Back to Journals » International Medical Case Reports Journal » Volume 16
Primary Tracheobronchial Amyloidosis Presenting with an Upper Airway Obstruction
Authors Demissie Z , Zemedkun N , Demile A, Nega B, Bekuretsion Y, Getachew B, Berhanu B
Received 2 September 2023
Accepted for publication 11 November 2023
Published 17 November 2023 Volume 2023:16 Pages 757—761
DOI https://doi.org/10.2147/IMCRJ.S438379
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Zekewos Demissie,1 Nahom Zemedkun,1 Abraham Demile,1 Berhanu Nega,2 Yonas Bekuretsion,3 Bisrat Getachew,4 Bethelhem Berhanu5
1Department of Internal Medicine, Lancet General Hospital, Addis Ababa, Ethiopia; 2Department of Surgery, Division of Cardiothoracic Surgery, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Pathology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 4Department of Otolaryngology, Head and Neck Surgery, St. Paul Millennium Medical College, Addis Ababa, Ethiopia; 5Department of Radiology, St. Paul Millennium Medical College, Addis Ababa, Ethiopia
Correspondence: Zekewos Demissie, Email [email protected]
Abstract: This is a case of localized primary tracheobronchial amyloidosis in a patient who presented with upper airway obstruction. Tracheobronchial amyloidosis is a type of localized bronchopulmonary amyloidosis that is frequently overlooked. It involves the deposition of amyloid protein on the tracheal and bronchial tissue, leading to progressive tracheal stenosis and airway obstruction that can be seen on imaging as a tracheal mass. Because of its significant diagnostic difficulty and therapeutic conundrum, it ought to be considered in the differential diagnosis of upper airway symptoms with an unknown etiology.
Keywords: tracheobronchial amyloidosis, tracheal mass, upper airway obstruction
Introduction
Amyloidosis is a disease that affects many organs’ normal function and is brought on by the extracellular and/or intracellular buildup of pathogenic amyloid fibrils.1 Various processes have been hypothesized to explain amyloidogenesis, including mutations in thermodynamic and kinetic pathways, nonphysiological proteolysis, and deficient or absent physiological proteolysis.2 These aberrant proteins are either the result of systemic precipitation following local production or a localized expression of deposition sites.3
In the past, amyloidosis was categorized as primary or secondary depending on whether it was accompanied by other clinical disorders or a chronic inflammatory disease. At present, it is defined by the biochemical makeup of the proteins that make up the fibril deposits and categorized based on its involvement in the body, whether it is systemic or localized and whether it is acquired or hereditary. Ocular, tracheobronchial, central nervous system, and other sites are examples of primarily localized amyloidosis.3
Given that a majority of patients are asymptomatic and those who are symptomatic exhibit aspecific symptoms, it presents a serious diagnostic hurdle. This is applicable to both systemic and localized amyloidosis. Apple-green birefringence on Congo red staining, amorphous eosinophil appearance on hematoxylin–eosin staining, fibrillar appearance on electron microscopy, and other tests confirm the diagnosis.4 Primary tracheobronchial amyloidosis is a rare occurrence of primary deposition of misfolded amyloid fibrils in the tracheobronchial tissue without other site involvement. We describe a particularly rare case of tracheal amyloidosis that manifested as upper airway obstruction.
Case Presentation
A 69-year-old man with no history of known medical conditions presented at the emergency room of Lancet General Hospital complaining of a daylong upper airway blockage. Prior to his admittance, he had experienced hoarse speech for 3 weeks, but he denied having hemoptysis, weight loss, or dysphagia. He was found to be in respiratory distress during the physical examination, with an audible biphasic stridor and a breathing rate of 40 breaths per minute. He had intense intercostal retraction together with decreased air entry over bilateral lung fields upon auscultation. At that time, his pulse rate was 110 beats per minute and his oxygen saturation was 71% on room air, but his other vital signs were in the normal range.
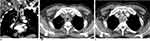
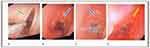
A 2 cm–thick circumferential nodular thickening originating from the posterior tracheal wall blocking the lumen was revealed by a CT scan of the chest and neck. An emergency tracheostomy was performed and the airway was protected. Afterward, bronchoscopy with bronchoscopic biopsy was done and biopsy was taken from the tracheal mass, which showed a nodule on the upper part of the trachea. The biopsy result suggested a classic histochemical finding of amyloidosis, with amorphous deposits demonstrating an apple-green birefringence highlighted with a Congo red stain under polarized light microscopy.
Initial laboratory investigations that included a complete blood count, comprehensive metabolic panel, and thyroid function test were all normal. No evidence of tuberculosis was found. VDRL, viral markers for hepatitis B and C, and HIV tests were all negative, and 24-hour urine protein was 24 mg/2400 mL. Peripheral morphology, bone marrow exam, SPEP with immunofixation, and free light-chain assay were unrevealing.
In our patient, we only provided tracheostomy for airway obstruction after failed endotracheal intubation and bronchoscopic biopsy and mucosal resection had been tried. We were unable to administer any direct amyloidosis therapy or other experimental treatments. He finally passed away from massive bleeding from the tracheostomy site. Neck and chest CT scan showing nodular tracheal thickening, the endoscopic findings, and the Congo red stain are shown in Figures 1–3.
 |
Figure 3 Section of tracheal sample showing acanthotic squamous epithelium with subepithelial deposition of pink hyaline extracellular material. Congo red stain was positive. |
Discussion
There are three distinct forms of respiratory amyloidosis: localized tracheobronchial amyloidosis (TBA), diffuse parenchymal amyloidosis, and nodular pulmonary amyloidosis. TBA accounts for 25%–50% of localized pulmonary amyloidosis. Nodular pulmonary amyloidosis typically involves localized nodular amyloid deposits and is generally not considered harmful. On the other hand, diffuse parenchymal amyloidosis is frequently linked to systemic illness. TBA, which is usually acquired, is occasionally manifested multifocally and can be seen in the central airways.5–7
The clinical signs of the TBA vary depending on the site involved. Although some patients may not exhibit any symptoms, the most frequent presenting symptoms are coughing, wheezing, dyspnea, hemoptysis, and stridor.7 Initially experiencing progressive dyspnea, our patient eventually developed hoarseness of voice and stridor on a physical examination.
Bronchoscopy is the cornerstone in the diagnosis, treatment, and monitoring of TBA patients. Multiple nodules or masses within airway lumens, luminal stenosis, thickening of the bronchial wall, and brittle, pale, and edematous mucosa are the main findings on bronchoscopic examination.8,9 A definitive diagnosis can be made with a tissue-specimen biopsy with appropriate staining techniques. The histological findings characteristic of amyloidosis include the classic apple-green birefringence seen with Congo red staining on polarized microscopy.4
To identify the exact protein components that have been deposited, additional tests can be carried out, such as mass spectrometry and immunofixation electrophoresis.10 Mapping the extent of the lesions with imaging techniques like computed tomography or magnetic resonance imaging might also be helpful, because during laryngoscopy, the lesions might appear to be smaller than they actually are. Laryngeal amyloidosis and TBA are visible on CT scan as a prominent nodular thickening of the laryngeal and tracheobronchial soft tissue with high density.6,11
The treatment of TBA is still being researched, and the findings are conflicting and mixed. There have been numerous interventions tested, including mucosal resection, external beam radiation therapy, and airway recanalization via bronchoscopy. Nevertheless, there are a number of variables that affect the management, such as the severity of involvement, the symptoms, and the likelihood of resection. Low-dose radiation therapy is the primary treatment for tracheobronchial amyloidosis, as plasma cells are sensitive to radiation. However, the exact mechanism by which external beam radiation therapy affects amyloid deposits in this disease is still unclear.6,8,9
Laser ablation therapy has been employed, but it was found to have little to no effect on the progression of the disease, particularly in cases of diffuse tracheobronchial amyloidosis. There is no convincing evidence that the combination of chemotherapeutic drugs is useful for treating individuals with diffuse tracheobronchial amyloidosis, despite the fact that this treatment is used to treat the systemic form of the disease.6,12 A tracheostomy or laryngectomy may occasionally be necessary to create a safe airway in patients with advanced local illness. The removal of all the amyloid tissue is quite difficult. Even locating it can be challenging at times, and getting rid of all the submucosal deposits can be challenging as well.6 In our patient, we undertook only tracheostomy for airway protection, bronchoscopic biopsy, and mucosal resection.
The prognosis of patients with TBA can vary depending on the level of impairment and the patient’s clinical characteristics. There have been no systematic reviews conducted, and most of the available literature consists of case reports or small case series. According to one study, the disease can lead to a mortality rate of around 30% after 7–12 years due to progressive obstructive disease. Another study estimated a 30%–50% 5-year survival rate, particularly in patients with diffuse tracheobronchial amyloidosis.8,10,12 To track the course of the disease and evaluate whether any intervention is required, it is critical to closely monitor the development of any new pulmonary symptoms and to routinely undertake bronchoscopy, pulmonary function tests, and chest CTs.10
It is crucial for physicians to be aware of tracheobronchial amyloidosis in the differential diagnosis of upper airway symptoms with an unidentified cause, because patients and providers may overlook aspecific respiratory symptoms like chronic coughing, hoarseness, and dyspnea for years. These symptoms can quickly progress the disease and ultimately prove fatal.
Conclusion
Primary tracheobronchial amyloidosis is a rare presentation of amyloidosis with aspecific upper airway symptomatology. TBA can present as a tracheal mass and subsequent upper airway obstruction. Histopathological diagnosis can be made through tissue biopsy using Congo red staining to show an apple-green birefringence. The diagnosis requires a high index of suspicion to complete the necessary investigations and monitor progression of the disease. There is no definitive intervention established for its treatment.
Ethics Approval
According to the review board policy of our institution, ethics approval was not necessary. Written consent was obtained from the patient’s family for publication of this case report and associated images.
Acknowledgment
We express our gratitude to the patient’s family for supplying all pertinent details, and also to the health-care providers involved in the management of this patient.
Funding
There is no funding to report.
Disclosure
All authors declare no competing interests of any kind in this publication.
References
1. Bustamante JG, Zaidi SRH. Amyloidosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. PMID: 29261990.
2. Buxbaum JN. The systemic amyloidoses. Curr Opin Rheumatol. 2004;16(1):67–75. PMID: 14673392. doi:10.1097/00002281-200401000-00013
3. Westermark P, Benson MD, Buxbaum JN, et al. A primer of amyloid nomenclature. Amyloid. 2007;14(3):179–183. PMID: 17701465. doi:10.1080/13506120701460923
4. Chuang E, Hori AM, Hesketh CD, Shorter J. Amyloid assembly and disassembly. J Cell Sci. 2018;131(8):jcs189928. PMID: 29654159; PMCID: PMC5963839. doi:10.1242/jcs.189928
5. Arulanantham J, Officer C, O’Connor C, et al. Localized tracheobronchial amyloidosis: a rare case presentation and tailored management approaches. Respirol Case Rep. 2021;9(9):e0820. PMID: 34401188; PMCID: PMC8355433. doi:10.1002/rcr2.820
6. Wang Q, Chen H, Wang S. Laryngo-tracheobronchial amyloidosis: a case report and review of literature. Int J Clin Exp Pathol. 2014;7(10):7088–7093. PMID: 25400802; PMCID: PMC4230125.
7. Tanrıverdi E, Akif Özgül M, Uzun O, et al. Tracheobronchial amyloidosis mimicking tracheal tumor. Case Rep Med. 2016;2016:4. doi:10.1155/2016/1084063
8. Sommer P, Kumar G, Lipchik RJ, Patel JJ. Tracheobronchial amyloidosis managed with multimodality therapies. Ther Adv Respir Dis. 2014;8(2):48–52. PMID: 24594977. doi:10.1177/1753465814524470
9. Lu X, He B, Wang G, He B, Wang L, Chen Q. Bronchoscopic diagnosis and treatment of primary tracheobronchial amyloidosis: a retrospective analysis from China. Biomed Res Int. 2017;2017:3425812. PMID: 28197412; PMCID: PMC5288517. doi:10.1155/2017/3425812
10. Crain MA, Lakhani DA, Balar AB, Hogg JP, Adelanwa A, Hailemichael E. Tracheobronchial amyloidosis: a case report and review of literature. Radiol Case Rep. 2021;16(9):2399–2403. PMID: 34257768; PMCID: PMC8260753. doi:10.1016/j.radcr.2021.05.082
11. Mangla L, Vadala R, Kadli SK, Prajapat D, Talwar D. Tracheobronchial amyloidosis: an uncommon disease with a common presentation. Respirol Case Rep. 2020;8(7):e00630. PMID: 32765884; PMCID: PMC7396321. doi:10.1002/rcr2.630
12. Chatkin G, Pipkin M, Pinto JAF, da Silva VD, Chatkin JM. Amiloidose traqueobrônquica primária. J Brasil De Pneumol. 2008;34(7):528–531. doi:10.1590/S1806-37132008000700013
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.