Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Primary Cutaneous Anaplastic Large Cell Lymphoma Arising in a Patient with Rhupus Syndrome and Sjogren’s Syndrome
Authors Gao Z, Xu Q, Chen X , Mao D, Zhang J, Jin J
Received 17 March 2022
Accepted for publication 19 May 2022
Published 30 May 2022 Volume 2022:15 Pages 975—979
DOI https://doi.org/10.2147/CCID.S366789
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Zirui Gao, Qianxi Xu, Xue Chen, Dandan Mao, Jianzhong Zhang, Jiang Jin
Department of Dermatology, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Jiang Jin, Department of Dermatology, Peking University People’s Hospital, No. 11 Xizhimen South Street, Xicheng District, Beijing, 100044, People’s Republic of China, Email [email protected]
Abstract: Rhupus syndrome, as an overlap syndrome of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), is relatively rare because of their substantially different immunopathological mechanisms. Herein, we report the first case of primary cutaneous anaplastic large cell lymphoma (PC-ALCL) in a patient with rhupus syndrome and Sjogren’s syndrome and review the relevant literature. A 52-year-old Chinese woman with a history of rhupus syndrome and Sjogren’s syndrome was treated with methotrexate, who developed gradually increasing nodules on the waist. Histopathological studies showed that the dermis and subcutaneous tissue were infiltrated with medium-to-large, atypical lymphocytes with the oval nucleus. The tumor cells showed CD3-, CD4-, CD8-, CD30+, LCA+, and EBV-encoded RNA (EBER) in situ hybridization (ISH) was positive. Therefore, the patient was diagnosed with PC-ALCL. Both immune disorders and EBV infection may be related to the onset of PL-ALCL, and further studies are needed to clarify the pathogenesis.
Keywords: lymphoproliferative disease, rheumatoid arthritis, systemic lupus erythematosus, Sjogren’s syndrome, methotrexate
Introduction
Primary cutaneous CD30-positive lymphoproliferative diseases (LPD) are the second most common subgroup of cutaneous T-cell lymphomas after mycosis fungoides, constituting approximately 30% of cutaneous T-cell neoplasms. According to the recent World Health Organization classification, this group includes primary cutaneous anaplastic large cell lymphoma (PC-ALCL), lymphomatoid papulosis (LyP), and borderline cases.1,2 Differential diagnosis of three clinical entities is based on clinical findings and the evolution of lesions.1,2 In contrast to PC-ALCL, LyP is characterized by a chronic course of years to decades of recurrent papulonodular lesions that are usually smaller (<1 cm) and resolve spontaneously within a few weeks or months.2,3
Herein, we report a rare case of PC-ALCL in a patient with rhupus syndrome and Sjogren’s syndrome. “Rhupus syndrome” is a rare and incompletely understood syndrome and commonly considered to feature the presence of symptoms of both SLE and RA in the same patient.4 To our knowledge, this is the first association between PC-ALCL with rhupus syndrome and Sjogren’s syndrome.
Case Report
A 52-year-old Asian woman had allergic rhinitis for 40 years, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and Sjogren’s syndrome (SS) for 6 years, and been treated with methotrexate (MTX) for 3 years. Other treatments for autoimmune diseases included steroids, Iguratimod, and Leflunomide (which was discontinued after 3 months due to leukopenia).
Eighteen months before presenting at our institution, a small nodule was noticed on her waist without any conscious symptoms. The cutaneous lesion was initially treated as furuncle with topical antibiotics, the lesion gradually increased in size, and the center of the lesion developed an ulcer a few months later, which prompted the patient to seek medical attention in our hospital. Grossly, the lesion measured 47mm × 41mm in size, with an ulceration of 23mm × 18mm in the center, and the surrounding skin demonstrated irregular pale red erythema (Figure 1).
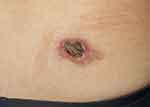 |
Figure 1 An ulcerated red lesion with peripheral erythema on the waist. |
The histopathological examination showed that the ulceration was covered with necrotic tissue. Infiltrating growth of medium-to-large atypical lymphocytes was observed in the surrounding fibrous tissues. The atypical lymphocytes had medium-to-large and oval nuclei (Figure 2A). Immunohistochemistry (IHC) demonstrated diffuse positivity for LCA and CD30 (Figure 2B). The neoplastic cells did not express CD2, CD5, CD20 (Figure 2C), CD3 (Figure 2D), CD4, CD7, CD8, CD56, CK, PAX5, or anaplastic lymphoma kinase (ALK) and the Ki-67 value was over 70%. EBV-encoded RNA (EBER) in situ hybridization (ISH) was positive (Figure 2E). Serum EBV DNA was negative.
Laboratory examination demonstrated anemia and lymphopenia with a decreasing absolute lymphocyte count (ALC) of 0.54 × 109/L. Computed tomography (CT) scan of the chest, abdomen, and pelvis did not show any evidence of systemic involvement by lymphoma.
Accordingly, diagnosis of primary cutaneous anaplastic large cell lymphoma (null-cell type) was made. The patient was treated with local irradiation. And after 30 times of irradiation, the lesion was crusted and the central ulcer healed (Figure 3), and until now there is no sign of recurrence.
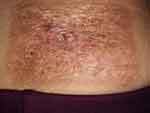 |
Figure 3 After radiotherapy, the lesion showed a clear border and the central ulcer healed, surrounded by radiation dermatitis. |
Discussion
PC-ALCL usually presents as asymptomatic solitary, grouped, or multifocal nodules on the upper half of the body, and the lesions persist over weeks to months.3 PC-ALCL does not infrequently show spontaneous regression (20–42% of cases), but up to half of the cases can recur.3 It mainly affects adults aged 50 to 70 years old, but pediatric and congenital cases have been described.3 Histologically, there are dense dermal infiltrates that are arranged in sheets, extending to the entire dermis and sometimes to the subcutaneous tissue. The infiltrate is comprised of large, irregular polygonal cells. “Hallmark” large cells with abundant eosinophilic or amphophilic cytoplasm and horseshoe-shaped nuclei are characteristic but may not be present. There were large, irregular polygonal cells. The typical nucleus was large and irregular, and the cytoplasm was rich, pale, or eosinophilic. Small reactive lymphocytes and eosinophil infiltration can be seen in and around the tumor.1,3 CD30 is expressed by at least 75% of the tumor cells in PC-ALCL.1 CD4 or CD8 is expressed in most cases with variable loss of pan-T cell antigens (CD2, CD3, CD5).1 PC-ALCL is generally found to be anaplastic lymphoma kinase (ALK)-negative, and ALK expression in skin lesions of ALCL evokes high suspicion of secondary skin involvement by underlying systemic ALCL.1,3
Large-scale cohort study has ascertained that persons with a history of some autoimmune diseases (ADs) have increased risk of Non-Hodgkin’s Lymphoma (NHL).5,6 Notably, Fallah et al found that there was a tendency toward higher standardized incidence ratios (SIRs) for cutaneous/peripheral T-cell and anaplastic large T- and null cell subtypes.7 The direct correlation between ADs and CD30+ PC-ALCL has not been studied but several studies have demonstrated an increased level of serum sCD30 (CD30 released as a soluble truncated form) or CD30 cells or both in RA, SS, and SLE conditions,8,9 which may involve the immunomodulation.10 Although the mechanism is still unclear, with rhupus and SS, upregulation of CD30+ lymphocytes may contribute to the onset of CD30-positive LPD.
Notably, our patient had a long-term history of exposure to MTX. MTX has been widely used to treat CD30+ lymphoproliferative disorders, especially lymphomatoid papulosis.1,11 And evidences about the efficacy of low-dose MTX in the treatment of PC-ALCL are increasing.12–14 Park et al14 assessed the long-term follow-up data of 7 patients with solitary or localized PC-ALCL and of them, 6 (85.7%) showed a complete response and 1 (14.3%) showed partial remission. Therefore in our case, MTX was continued and the patient was treated with radiotherapy.
Over 90% of adults worldwide harbor lifelong latent EBV, and EBV has been implicated in the development of a wide range of B-cell lymphoproliferative disorders such as Burkitt’s Lymphoma.15 The role of EBV in ALCL is still controversial. Some reports indicate that EBV is important in the pathogenesis of ALCL,17 while a series of 64 cases16 showed that there is no role for EBV in ALCL. In our case, the patients were found EBER positive and it may contribute to the PC-ALCL but considering the high infection rate of EBV, it’s hard to be proven.
In conclusion, we report a rare case of CD30-positive PC-ALCL that arose in rhupus syndrome and Sjogren’s syndrome. The patient was not correctly diagnosed until 18 months after the onset of the mass. Dermatologists should be aware of the development of cutaneous lymphomas under a background of immune disorders.
Abbreviations
PL-ALCL, primary cutaneous anaplastic large cell lymphoma; LPD, lymphoproliferative disease; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; SS, Sjogren’s syndrome; MTX, methotrexate; ALK, anaplastic lymphoma kinase.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The patient has given consent to publish the data and the study was approved by the Ethical Committee of our institution.
Funding
No funding or sponsorship was received for the conduct of this study or the preparation of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma *. Blood. 2011;118(15):4024–4035. doi:10.1182/blood-2011-05-351346
2. Perry E, Karajgikar J, Tabbara IA. Primary cutaneous anaplastic large-cell lymphoma. Am J Clin Oncol. 2013;36(5):526–529. doi:10.1097/COC.0b013e3182185aa2
3. Brown A, Fernandez-Pol S, Kim J. Primary cutaneous anaplastic large cell lymphoma. J Cutan Pathol. 2017;44(6):570–577. doi:10.1111/cup.12937
4. Liu T, Li G, Mu R, Ye H, Li W, Li Z. Clinical and laboratory profiles of rhupus syndrome in a Chinese population: a single-centre study of 51 patients. Lupus. 2014;23(9):958–963. doi:10.1177/0961203314526439
5. Anderson LA, Gadalla S, Morton LM, et al. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int j Cancer. 2009;125(2):398–405. doi:10.1002/ijc.24287
6. Mellemkjaer L, Pfeiffer RM, Engels EA, et al. Autoimmune disease in individuals and close family members and susceptibility to non-Hodgkin’s lymphoma. Arthritis Rheum. 2008;58(3):657–666. doi:10.1002/art.23267
7. Fallah M, Liu X, Ji J, Forsti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25(10):2025–2030. doi:10.1093/annonc/mdu365
8. Gerli R, Lunardi C, Vinante F, Bistoni O, Pizzolo G, Pitzalis C. Role of CD30+ T cells in rheumatoid arthritis: a counter-regulatory paradigm for Th1-driven diseases. Trends Immunol. 2001;22(2):72–77. doi:10.1016/s1471-4906(00)01829-9
9. Cabrera CM, Urra JM, Carreño A, Zamorano J. Differential expression of CD30 on CD3 T lymphocytes in patients with systemic lupus erythematosus. Scand J Immunol. 2013;78(3):306–312. doi:10.1111/sji.12088
10. Pierce JM, Mehta A. Diagnostic, prognostic and therapeutic role of CD30 in lymphoma. Expert Rev Hematol. 2017;10(1):29–37. doi:10.1080/17474086.2017.1270202
11. Vonderheid EC, Sajjadian A, Kadin ME. Methotrexate is effective therapy for lymphomatoid papulosis and other primary cutaneous CD30-positive lymphoproliferative disorders. J Am Acad Dermatol. 1996;34(3):470–481. doi:10.1016/s0190-9622(96)90442-9
12. Fujita H, Nagatani T, Miyazawa M, et al. Primary cutaneous anaplastic large cell lymphoma successfully treated with low-dose oral methotrexate. Eur J Dermatol. 2008;18(3):360–361. doi:10.1684/ejd.2008.0420
13. Yokoi I, Ishikawa E, Koura A, et al. Successful treatment of primary cutaneous anaplastic large cell lymphoma with intralesional methotrexate therapy. Acta Derm Venereol. 2014;94(3):319–320. doi:10.2340/00015555-1692
14. Park JB, Yang MH, Kwon DI, et al. Low-dose methotrexate treatment for solitary or localized primary cutaneous anaplastic large cell lymphoma: a long-term follow-up study. Acta Derm Venereol. 2020;100(4):adv00069. doi:10.2340/00015555-3413
15. Carbone A, Gloghini A, Dotti G. EBV-associated lymphoproliferative disorders: classification and treatment. oncologist. 2008;13(5):577–585. doi:10.1634/theoncologist.2008-0036
16. Herling M, Rassidakis GZ, Jones D, Schmitt-Graeff A, Sarris AH, Medeiros LJ. Absence of Epstein-Barr virus in anaplastic large cell lymphoma: a study of 64 cases classified according to World Health Organization criteria. Hum Pathol. 2004;35(4):455–459. doi:10.1016/j.humpath.2003.10.013
17. Noorali S, Pervez S, Yaqoob N, et al. Prevalence and characterization of anaplastic large cell lymphoma and its association with Epstein-Barr virus in Pakistani patients. Pathol Res Pract. 2004;200(10):669–679. doi:10.1016/j.prp.2004.08.004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

