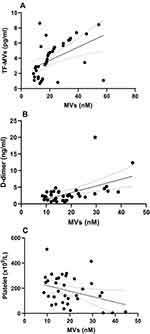 |
Figure 2 Scatter plot between microvesicles and (A) tissue factor-bearing microvesicles, (B) D-dimer levels and (C) platelet count. |
Discussion
Our findings showed abnormalities in the hematological parameters, including increased WBC count (leukocytosis), neutropenia and lymphopenia, low red blood cell (RBC) count, low hemoglobin concentration, and low platelet count. These results are consistent with previous reports in COVID-19 patients elsewhere.21,29,30
In addition, like previous reports,20,21 the current study reports elevated levels of D-dimer in COVID-19 patients. Elevated D-dimer levels are associated with increased mortality in these patients.19,31 Moreover, PT and aPTT were found to be prolonged in our patient group as compared with the control group. A recent study demonstrated longer PT and aPTT among COVID-19 non-survivors compared to survivors on admission.31 Thrombocytopenia, high D-dimer, and prolonged PT have been proposed to contribute to the hypercoagulability in COVID-19 patients,21 which may result in DIC;31 coagulopathy in COVID-19 is accompanied with vascular/endothelial damage.32
The current study shows elevated levels of procoagulant MVs in the patient group as compared to healthy controls. Elevated MVs have been reported in COVID-19 infection18–23 lung diseases,33 other viral diseases,17 and inflammatory conditions,13 which have been associated with tendency for thrombosis. Interestingly, elevated levels of MVs derived from endothelial cells and platelets have been reported in COVID-19 with malignancy as compared to healthy controls.21 Elevated levels of MVs have been reported elsewhere to contribute to COVID-19 severity and mortality.18,23
Because of their procoagulant nature, MVs can assemble coagulation complexes and support thrombin generation.34,35 The prothrombotic changes in patients with COVID-19 are driven by many factors including increased thrombin generation and decreased fibrinolytic activity.36 The procoagulant surface of MVs is similar to the surface of activated platelets, which supports the assembly of coagulation cascade in healthy individuals.28 Furthermore, the surface of MVs are more procoagulant (50–100-fold) than the surface of activated platelets.27 MVs transfer active mediators and are considered a reliable marker of vascular damage. In addition, MVs modulate inflammatory responses by inducing endothelial cells to release TF, interleukin-6 (IL-6), cytokines, and other inflammatory modulators, which lead to attract and increase other cells’ adhesiveness to the damaged endothelium.37–40 The level of plasma endothelial-derived MVs has been suggested to be an early marker for lung diseases.41,42 Endothelial-derived MVs have been shown to carry angiotensin-converting enzyme (ACE),43,44 including in cases of lung injury,44 which has been linked to thrombosis in COVID-19.45
Tissue factor is the principal activator of the coagulation cascade, which is another procoagulant player.46,47 Although TF is normally found in the sub-endothelium matrix and TF is sequestrated from the circulation, some studies have demonstrated that TF-bearing MVs13,17 or soluble TF48,49 can be found in the blood circulation in many inflammatory diseases. Elevated levels of TF and increased TF patient with COVID-19 compared to non-COVID-19 patients.18,50 The current study found significantly increased levels of TF-bearing MVs in the circulation of patient group, which is similar to previously published findings related to COVID-1918,21 and viral infections.17 In COVID-19, TF-bearing MVs have been suggested to play a role in thrombosis and even the severity and mortality.18
There is a growing recognition of MVs as key players in hemostasis, thrombosis, inflammation, and other pathological mechanisms because of their procoagulant nature (PS and TF), by which they contain and express biologically active mediators. Thus, understanding the underlying pathophysiology of many diseases could be a new discovery for potentially promising new therapeutic strategies.15,51,52 The PLR, NLR, and PNR have been proposed to be associated with inflammation and the severity of many diseases, including COVID-19.53–59 In the current study, the PLR and NLR were higher in the patient group as compared with the control group; this finding is similar to previously reported findings.21 An elevated NLR has been reported to be associated with poor outcomes and mortality in COVID-19.60–64 In the current study, we could not find any correlation between MVs or TF-bearing MVs and NLR, PLR, and PNR. In contrast, we found a significant positive correlation of MVs with TF-bearing MVs and D-dimer levels. This is similar to the result of a recently published reports.18,19,21 At the same time, we found a significant negative correlation with platelet count; Zahran et al also reported a negative correlation between MVs derived from platelets and MVs derived from endothelial cells with platelet count.21 Elevated levels of MVs, TF and D-dimer have been suggested to be associated with thrombosis, severity and mortality in COVID-19.18,22
As similar to many other studies, this research has some limitations. The study did not consider any thrombotic data and the comorbidities of the patients, and it did not follow up with the patient outcomes. In addition, we used only one study application (functional assay) to evaluate the procoagulant activity of MVs and TF-bearing MVs. Combining the functional assay with other applications is highly recommended, such as by utilizing flow cytometry for antigenic features and nanoparticle tracing analysis for precise size determination; this represents a future research direction. Although the sample size was relatively small, the data generated in the current study are in line with the literature on viral infection and add to the growing literature on the procoagulant activity of COVID-19.
Conclusions
The findings of the current study showed significantly elevated levels of MVs and TF-bearing MVs in patients with COVID-19; these high levels are associated with increased procoagulant activity of patients with COVID-19 and therefore lead to increased risk of hypercoagulability in COVID-19 and subsequently increase the risk of prothrombotic tendency. Although the current study showed the significant procoagulant activity of the MVs and TF-bearing MVs in COVID-19, further research is needed to explain the exact mechanism of hypercoagulability in relation to other factors, such as endothelial damage markers and the origin of MVs. MVs role in driving procoagulant activity in COVID-19 may represent a novel and potentially key role as early marker in predicting inflammatory response and thrombotic events.
Ethical Considerations
The current study was approved by the Jazan Health Ethics Committee, Ministry of Health, and was carried out according to the Declaration of Helsinki. Informed consent was waived off by the Ethics committee on special request to avoid close contact with the COVID-19 patients. Personal identification and bioinformation of study subjects were neither collected nor disclosed and the data were kept anonymously and confidentiality.
Acknowledgments
The authors acknowledge the Medical Research Center, Jazan University, Saudi Arabia, for providing some technical facilities.
Author Contributions
All authors have made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors have read the journal’s policy and declare no conflict of interest.
References
1. Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455–459. doi:10.1002/jmv.25688
2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
3. Che Mohd Nassir CMN, Hashim S, Wong KK, et al. COVID-19 infection and circulating microparticles-reviewing evidence as microthrombogenic risk factor for cerebral small vessel disease. Mol Neurobiol. 2021;58(8):4188–4215. doi:10.1007/s12035-021-02457-z
4. Akhter MS, Hamali HA, Mobarki AA, Rashid H, Oldenburg J, Biswas A. Sars-cov-2 infection: modulator of pulmonary embolism paradigm. J Clin Med. 2021;10(5):1–20. doi:10.3390/jcm10051064
5. Abid Hussein MN, Böing AN, Sturk A, Hau CM, Nieuwland R. Inhibition of microparticle release triggers endothelial cell apoptosis and detachment. Thromb Haemost. 2007;98(11):1096–1107. doi:10.1160/TH05-04-0231
6. Essayagh S, Brisset A-C, Terrisse A-D, et al. Microparticles from apoptotic vascular smooth muscle cells induce endothelial dysfunction, a phenomenon prevented by beta3-integrin antagonists. Thromb Haemost. 2005;94(4):853–858. doi:10.1160/TH04-12-0786
7. Rousseau M, Belleannee C, Duchez AC, et al. Detection and quantification of microparticles from different cellular lineages using flow cytometry. Evaluation of the impact of secreted phospholipase A2 on microparticle assessment. PLoS One. 2015;10(1):1–27. doi:10.1371/journal.pone.0116812
8. Iwai K, Minamisawa T, Suga K, Yajima Y, Shiba K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J Extracell Vesicles. 2016;5(1):30829. doi:10.3402/jev.v5.30829
9. Berckmans RJ, Sturk A, Van Tienen LM, Schaap MCL, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011;117(11):3172–3180. doi:10.1182/blood-2010-06-290460
10. Shill DD, Lansford KA, Hempel HK, Call JA, Murrow JR, Jenkins NT. Effect of exercise intensity on circulating microparticles in men and women. Exp Physiol. 2018;103(5):693–700. doi:10.1113/EP086644
11. Aharon A, Brenner B. Microparticles and pregnancy complications. Thromb Res. 2011;127(Suppl):S67–71. doi:10.1016/S0049-3848(11)70019-6
12. VanWijk MJ, Nieuwland R, Boer K, van der Post JAM, VanBavel E, Sturk A. Microparticle subpopulations are increased in preeclampsia: possible involvement in vascular dysfunction? Am J Obstet Gynecol. 2002;187(2):450–456. doi:10.1067/mob.2002.124279
13. Hamali H, Elhussein O, Jamil A, Hussain S, Alshraim M, Alshehri A. Elevated levels of pro‑coagulant microvesicles in children in‑steady state sickle cell disease. J Appl Hematol. 2015;6:115–118. doi:10.4103/1658-5127.165650
14. Nieuwland R, Berckmans RJ, McGregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95(3):930–935. doi:10.1182/blood.V95.3.930.003k46_930_935
15. Burton JO, Hamali HA, Singh R, et al. Elevated levels of procoagulant plasma microvesicles in dialysis patients. PLoS One. 2013;8(8):e72663. doi:10.1371/journal.pone.0072663
16. Bidot L, Jy W, Bidot CJ, et al. Microparticle-mediated thrombin generation assay: increased activity in patients with recurrent thrombosis. J Thromb Haemost. 2008;6(6):913–919. doi:10.1111/j.1538-7836.2008.02963.x
17. Hamali HA, Mobarki AA, Akhter MS, et al. Elevated levels of procoagulant microvesicles in patients with dengue fever. Future Virol. 2020;15(10):701–706. doi:10.2217/fvl-2020-0202
18. Rosell A, Havervall S, von Meijenfeldt F, et al. Patients With COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality-brief report. Arterioscler Thromb Vasc Biol. 2021;41(2):878–882. doi:10.1161/ATVBAHA.120.315547
19. Guervilly C, Bonifay A, Burtey S, et al. Dissemination of extreme levels of extracellular vesicles: tissue factor activity in patients with severe COVID-19. Blood Adv. 2021;5(3):628–634. doi:10.1182/bloodadvances.2020003308
20. Morel O, Marchandot B, Jesel L, et al. Microparticles in COVID-19 as a link between lung injury extension and thrombosis. ERJ Open Res. 2021;7(2):00954–02020. doi:10.1183/23120541.00954-2020
21. Zahran AM, El-Badawy O, Ali WA, Mahran ZG, Mahran EE, Rayan A. Circulating microparticles and activated platelets as novel prognostic biomarkers in COVID-19; relation to cancer. PLoS One. 2021;16(2):1–17. doi:10.1371/journal.pone.0246806
22. Campbell RA, Hisada Y, Denorme F, et al. Comparison of the coagulopathies associated with COVID-19 and sepsis. Res Pract Thromb Haemost. 2021;5(4):e12525. doi:10.1002/rth2.12525
23. Balbi C, Burrello J, Bolis S, et al. Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine. 2021;67:103369. doi:10.1016/j.ebiom.2021.103369
24. Krishnamachary B, Cook C, Kumar A, Spikes L, Chalise P, Dhillon NK. Extracellular vesicle-mediated endothelial apoptosis and EV-associated proteins correlate with COVID-19 disease severity. J Extracell Vesicles. 2021;10(9):e12117. doi:10.1002/jev2.12117
25. Zwaal RFA, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62(9):971–988. doi:10.1007/s00018-005-4527-3
26. Wolberg AS, Monroe DM, Roberts HR, Hoffman MR. Tissue factor de-encryption: ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coagul Fibrinolysis. 1999;10(4):201–210. doi:10.1097/00001721-199906000-00007
27. Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–434. doi:10.1160/TH06-06-0313
28. Berckmans RJ, Nieuwland R, Böing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85(4):639–646. doi:10.1055/s-0037-1615646
29. Pozdnyakova O, Connell NT, Battinelli EM, Connors JM, Fell G, Kim AS. Clinical significance of CBC and WBC morphology in the diagnosis and clinical course of COVID-19 infection. Am J Clin Pathol. 2021;155(3):364–375. doi:10.1093/ajcp/aqaa231
30. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi:10.1172/JCI137244
31. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi:10.1111/jth.14768
32. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi:10.1056/NEJMoa2015432
33. Nieri D, Neri T, Petrini S, Vagaggini B, Paggiaro P, Celi A. Cell-derived microparticles and the lung. Eur Respir Rev. 2016;25(141):266–277. doi:10.1183/16000617.0009-2016
34. Joop K, Berckmans RJ, Nieuwland R, et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost. 2001;85(5):810–820. doi:10.1055/s-0037-1615753
35. Daniel L, Fakhouri F, Joly D, et al. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int. 2006;69(8):1416–1423. doi:10.1038/sj.ki.5000306
36. von Meijenfeldt FA, Havervall S, Adelmeijer J, et al. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv. 2021;5(3):756–759. doi:10.1182/bloodadvances.2020003968
37. Barry OP, Pratico D, Lawson JA, FitzGerald GA. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99(9):2118–2127. doi:10.1172/JCI119385
38. Nomura S, Tandon NN, Nakamura T, Cone J, Fukuhara S, Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158(2):277–287. doi:10.1016/s0021-9150(01)00433-6
39. Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998;161(8):4382–4387.
40. Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25(7):1512–1518. doi:10.1161/01.ATV.0000170133.43608.37
41. Gordon C, Gudi K, Krause A, et al. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011;184(2):224–232. doi:10.1164/rccm.201012-2061OC
42. Bakouboula B, Morel O, Faure A, et al. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177(5):536–543. doi:10.1164/rccm.200706-840OC
43. Abbas M, Jesel L, Auger C, et al. Endothelial microparticles from acute coronary syndrome patients induce premature coronary artery endothelial cell aging and thrombogenicity: role of the Ang II/AT1 receptor/NADPH oxidase-mediated activation of MAPKs and PI3-Kinase pathways. Circulation. 2017;135(3):280–296. doi:10.1161/CIRCULATIONAHA.116.017513
44. Takei Y, Yamada M, Saito K, et al. Increase in circulating ACE-positive endothelial microparticles during acute lung injury. Eur Respir J. 2019;54(4):1801188. doi:10.1183/13993003.01188-2018
45. Dalan R, Boehm BO. Thrombosis post COVID-19 vaccinations: potential link to ACE pathways. Thromb Res. 2021;206:137–138. doi:10.1016/j.thromres.2021.08.018
46. Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27(8):1687–1693. doi:10.1161/ATVBAHA.107.141911
47. Giesen PLA, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci USA. 1999;96(5):2311–2315. doi:10.1073/pnas.96.5.2311
48. Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96(12):1233–1239. doi:10.1161/01.RES.0000171805.24799.fa
49. Bogdanov VY, Versteeg HH. Soluble tissue factor in the 21st Century: definitions, biochemistry, and pathophysiological role in thrombus formation. Semin Thromb Hemost. 2015;41(7):700–707. doi:10.1055/s-0035-1556049
50. Subrahmanian S, Borczuk A, Salvatore S, et al. Tissue factor upregulation is associated with SARS-CoV-2 in the lungs of COVID-19 patients. J Thromb Haemost. 2021;19(9):2268–2274. doi:10.1111/jth.15451
51. Nomura S, Shouzu A, Omoto S, Nishikawa M, Iwasaka T. Effects of losartan and simvastatin on monocyte-derived microparticles in hypertensive patients with and without type 2 diabetes mellitus. Clin Appl Thromb. 2004;10(2):133–141. doi:10.1177/107602960401000203
52. Lee YJ, Jy W, Horstman LL, et al. Elevated platelet microparticles in transient ischemic attacks, lacunar infarcts, and multiinfarct dementias. Thromb Res. 1993;72(4):295–304. doi:10.1016/0049-3848(93)90138-e
53. Wang T, Du Z, Zhu F, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228):E52. doi:10.1016/S0140-6736(20)30558-4
54. Kerboua KE. NLR: a cost-effective nomogram to guide therapeutic interventions in COVID-19. Immunol Invest. 2021;50(1):92–100. doi:10.1080/08820139.2020.1773850
55. Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):1–12. doi:10.1186/s12967-020-02374-0
56. Ozcelik N, Ozyurt S, Yilmaz Kara B, Gumus A, Sahin U. The value of the platelet count and platelet indices in differentiation of COVID-19 and influenza pneumonia. J Med Virol. 2021;93(4):2221–2226. doi:10.1002/jmv.26645
57. Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38(3):641–647. doi:10.1016/j.ajem.2019.10.023
58. Templeton AJ, Mcnamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. JNCI. 2014;106(6). doi:10.1093/jnci/dju124
59. Mobarki AA, Dobie G, Saboor M, et al. MPR and NLR as prognostic markers in ICU-admitted patients with covid-19 in Jazan, Saudi Arabia. Infect Drug Resist. 2021;14:4859–4864. doi:10.2147/IDR.S342259
60. Zeng Z, Feng S, Chen G, Wu J. Predictive value of the neutrophil to lymphocyte ratio for disease deterioration and serious adverse outcomes in patients with COVID-19: a prospective cohort study. BMC Infect Dis. 2021;21(1):80. doi:10.1186/s12879-021-05796-3
61. Tatum D, Taghavi S, Houghton A, Stover J, Toraih E, Duchesne J. Neutrophil-to-lymphocyte ratio and outcomes in Louisiana COVID-19 patients. Shock. 2020;54(5):652–658. doi:10.1097/SHK.0000000000001585
62. Li X, Liu C, Mao Z, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care. 2020;24(1):647. doi:10.1186/s13054-020-03374-8
63. Sayed AA, Allam AA, Sayed AI, Alraey MA, Joseph MV. The use of neutrophil-to-lymphocyte ratio (NLR) as a marker for COVID-19 infection in Saudi Arabia. Saudi Med J. 2021;42(4):370LP- 376. doi:10.15537/smj.2021.42.4.20200818
64. Yang A-P, Liu J-P, Tao W-Q, Li H-M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi:10.1016/j.intimp.2020.106504
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






