Back to Journals » International Medical Case Reports Journal » Volume 17
Primary Intranasal Melanotic Mucosal Melanoma – A Case Report and Literature Review
Authors Gashaw SD , Birhanu WD , Alemayehu F
Received 9 January 2024
Accepted for publication 9 April 2024
Published 14 April 2024 Volume 2024:17 Pages 335—339
DOI https://doi.org/10.2147/IMCRJ.S458817
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Vinay Kumar
Samson Dires Gashaw, Waltengus Demissie Birhanu, Fitsum Alemayehu
Department of Otolaryngology, Head and Neck Surgery, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Correspondence: Waltengus Demissie Birhanu, Email [email protected]
Background: Intranasal mucosal melanoma is a rare form of melanoma. Presenting as the features of occult malignancy and rapid in its progression. Presented with nonspecific symptoms. So far, no specific risk factor has been identified. The histopathological and immunohistochemical examination helps to confirm the diagnosis. Here, we present a case of primary intranasal melanotic mucosal melanoma and literature review.
Case Report: The authors present a patient with primary right intranasal melanotic mucosal melanoma.
Conclusion: An endoscopic medial maxillectomy was done, and the patient was linked to the oncology department for radiotherapy.
Keywords: mucosal melanoma, ectodermal, endoscopic medial maxillectomy
Introduction
Mucosal melanoma is a rare sub type, representing 1% of all melanoma diagnoses. As compared to cutaneous melanoma, patients with mucosal melanoma often present with more advanced disease and prognosis is significantly worse.1 Its incidence is slightly higher in men, in whites, and in over 60 s. It accounts for 0.5–2% of all melanomas.2,3
Melanocytes found in extracutaneous epithelial tissues of ectodermal origin are the source of mucosal melanoma. Except for the conjunctiva, ultraviolet radiation exposure does not cause melanoma; nonetheless, the etiologic causes of carcinogenesis are unknown. Any mucosal surface can become affected by mucosal melanoma, although the sinonasal tract, oral cavity, female genital system, the anorectum, and urinary tracts are the most prevalent locations for initial lesions to appear.4–7 The high density of melanocytes in the nasal cavity and paranasal sinuses compared to other mucosal sites explains the relative elevated frequency of primary mucosal melanomas in these areas. Surgery is the main modality of management.2
Case Presentation
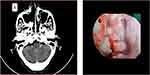
Sixty-five years old female presented with right nasal obstruction and recurrent nasal bleeding of 1-year duration. On endoscopic examination, there was a fragile dark pigmented mass arising from the lateral nasal wall, which completely fills the nasal cavity, and bleeds with easy manipulation (Figure 1). Multiplanar contrast computed tomography (CT) of paranasal sinus (Figure 2A–C) and neck CT was done and revealed an enhancing soft tissue mass filling and mildly expanding the right nasal cavity, otherwise no lymphadenopathy seen. Punch biopsy comes as mucosal melanoma. Routine hematologic and biochemical tests were in normal range. Abdominopelvic ultrasound and chest CT with contrast showed no abnormality. Therefore, primary melanoma of nasal cavity was entertained due to absence of previous or concurrent pigmented lesions elsewhere. Later, the patient managed with endoscopic medial maxillectomy.
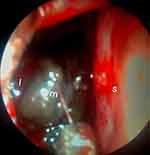 |
Figure 1 Shows per-operative nasal endoscopy. Abbreviations: I, lateral nasal wall; S, septum; m, mass. |
 |
Figure 2 Axial (A) coronal (B) and sagittal view (C) of contrast CT of para-nasal sinus. |
During operation under general anesthesia, Using nasal endoscopy. The mass removed with Blakesley forceps. After removal of the bulk of mass, the origin of the mass was found to be the anterior 2/3 of the right inferior turbinate. Right side Endoscopic medial maxillectomy was done with free margin and the nasolacrimal sac identified and preserved (Figure 3). In addition, the maxillary sinus mucosa was removed and subjected to histopathology with a separate container.
 |
Figure 3 Intra-operative nasal endoscopy. Abbreviations: M, maxillary sinus; IT, posterior end of inferior turbinate; MT, middle turbinate; S, septum. |
No Postoperative complication observed. Subsequently, adequate anti pain medication was given, and the patient discharged with 1 week follow up.
Excision biopsy shows solid sheet of highly pleomorphic cells with membrane irregularity and cherry red nucleoli admixed with dark brown pigment deposition with area of mixed inflammatory infiltrate (Figure 4A), histopathology from maxillary sinus shows respiratory epithelium lined tissue, no feature of malignancy seen (Figure 4B). Third week nasal endoscopic examination and fourth week contrast para-nasal sinus CT (Figure 5). And the patient is linked to the oncology department for radiotherapy.
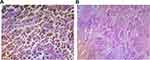 |
Figure 4 (A) Post operative histopathology from right nasal cavity. (B) Histopathology from right maxillary sinus. |
Discussion
Mucosal melanomas (MM) represent 1.3% of all melanomas, for this reason we want to report and discuss this case; 55% of them are found in the head and neck area, primarily in the sinonasal and oropharyngeal cavity. Due to their high prevalence of local recurrence and risk of metastasis, sinonasal mucosal melanomas have a dismal survival rate.7 The diagnosis of mucosal melanomas is frequently delayed due to their lack of appearance and the absence of symptoms in the early stages.6,8,9
Symptoms of sinonasal mucosal melanomas include epistaxis, unilateral nasal obstruction, and loss of smell. In addition, mucosal melanoma frequently presents as a pigmented, polypoid mass, leading to initial misdiagnosis as a benign lesion.10 Other manifestations are rhinorrhea, pain, and lacrimation in case of invasion of the inferior meatus and lacrimal duct.2
Magnetic resonance imaging (MRI) and computed tomography (CT) of the facial bones are crucial diagnostic and staging tools. MRI diagnostic sequences typically include T1, T1 post-gadolinium, and T2. A definitive diagnosis is made more challenging by the uneven contrast enhancement seen in T1 and T2 images of malignant melanomas. The best method for determining the anatomical involvement of other structures, including the skull and brain, is MRI. Last but not least, positron emission tomography (PET) is an additional test for tumor staging that evaluates distant metastases and directs the therapeutic course.2,10 PET is not available in our setup.
To correctly diagnose mucosal melanoma, immunohistochemical staining analysis for S-100, HMB-45, Melan-A, microphthalmia transcription factor, tyrosinase, and Mart-1 is essential, but we were not able to do immunohistochemistry due to financial reasons. Mucosal melanomas also frequently exhibit angioinvasion, multicentricity, and a lack of lymphoplasmacytic response to the tumor, among other histopathologic characteristics.6,8,9
Intracellular melanin is one of the primary histologic characteristics of melanoma; nevertheless, mucosal melanoma lesions can lack melanin pigmentation (amelanotic melanoma), making the diagnosis even more challenging. The dopa reaction, which shows tyrosinase activity, can be used to identify dopa-positive melanocytes, although it requires fresh tissue.9
TNM staging starts at stage III, which is indicative of the poor prognosis of these patients with 5-year survival rates of approximately 30%.6,8,11
Surgical treatment is the primary therapeutic option. Wide excision and lymphadenectomy (for locoregional lymph node metastases) are the treatments of choice in locally advanced disease. In our case we managed with endoscopic medial maxillectomy because of its least invasive and offer fast recovery, there were no clinically detectable lymph nodes. Postoperative radiation therapy can improve local control because high chance of local recurrence but not survival benefit.2,6,12 Despite low occurrence of BRAF mutation is sinonasal mucosal melanoma the possibility that patients with BRAF or KIT gene mutations may benefit from immunotherapy.8
Compared to surgery alone or RT alone, postoperative radiation after surgery produced the best locoregional control which has significant impact on patients quality of life. There is no benefit to combination therapy for survival.8 Immunotherapy and chemotherapy have been used as adjuvant or palliative therapies.7
Conclusion
To sum up, Sino nasal melanomas are a rare type of tumor that primarily affects older people. The preferred course of treatment is still surgical resection with wide surgical margins. Despite this, local recurrence is the primary reason for treatment failure, and distant metastasis occurs often. It seems vital to conduct studies targeted at developing novel therapeutic techniques to enhance the prognosis.
Consent Information
The patient granted her written permission for the publication of their clinical information and clinical photos. Institutional approval was not required to publish this case report.
Funding
No funding sources are to be disclosed.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Yentz S, Lao CD. Immunotherapy for mucosal melanoma. Ann Transl Med. 2019;7(S3):S118–S118. doi:10.21037/atm.2019.05.62
2. Lombardo N, Della CM, Pelaia C, et al. Primary mucosal melanoma presenting with a unilateral nasal obstruction of the left inferior turbinate. Medicine. 2021;57(4):2–6.
3. Alves ISS, Berriel LGS, Alves RT, et al. Sinonasal melanoma: a case report and literature review. Case Rep Oncol Med. 2017;2017. doi:10.1155/2017/8201301
4. Broit N, Johansson PA, Rodgers CB, et al. Meta-analysis and systematic review of the genomics of mucosal melanoma. Mol Cancer Res. 2021;19(6):991–1004. doi:10.1158/1541-7786.MCR-20-0839
5. Pfister DG, Ang K, Brizel DM, et al. Mucosal melanoma of the head and neck clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;10(3):320–338. doi:10.6004/jnccn.2012.0033
6. Plavc G, But-Hadžić J, Aničin A, Lanišnik B, Didanović V, Strojan P. Mucosal melanoma of the head and neck: a population-based study from Slovenia, 1985–2013. Radiat Oncol. 2016;11(1). doi:10.1186/s13014-016-0712-9
7. Díaz Molina JP, Rodrigo Tapia JP, Llorente Pendas JL, Nieto CS. Sinonasal mucosal melanomas. Review of 17 cases. Acta Otorrinolaringol Esp. 2008;59(10):489–493. doi:10.1016/S0001-6519(08)75518-3
8. Wahid NW, Meghji S, Barnes M. Nasal mucosal melanoma. Lancet Oncol. 2019;20(5):e284. doi:10.1016/S1470-2045(19)30179-2
9. Seetharamu N, Ott PA, Pavlick AC. Mucosal melanomas: a case-based review of the literature. Oncologist. 2010;15(7):772–781. doi:10.1634/theoncologist.2010-0067
10. Behranwala R, Loku Waduge BH, Teo B. Nasal mucosal melanoma as a cause of epistaxis. BMJ Case Rep. 2019;12(7):12–15. doi:10.1136/bcr-2018-228640
11. Lian B, Cui CL, Zhou L, et al. The natural history and patterns of metastases from mucosal melanoma: an analysis of 706 prospectively-followed patients background: we examined whether mucosal melanomas are different in their clinical course; 2016. Available from: http://annonc.oxfordjournals.org/.
12. Andrianakis A, Kiss P, Pomberger M, Wolf A, Thurnher D, Tomazic PV. Sinonasal mucosal melanoma: treatment strategies and survival rates for a rare disease entity: a single center experience and review of literature. Wien Klin Wochenschr. 2021;133(21–22):1137–1147. doi:10.1007/s00508-021-01847-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.