Back to Journals » Hepatic Medicine: Evidence and Research » Volume 15
Primary Biliary Cholangitis: Promising Emerging Innovative Therapies and Their Impact on GLOBE Scores
Authors Sohal A , Kowdley KV
Received 28 March 2023
Accepted for publication 30 May 2023
Published 8 June 2023 Volume 2023:15 Pages 63—77
DOI https://doi.org/10.2147/HMER.S361077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Aalam Sohal,1 Kris V Kowdley1,2
1Department of Hepatology, Liver Institute Northwest, Seattle, WA, USA; 2Department of Gastroenterology and Hepatology, Elson Floyd College of Medicine, Spokane, WA, USA
Correspondence: Kris V Kowdley, Liver Institute Northwest, 3216 NE 45th Pl Suite 212, Seattle, WA, 98105, USA, Email [email protected]
Abstract: Primary biliary cholangitis (PBC), previously referred to as primary biliary cirrhosis, is an autoimmune disorder leading to the destruction of intra-hepatic bile ducts. If untreated, progressive bile duct damage and cholestasis can lead to ductopenia and result in cirrhosis. Ursodiol, the first drug approved for PBC, has changed the natural history of this disease and improved patient outcomes. Subsequently, several new prediction models incorporating a response to ursodiol were developed. These include the GLOBE score, which was shown to predict long-term outcomes in patients with PBC. In 2016, obeticholic acid (OCA) became the second drug to be approved by the FDA, predominantly based on improvement in alkaline phosphatase (ALP) levels. This trial has subsequently influenced the design of clinical trials. Several drugs are currently being evaluated as therapeutic options for PBC, with improvement in ALP being a main endpoint. In this review, we will discuss the impact of new therapies on GLOBE scores in patients with PBC.
Keywords: PBC, GLOBE scores, treatments, therapeutic options
Introduction
Primary biliary cholangitis (PBC) is an autoimmune disorder wherein intrahepatic bile ducts are targeted by lymphocytes.1 This results in periportal inflammation and cholestasis and may ultimately lead to cirrhosis and complications of chronic liver disease.1 The incidence and prevalence of PBC varies by region: the highest prevalence was reported to be in North America (21.81 per 100,000 persons), followed by Europe (14.59 per 100,000 persons), and the lowest prevalence was reported in the Asia-Pacific region (9.82 per 100,000 persons).2 Primary biliary cholangitis predominantly affects women, although men comprise approximately 10% of cases.3,4 More recent studies have shown increased prevalence among men in recent years.5 Men with PBC have a worse prognosis, possibly due to delayed disease recognition.6
There have been significant advances that have improved the staging, natural history, prognostication, and treatment of PBC in recent years.7 An important development is a change in the name from “primary biliary cirrhosis” to “primary biliary cholangitis” in recognition of the fact that most patients with the disease do not have cirrhosis.8 Multiple risk stratification scores based on new predictive models and novel therapeutic options have been developed that have improved the management of primary biliary cholangitis.9 This article will discuss the approved treatments and emerging therapeutic options for managing PBC.
Pathophysiology
Before discussing the therapeutic options for PBC, it is pertinent to understand the pathophysiology of this disease. Biliary epithelial cells (BECs) form the lining of the biliary tree and play a role in bile formation.10 This process is mediated via apical and basolateral exchangers and transmembrane channels.10 Studies have also reported that a defect in the “Biliary bicarbonate umbrella” may be responsible for initiating injury in primary biliary cholangitis.11 Normal BEC cellular integrity is maintained by appropriate bicarbonate production. Anion exchanger-2 (AE2), a chloride-bicarbonate exchange port expressed by BEC, regulates intracellular pH and biliary HCO3 secretion.12–14 This bicarbonate layer is protective against the acidic environment produced by bile acids. In patients with PBC, there is a downregulation of AE2, leading to an alkaline intracellular environment.15,16 This leads to the acidification of bile salts, rendering them more hydrophobic and cell membrane permeable, sensitizing the BECs to bile-salt-induced apoptosis.17
Apoptosis of BECs is a key step in the pathogenesis of PBC. In normal patients, during apoptosis, PDC-E2 in the BECs is modified through the covalent binding of glutathione.18 In patients with PBC, this modification does not occur, and mitochondrial PDC-E2 remains immunologically intact,18 which is recognized by circulating anti-mitochondrial antibodies (AMA), present in 90–95% of patients with PBC.19 The antimitochondrial antibody (AMA) targets PDC-E2 within the apoptotic BECs,19,20 resulting in antigen–antibody complexes and widespread immune activation. The inflammatory infiltrates comprise CD4+ T cells, CD8+ T cells, B lymphocytes, plasma cells, and variable eosinophils.21 They localize around the portal tracts and recognize antigenic sequences within the mitochondrial complexes, leading to targeted biliary injury.22,23 Regulatory T cells (Treg) cells are also reduced in patients with PBC, a defect that facilitates the autoimmune process.24 Following initial bile duct injury, the process of cholestatic injury and cell death persists in PBC, eventually leading to the destruction of intrahepatic bile ducts, cholestasis, and fibrosis.
Approved Treatments and the Risk Stratification Scores
Ursodeoxycholic Acid (UDCA)
Ursodiol has been a component of traditional Chinese medicine for over 3000 years.25 Ursodeoxycholic acid (UDCA) was the first drug approved by the US FDA for primary biliary cirrhosis in 1997.26 UDCA is absorbed by passive non-ionic diffusion, mainly in the small intestine.27 It is then extracted from the portal circulation by the liver, where it undergoes conjugation with glycine and taurine,28 and is then secreted into the bile and undergoes enterohepatic circulation.27
Multiple possible mechanisms of action of UDCA have been described. (1) UDCA replaces more toxic hydrophobic bile acids and protects injured cholangiocytes by reducing their concentration in the bile. (2) UDCA also stimulates the bile acid secretion and detoxification of hydrophobic bile acids. (3) UDCA immunomodulates the humoral immune system (4) and may inhibit the apoptosis of hepatocytes.29
UDCA has been shown to alter the natural course of PBC.30 EASL guidelines recommend the administration of ursodiol at a dose of 13–15 mg/kg/day in patients with PBC.31 A cohort study including 3902 patients with PBC found that treatment with UDCA prolonged liver transplant-free survival; irrespective of the stage of the disease, UDCA significantly reduced the risk of LT or death (hazard ratio-0.46, 95% CI-0.40–0.52, p<0.001).32 The findings were confirmed in a meta-analysis of seven randomized controlled trials and six reports of their follow-up.33 Long-term treatment with UDCA, compared to placebo, reduced the incidence of liver transplantation and death. Common side effects of UDCA include hair thinning, weight gain, and flatulence.34
Risk-Stratification Scores
Before the approval of ursodiol for the prognostication of patients with PBC, multiple prediction models were developed.35–42 Christensen et al were the first to develop a prediction model and reported that elevated bilirubin, older age, and cirrhosis were independently associated with poor prognosis.39 Dickson et al, in 1989, developed the Mayo Risk Score (MRS) for predicting prognosis in PBC patients.36 They reported that bilirubin, albumin, prothrombin time, age, and severity of edema were independent predictors of prognosis.36 This model was initially developed to assess the ideal time for a liver transplant but was later adjusted in 1994 to predict a 2-year prognosis.37
The scores mentioned above, developed in the pre-UDCA era, were not useful after the approval of ursodiol, as ursodiol altered the natural course of the disease.32,33 Multiple studies reported that ursodiol use was associated with improved ALP and bilirubin levels, which are markers of disease activity.43,44 Lammers et al, in their study of 4845 patients from the Global PBC Study Group, reported that ALP levels and bilirubin are markers of disease activity in PBC and can predict long-term survival.44 In this study, ALP >2 ULN and bilirubin >ULN were noted to be independent predictors of liver transplantation and death.44 ALP levels and bilirubin levels one year after the use of UDCA were shown to predict long-term outcomes.44
Subsequently, multiple scores assessing biochemical response criteria to UDCA, such as the Barcelona, Rotterdam, Paris, and Toronto criteria, were developed.45–49 Information regarding these scores is presented in Table 1. Prediction models incorporating more variables were then developed to improve prognostication in patients with PBC. The GLOBE score was introduced in 2015 by the GLOBE-PBC Study Group.50 This score was developed using a cohort of 2488 UDCA-treated patients and was validated in 1634 UDCA-treated patients. This score consists of age at baseline as well as total bilirubin (TB), ALP, albumin, and platelet count after 12 months of ursodiol.50 This score estimates the risk of liver transplantation or mortality after a year of UDCA therapy. It is available on GLOBAL-PBC website (https://www.globalpbc.com/globe), and their calculator is presented in Figure 1. Multiple subsequent studies have reported that this score is beneficial in assessing the risk of worse outcomes in UDCA-treated patients over a longer period.51–53 Another score, the UK-PBC score, was developed using 1916 UDCA-treated patients and validated in a cohort of 1249 UDCA-treated patients.54 This score included baseline albumin, platelet count, bilirubin, aminotransferases, and alkaline phosphatase one year after treatment with UDCA. Both models have been shown to be superior in predicting outcomes in UDCA-non responders than Barcelona, Paris I/II, and Rotterdam criteria.55
 |
Table 1 Risk Stratification Scores in the Post-UDCA Era |
 |
Figure 1 GLOBE risk calculator on the GLOBAL-PBC website. Reproduced with permission from GlobalPBC.com. |
Obeticholic Acid (OCA)
Based on the previous studies and biochemical response criteria, it was noted that patients who did not achieve biochemical remission with UDCA had worse outcomes.45–49 About 40% of the patients on UDCA are non-responders, defined as ALP >1.67 ULN, after UDCA therapy.56 This led to the need to develop other therapeutic targets for managing patients with PBC.
In 2016, obeticholic acid (OCA) was approved for the treatment of PBC by the FDA as an adjunctive agent to UDCA or monotherapy in UDCA-intolerant patients.57 Obeticholic acid is derived from chenodeoxycholic acid and imparts 100 times the increased agonism for the farnesoid X receptor (FXR) than endogenous chenodeoxycholic acid.58 FXR activation inhibits the transcription of CYP7A1, which decreases the synthesis of bile acids.59 FXR agonist has also been shown to regulate bile acids through enterohepatic circulation.59 Activation of FXR agonists increases the secretion of fibroblast growth factor-19 (FGF-19), which also inhibits bile acid synthesis.59
In the POISE trial, a Phase 3 trial comparing OCA vs placebo in addition to UDCA in 216 patients with PBC, 46–47% of patients receiving OCA achieved the primary endpoint (alkaline phosphatase <1.67 times the upper limit of normal and normalization of total bilirubin) compared to 10% of the patients in the control group (p<0.001).60 FDA approval of the drug based on the reduction in the level of the surrogate marker ALP, which, as discussed previously, has been documented to be a marker of transplant-free survival.44 The open-label extension of this trial revealed that the results were durable after six years of follow-up.61 This study reported that patients treated with OCA had greater transplant-free survival than real-world controls. Another study assessing obeticholic acid monotherapy over six years found that individuals treated with 10 mg of OCA had a 53.9% reduction in alkaline phosphatase compared to 0.8% in the placebo group.62 A post hoc analysis by Harms et al in the POISE study reported that 12 months of OCA treatment resulted in the reduction of APRI and GLOBE scores.63 They reported that in patients who had GLOBE score >0.3 at baseline, the use of OCA 5-1 mg was associated with a greater percentage of patients achieving a GLOBE score <0.3, compared to placebo (27% vs 6%). Furthermore, a lower proportion of patients in the OCA treated group with GLOBE score≤0.3 at baseline were noted to progress to GLOBE score >0.3, compared to placebo (3–13% vs 33%).63
Pruritus is the most common side effect associated with OCA use.60 The underlying mechanism of action for this symptom is unknown. This side effect has been noted to be dose dependent. A 68% of the patients in the 10 mg group and 56% in the 5–10 mg range experienced placebo as a side effect in the POISE trial.60 Hepatotoxicity secondary to OCA use has been identified and postulated to be dose-related in patients with more advanced liver disease.64,65 Due to these findings, OCA is now contraindicated in patients with child Class B and C cirrhosis, and a black box warning has been issued to the medication packaging.66 Close monitoring of liver function tests is recommended in patients taking OCA. OCA use has also been shown to affect lipoprotein metabolism, with an increase in total cholesterol and LDL-cholesterol, and a decrease in HDL-C and triglycerides (TG).67
Emerging Therapies
Several subsequent studies have followed a study design similar to the POISE study. Most trials have documented changes in the ALP levels, with some studies reporting GLOBE scores. Even though some studies have not reported GLOBE scores, changes in the ALP translate to changes in the GLOBE score. In the following section, we will discuss the impact of emerging therapeutic options on ALP and GLOBE scores based on the published data.
Peroxisome Proliferator-Activated Receptors (PPAR) Agonists
Fibrates are FDA approved for treating patients with hyperlipidemia.68 These drugs target peroxisome proliferator-activated receptors (PPAR). There are three isoforms of PPAR- α, δ, and γ, with fibrates differing in their affinity for each isoform.69 PPARα induces the expression of genes involved in bile acid and lipid metabolism.69 Its induction also results in the downregulation of genes involved in immune-related pathways.70 PPARγ and δ activation has anti-inflammatory and anti-fibrotic properties.70,71
Given the mechanism of action of fibrates, they have been evaluated as a potential treatment option in patients with PBC. It was first noted in the 1960s that clofibrate use could reduce serum ALP, but this specific property of fibrates remained ignored before it was rediscovered in the 1990s.72 In 1999, a study by Iwasaki et al reported, for the first time, that the use of bezafibrate alone or in combination with UDCA was able to decrease or normalize ALP levels and improve related symptoms.73 Since then, multiple unblinded small-sized controlled studies in Japan and Western countries have consistently confirmed the role of fibrates in patients with PBC.74–82 The BEZURSO trial comparing bezafibrate to placebo showed that 14 of 45 patients who had an incomplete response to ursodeoxycholic acid (UDCA) achieved the primary endpoint of biochemical remission at month 24 (defined as normalization of total bilirubin, ALP, aminotransferases, albumin concentrations, and prothrombin index) after receiving a daily dose of 400 mg bezafibrate, compared with none of the 39 patients who received UDCA and placebo (p<0.001).83 Liver stiffness measured by vibration-controlled transient elastography showed a decrease of 15% in the bezafibrate (BZF) group compared to an increase of 22% in the placebo group.83 Some adverse effects noted in the trial were myopathy and increased serum creatinine. Three patients were also noted to have a severe elevation of aminotransferases resolved after discontinuing bezafibrate.83 Tanaka et al, in their study of 3908 patients receiving UDCA, reported that the using UDCA-BZF combination reduced the risk of all-cause as well as liver-related mortality. The requirement for LT was also reduced in the study.84 The Japan Primary Biliary Cholangitis Study Group showed that patients who received the combination of bezafibrate and UDCA had significantly increased transplant-free survival than predicted by the GLOBE score calculated before initiating bezafibrate.85 In this study, the mean GLOBE score improved from 0.504 (+/-0.08) before combination therapy to 0.115 (+/-0.085), after one year of combination therapy. Their study suggested that addition of bezafibrate to UDCA improves the long-term prognosis in patients with PBC.85 Information regarding the drugs currently under evaluation as well as their effect on GLOBE scores is presented in Table 2.
 |
Table 2 Therapies for PBC and Their Impact on GLOBE Scores |
Fenofibrate has also been studied as an add-on therapeutic option in patients with PBC.86–90 A recent meta-analysis by Guoyun et al reported that combining fenofibrate and UDCA could decrease ALP and GGT in UDCA-refractory patients with PBC.89 The data regarding the impact of fenofibrate on GLOBE scores is mixed. A study by Wang et al reported that the addition of fenofibrate to UDCA resulted in an improvement in the GLOBE and UK-PBC scores.90 On the contrary, Duan et al in their study of 39 patients with PBC, refractory to UDCA reported that 2-year combination therapy with UDCA and fenofibrate was not associated with improvement in the UK-PBC risk score and GLOBE score.91 Further studies are needed to estimate the true effect of fenofibrate on GLOBE scores. Fibrates have also been beneficial in decreasing pruritus, a common symptom in patients with PBC.92,93
AASLD guidelines currently recommend that fibrates be considered an “off-label” alternative therapy for patients with PBC who have an inadequate response to ursodiol.94 Other PPAR agonists are also in development. PPAR-δ, present in biliary epithelial cells, is involved in cholesterol trafficking and excretion.94 The receptor induces the expression of ABCA1, a phospholipid-transporting ATPase responsible for cholesterol efflux from biliary epithelial cells.95 PPAR-δ also plays a role in the macrophage clearance of apoptotic cells and reduces exposure to self-antigens.96,97 Seladelpar has been noted to be a potent, selective agonist of PPAR-δ.98 Seladelpar use was associated with a 53–63% reduction in ALP concentration compared to a 2% reduction in patients not taking the drug.98 Another trial evaluating 25 patients with Child-Pugh A cirrhosis showed that response in ALP concentrations in patients with cirrhosis was comparable to those without cirrhosis.99 There were no treatment-related adverse events during the 52-week study period. A phase 3 randomized placebo-controlled study (ENHANCE: NCT03602560) in 265 patients with PBC who were intolerant or UDCA non-responders reported that the use of seladelpar 10 mg resulted in normalization of ALP in 27% of the patients in the treatment group compared to placebo.100 An improvement in the total bilirubin and symptoms such as pruritus was also noted.100 This study was stopped prematurely because of the concern of hepatotoxicity noted during the end-of-treatment biopsy of the NASH study with seladelpar.101 A subsequent review showed no drug-related clinical, biochemical, or histological evidence of liver injury.102 Subsequently, trials of seladelpar for PBC were resumed.102 Another study reported that seladelpar treatment for one year was associated with improved symptoms such as fatigue and pruritus.103 A recent study by Hansen et al reported an improvement in the GLOBE score after two years of treatment with seladelpar.104 This study reported that seladelpar use resulted in a mean change (SD) from baseline in GLOBE score of −0.417 (0.27). This change corresponded to 34% lower likelihood of requiring liver transplantation or death, compared to no prior treatment with seladelpar.104
Elafibranor (GFT505), first used in treating non-alcoholic steatohepatitis (NASH), is a dual PPARα and δ agonist.105 The drug was subsequently evaluated in a Phase 2 trial as an adjunctive treatment for patients with PBC who did not respond to UDCA.106 Patients who received elafibranor showed a 41–68% reduction in ALP compared with a 3% increase in those receiving a placebo (p<0.001). Secondary composite endpoints including ALP <1.67 times the upper limit of normal, more than 15% decrease in ALP, and normal total bilirubin levels were met in 67–79% of patients receiving elafibranor, compared to 6.7% of the patients in the placebo group.106 This randomized placebo-controlled trial of elafibranor revealed that elafibranor increased the estimated transplant-free survival at 5, 10, or 15 years using a composite GLOBE score.106 A phase 3 study ELATIVE comparing the effect of elafibranor in patients with PBC is currently underway (ELATIVE- NCT04526665). Data on therapies currently in phase 3 trials is presented in Table 3. The drugs currently being evaluated in phase 3 clinical trials are provided in Table 2.
 |
Table 3 Drugs Currently in Phase 3 Clinical Trials for the Management of PBC |
Saroglitazar is a dual PPAR-α and PPAR-γ agonist that has shown promising results in mouse models of NASH.107 In a phase 2 study (EPICS), saroglitazar use was associated with a 50% decrease in ALP levels in 81 patients who did not respond to UDCA.108 An open-label phase-3 study evaluating saroglitazar as a potential treatment for PBC was terminated early because of a lack of enrollment.109 In this 16-week study of 7 patients, treatment with saroglitazar resulted in a rapid reduction in ALP levels at week 4. Another trial (EPICS-III) is currently enrolling patients (NCT05133336).
NOX Inhibitors
Nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase (NOX) 1 and NOX4 are key enzymes involved in the development of liver fibrosis.110 TGF-beta, through NOX1 and NOX4 signaling, initiates apoptosis of hepatocytes.111 They also mediate the differentiation of hepatic stem cells to myofibroblasts.110
Setanaxib (GKT137831) is a NOX1 and NOX4 inhibitor.112 It has been shown to be beneficial in patients with doxorubicin-induced cardiotoxicity.112 It is currently being evaluated as a management option for patients with idiopathic pulmonary fibrosis (NCT03865927) and primary biliary cholangitis. Interim efficacy results of the phase 2 clinical trial in 111 patients showed that the use of GKT137831 was associated with improvement in the markers of cholestasis, inflammation, and liver fibrosis.113 Their phase 2 study did not report GLOBE scores, but they likely improved based on the improvement in the ALP levels noted in the study.113 Due to the positive results, a phase 2/3 clinical trial, TRANSFORM (NCT05014672), is currently underway and open to enrollment. It is planned that 318 patients with PBC will be randomized to setanaxib 1200 mg, 600 mg, and placebo in a 1:1:1 fashion for 52 weeks.
Non-Bile Acid FXR Agonists
Non-steroidal farnesoid X receptor (FXR) agonists have been evaluated for patients with PBC. These include tropifexor (LJN452), cilofexor (GS-9674), and EDP-305.
Tropifexor has been evaluated in a phase 2 trial of 61 patients.114 In this study, tropifexor use was associated with an improvement in ALP and GGT levels. Tropifexor use was associated with a 26–72% reduction in GGT from baseline compared to placebo.114 The most frequent adverse event in the study was pruritus. An increase in mean VAS itch score and median PBC-40 itch domain scores was noted in patients receiving tropifexor.114 It was also noted that although the mean ALP was reduced, the ALP remained above the upper limit of normal value in all the treatment groups.114 It has been previously reported that FXR activation can induce ALP gene transcription, which may confound the downstream effects of ALP reduction.113–116 Since ALP is a component of GLOBE scores, this may impact the assessment of long-term prognosis in patients with PBC when GLOBE score or UK-PBC scores are used.
Cilofexor (GS-9674) has been shown to be beneficial in patients with NASH and PSC.117,118 A phase 2 study involving 71 patients with PBC, with ALP greater than 1.67 times the upper limit of normal (ULN) and elevated serum total bilirubin, showed that cilofexor was associated with significant improvement in ALP and GGT.119 The most common adverse event was grade 2 or 3 pruritus, noted in patients on high-dose cilofexor.119
In the INTREPID study, a 12-week trial comparing EDP-305 to placebo reported a reduction of ALP in 45% of patients on EDP-305 compared to 11% in the placebo group. The study, however, did not meet its primary endpoint of a 20% reduction in ALP.120
FGF-19 Analogues
Ileal enterocytes primarily produce FGF-19 in response to bile acid exposure.121 After reaching the liver, it binds to FGFR4/ßKlotho and inhibits hepatic bile acid synthesis by suppressing CYP7A1.121 FGF-19 is a crucial regulator of lipid and glucose metabolism.122 FGF-19 agonist suppresses lipogenesis and gluconeogenesis122 and promotes fatty acid oxidation and glycogen synthesis.122 Murine models have shown that deficiency of FGF15/19 and receptor FGFR4 resulted in increased production of cholesterol-7-alpha-hydroxylase, increased bile acid turnover, and impaired gall bladder filling.123
Aldafermin (NGM282) is a subcutaneously administered analog of FGF-19.124 It is non-mitogenic and is being developed as a therapeutic option for treating primary sclerosing cholangitis (PSC), PBC, and NASH.124–126 A double-blind phase 2 trial evaluating 45 patients with PBC with inadequate response to UDCA for 28 days reported that aldafermin significantly reduced ALP levels from baseline.126 The most common side effect of aldafermin reported in the phase 2 study was gastrointestinal disorders. A 21% of patients receiving NGM282 0.3 mg and 43% receiving NGM282 3 mg developed diarrhea.126 A phase 2b study evaluating the effect of extended treatment with NGM282 for 24 weeks in PBC patients has been completed, with results pending (NCT02135536).
Budesonide
Budesonide is a potent corticosteroid with a high first-pass metabolism in the liver, resulting in fewer systemic side effects.127 Budesonide and UDCA, when used together, have been noted to increase the expression of biliary chloride/bicarbonate anion exchanger 2.128 This increases bicarbonate secretion and stabilizes the biliary bicarbonate umbrella.128
A phase 3 trial reported that combination therapy with budesonide and UDCA was not associated with improved liver histology, but an improvement in biochemical markers of disease was demonstrated when secondary analyses were performed.129 A small change in the GLOBE score of 0.3 was also noted in the budesonide group.129 It is recommended that budesonide use is avoided in patients with cirrhosis due to uncontrolled systemic shunting of the drug and increased risk of portal vein thrombosis.130
IBAT Inhibitors
Pruritus is a frequent symptom reported in 60–70% of patients.131,132 Previous studies have shown that UDCA is ineffective in improving this symptom.133 Normally, primary bile acids undergo extensive enterohepatic circulation with reabsorption of 95% of the bile acids secreted into the duodenum.134 Apical sodium-dependent transporters (ASBT and IBAT) play a role in the reabsorption of bile acids in the ileum.134 Since bile acids have been implicated among pruritogenic substances in patients with PBC,135 Ileal bile acid transport (IBAT) inhibitors by decreasing the reabsorption of bile acids have been hypothesized to reduce itching.136 Phase 2 GLIMMER trial evaluated linerixibat in 147 patients with PBC and moderate itching.136 Although no statistically significant difference was noted in the worst daily itch scores between linerixibat and placebo, the study reported a statistically significant change in the monthly itch scores from baseline in patients on linerixibat 40 mg, 90 mg, and 180 mg compared to placebo.136 Diarrhea was the most frequent adverse event in this study. The GLIMMER study did note a higher change in serum ALP concentrations from baseline, but the differences were not clinically significant.136 Since the mechanism of action does not decrease bile acid synthesis, it is less likely that an improvement in the ALP levels will be noted in patients on IBAT inhibitors.136 Based on the findings of GLIMMER trial, a phase 3 clinical trial GLISTEN is underway (NCT-04950127). Maralaxibat, another IBAT inhibitor, has been evaluated in a phase 2 trial.137 In this study, no significant reductions in the itching scores were noted between the patients on placebo and on maralixibat. There were no significant differences in the changes in the level of ALP between placebo and maralixibat.137 Another trial evaluating volixibat in patients with PBC is underway, with results awaited (NCT05050136).
Other Drugs
Multiple other drugs such as immunomodulators, anti-retroviral therapy, antioxidants and mesenchymal stem cells138–150 are currently being evaluated as therapeutic options for the management of PBC. Majority of these studies are limited to small sample of patients and only report reductions in the ALP. We believe that further large-population studies are needed, before the impact of these drugs on GLOBE scores can be studied. A diagram depicting the drugs currently approved as well as those under investigation is presented in Figure 2.
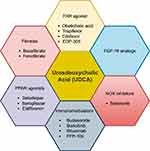 |
Figure 2 The current and potential therapies for the management of PBC. |
Conclusion
In summary, there have been substantial advances in the understanding of the pathophysiology, management, and prognostication models in patients with PBC. The approval of ursodiol and obeticholic acid has changed the natural history of PBC and the drug development processes for this rare disease. The drugs currently under investigation have a reduction in ALP as a primary endpoint in majority of the trials. Furthermore, the development of prognostication tools such as GLOBE scores and UK-PBC scores have improved our insight into the long-term outcomes. These scores are also currently being used as surrogate markers in clinical trials for the development of upcoming therapeutic options. We speculate that the approval of these upcoming therapies in the next decade will further change the management and help patients who do not respond to ursodiol and obeticholic acid. In addition, a flow diagram is provided in Figure 3 to assist the physicians in understanding how to approach PBC management.
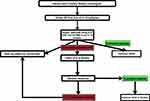 |
Figure 3 Flowchart describing the approach to a patient with PBC. |
Disclosure
Kris V. Kowdley declares research support from CymaBay Therapeutics; grants and/or contracts from 89Bio, Genfit, Gilead, GlaxoSmithKline, Hanmi, HighTide, Intercept, Madrigal, Mirum, NGM, Pfizer, Pliant, and Viking; royalties/licenses from UpToDate; consulting fees from 89Bio, Calliditas Therapeutics, CymaBay Therapeutics, Genfit, Gilead, Inipharm, Intercept, Madrigal, Mirum, NGM, and Pliant; payment/honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Gilead, and Intercept; participation in a data safety monitoring board or advisory board for CTI, Medpace and Labcorp; stock or stock options for Inipharm; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Sonic Insight. Aalam Sohal reports no conflicts of interest in this work.
References
1. Kowdley KV, Bowlus CL, Levy C, et al. Application of the latest advances in evidence-based medicine in primary biliary cholangitis. Am J Gastroenterol. 2023;118(2):232–242. doi:10.14309/ajg.0000000000002070
2. Lv T, Chen S, Li M, Zhang D, Kong Y, Jia J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36(6):1423–1434. doi:10.1111/jgh.15329
3. Nalbandian G, Van De Water J, Gish R, et al. Is there a serological difference between men and women with primary biliary cirrhosis? Am J Gastroenterol. 1999;94(9):2482–2486. doi:10.1111/j.1572-0241.1999.01380.x
4. Selmi C, Meda F, Kasangian A, et al. Experimental evidence on the immunopathogenesis of primary biliary cirrhosis. Cell Mol Immunol. 2010;7(1):1–10. doi:10.1038/cmi.2009.104
5. Fan X, Wang T, Shen Y, Xi X, Yang L. Underestimated male prevalence of primary biliary cholangitis in China: results of a 16-yr cohort study involving 769 patients. Sci Rep. 2017;7(1):6560. doi:10.1038/s41598-017-06807-7
6. Abdulkarim M, Zenouzi R, Sebode M, et al. Sex differences in clinical presentation and prognosis in patients with primary biliary cholangitis. Scand J Gastroenterol. 2019;54(11):1391–1396. doi:10.1080/00365521.2019.1683226
7. Huang YQ. Recent advances in the diagnosis and treatment of primary biliary cholangitis. World J Hepatol. 2016;8(33):1419–1441. doi:10.4254/wjh.v8.i33.1419
8. Beuers U, Gershwin ME, Gish RG, et al. Changing nomenclature for PBC: from ‘cirrhosis’ to ‘cholangitis’. Am J Gastroenterol. 2015;110(11):1536–1538. doi:10.1038/ajg.2015.312
9. Goet JC, Harms MH, Carbone M, Hansen BE. Risk stratification and prognostic modelling in primary biliary cholangitis. Best Pract Res Clin Gastroenterol. 2018;34–35:95–106. doi:10.1016/j.bpg.2018.06.006
10. Bogert PT, LaRusso NF. Cholangiocyte biology. Curr Opin Gastroenterol. 2007;23(3):299–305. doi:10.1097/MOG.0b013e3280b079fb
11. Sasaki M, Sato Y, Nakanuma Y. An impaired biliary bicarbonate umbrella may be involved in dysregulated autophagy in primary biliary cholangitis. Lab Invest. 2018;98(6):745–754. doi:10.1038/s41374-018-0045-4
12. Strazzabosco M. Transport systems in cholangiocytes: their role in bile formation and cholestasis. Yale J Biol Med. 1997;70:427–434.
13. Banales JM, Arenas F, Rodríguez-Ortigosa CM, et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266–275. doi:10.1002/hep.21042
14. Martínez-Ansó E, Castillo JE, Díez J, et al. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406. doi:10.1002/hep.1840190613
15. Melero S, Spirlì C, Zsembery A, et al. Defective regulation of cholangiocyte Cl−/HCO3− and Na+/H+ exchanger activities in primary biliary cirrhosis. Hepatology. 2002;35:1513–1521. doi:10.1053/jhep.2002.33634
16. Medina JF, Martínez-Ansó E, Vázquez JJ, Prieto J. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology. 1997;25:12–17. doi:10.1002/hep.510250104
17. Chang JC, Go S, de Waart DR, et al. Soluble adenylyl cyclase regulates bile salt-induced apoptosis in human cholangiocytes. Hepatology. 2016;64:522–534. doi:10.1002/hep.28550
18. Lleo A, Bowlus CL, Yang X, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52(3):987. doi:10.1002/hep.23783
19. Bogdanos DP, Invernizzi P, Mackay IR, Vergani D. Autoimmune liver serology: current diagnostic and clinical challenges. World J Gastroenterol. 2008;14:3374–3387. doi:10.3748/wjg.14.3374
20. Dahnrich C, Pares A, Caballeria L, et al. New ELISA for detecting primary biliary cirrhosis-specific antimitochondrial antibodies. Clin Chem. 2009;55:978–985. doi:10.1373/clinchem.2008.118299
21. Tsuneyama K, Baba H, Morimoto Y, et al. Primary biliary cholangitis: its pathological characteristics and immunopathological mechanisms. J Med Invest. 2017;64:7–13. doi:10.2152/jmi.64.7
22. Kita H, Lian ZX, Van de Water J, et al. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: t cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2020;195:113–123. doi:10.1084/jem.20010956
23. Shimoda S, Miyakawa H, Nakamura M, et al. CD4 T-cell autoreactivity to the mitochondrial autoantigen PDC-E2 in AMA-negative primary biliary cirrhosis. J Autoimm. 2008;31:110–115. doi:10.1016/j.jaut.2008.05.00
24. Lan RY, Cheng C, Lian ZX, et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729–737. doi:10.1002/hep.21123
25. Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid mechanisms of action and clinical use in hepatobiliary disorders. J Hepatol. 2001;35:134–146. doi:10.1016/S0168-8278(01)00092-7
26. Drug Approval Package: Urso (Ursodiol) NDA# 020675. (n.d.) Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/20675a.cfm.
27. Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol Suppl. 1994;204:1–15. doi:10.3109/00365529409103618
28. Úriz M, Sáez E, Prieto J, Medina JF, Banales JM. Ursodeoxycholic acid is conjugated with taurine to promote secretin-stimulated biliary hydrocholeresis in the normal rat. PLoS One. 2011;6(12):e28717. doi:10.1371/journal.pone.0028717
29. Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi:10.1053/jhep.2002.36088
30. Tanaka A. Current understanding of primary biliary cholangitis. Clin Mol Hepatol. 2021;27(1):1–21. doi:10.3350/cmh.2020.0028
31. European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–172. doi:10.1016/j.jhep.2017.03.022
32. Harms MH, van Buuren HR, Corpechot C, et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71(2):357–365. doi:10.1016/j.jhep.2019.04.001
33. Shi J, Wu C, Lin Y, Chen YX, Zhu L, Xie WF. Long-term effects of mid-dose ursodeoxycholic acid in primary biliary cirrhosis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101(7):1529–1538. doi:10.1111/j.1572-0241.2006.00634.x
34. Onofrio FQ, Hirschfield GM, Gulamhusein AF. A practical review of primary biliary cholangitis for the gastroenterologist. Gastroenterol Hepatol (N Y). 2019;15(3):145–154.
35. Roll J, Boyer JL, Barry D, Klatskin G. The prognostic importance of clinical and histologic features in asymptomatic and symptomatic primary biliary cirrhosis. N Engl J Med. 1983;308(1):1–7. doi:10.1056/NEJM198301063080101
36. Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10(1):1–7. doi:10.1002/hep.1840100102
37. Murtaugh PA, Dickson ER, Van Dam GM, et al. Primary biliary cirrhosis: prediction of short-term survival based on repeated patient visits. Hepatology. 1994;20(1 Pt 1):126–134. doi:10.1002/hep.1840200120
38. Kim WR, Wiesner RH, Poterucha JJ, et al. Adaptation of the Mayo primary biliary cirrhosis natural history model for application in liver transplant candidates. Liver Transpl. 2000;6(4):489–494. doi:10.1053/jlts.2000.6503
39. Christensen E, Neuberger J, Crowe J, et al. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology. 1985;89(5):1084–1091. doi:10.1016/0016-5085(85)90213-6
40. Christensen E, Altman DG, Neuberger J, De Stavola BL, Tygstrup N, Williams R. Updating prognosis in primary biliary cirrhosis using a time-dependent Cox regression model. PBC1 and PBC2 trial groups. Gastroenterology. 1993;105(6):1865–1876. doi:10.1016/0016-5085(93)91086-W
41. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi:10.1053/jhep.2001.22172
42. Angulo P, Lindor KD, Therneau TM, et al. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver. 1999;19(2):115–121. doi:10.1111/j.1478-3231.1999.tb00020.x
43. Batta AK, Salen G, Mirchandani R, et al. Effect of long-term treatment with ursodiol on clinical and biochemical features and biliary bile acid metabolism in patients with primary biliary cirrhosis. Am J Gastroenterol. 1993;88(5):691–700.
44. Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147(6):1338–e15. doi:10.1053/j.gastro.2014.08.029
45. Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130(3):715–720. doi:10.1053/j.gastro.2005.12.029
46. Kuiper EM, Hansen BE, de Vries RA, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136(4):1281–1287. doi:10.1053/j.gastro.2009.01.003
47. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48(3):871–877. doi:10.1002/hep.22428
48. Corpechot C, Chazouilleres O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 2011;55(6):1361–1367. doi:10.1016/j.jhep.2011.02.031
49. Kumagi T, Guindi M, Fischer SE, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105(10):2186–2194. doi:10.1038/ajg.2010.216
50. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149(7):1804–12 e4. doi:10.1053/j.gastro.2015.07.061
51. Carbone M, Sharp SJ, Flack S, et al. The UKPBC risk scores: derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cirrhosis. Hepatology. 2015;63:930–950. doi:10.1002/hep.28017
52. Goet JC, Lammers WJ, Floreani A, et al. The GLOBE score identifies PBC patients at increased risk of liver transplantation or death in different age-categories over time. J Hepatol. 2017;66(1):S543–S4 . doi:10.1016/S0168-8278(17)31494-0
53. Goet LW, Janssen HL, Hirschfield GM, et al. The GLOBE score identifies PBC patients at increased risk of liver transplantation or death during follow-up. Hepatology. 2016;2016:189A–190A.
54. Lammers WJ, Goet JC, Mayo MJ, et al. Time-dependent factors associated with diminished transplant-free survival in patients with primary biliary cholangitis and an optimal response to ursodeoxycholic acid after one year of treatment. Hepatology. 2017;66(1):Suppl 157A–8A.
55. Yoo J, Cho EJ, Lee B, et al. Prognostic value of biochemical response models for primary biliary cholangitis and the additional role of the neutrophil-to-lymphocyte ratio. Gut Liver. 2018;12(6):714–721. doi:10.5009/gnl18271
56. Goel A, Kim WR. Natural history of primary biliary cholangitis in the ursodeoxycholic acid era: role of scoring systems. Clin Liver Dis. 2018;22:563–578. doi:10.1016/j.cld.2018.03.007
57. Food and Drug Administration. FDA approves ocaliva for rare, chronic liver disease. U.S Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-ocaliva-rare-chronic-liver-disease.
58. Pellicciari R, Fiorucci S, Camaioni E, et al. 6α-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi:10.1021/jm025529g
59. Smith SM, Pegram AH. Obeticholic acid: a farnesoid X receptor agonist for primary biliary cholangitis. J Pharm Technol. 2017;33(2):66–71. doi:10.1177/8755122516687122
60. Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. doi:10.1056/NEJMoa1509840
61. Murillo Perez CF, Fisher H, Hiu S, et al. Greater transplant-free survival in patients receiving obeticholic acid for primary biliary cholangitis in a clinical trial setting compared to real-world external controls. Gastroenterology. 2022;163(6):1630–1642.e3. doi:10.1053/j.gastro.2022.08.054
62. Kowdley KV, Luketic V, Chapman R, et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018;67:1890–1902. doi:10.1002/hep.29569
63. Harms MH, Hirschfield GM, Floreani A, et al. Obeticholic acid is associated with improvements in AST-to-platelet ratio index and GLOBE score in patients with primary biliary cholangitis. JHEP Rep. 2020;3(1):100191. doi:10.1016/j.jhepr.2020.100191
64. Eaton JE, Vuppalanchi R, Reddy R, et al. Liver injury in patients with cholestatic liver disease treated with obeticholic acid. Hepatology. 2020;71:1511–1514. doi:10.1002/hep.31017
65. Aschenbrenner DS. Excessive dosing of obeticholic acid may increase risk of liver damage. Am J Nurs. 2018;118(2):46.
66. US Food and Drug Administration. FDA Adds Boxed Warning to Highlight Correct Dosing of Ocaliva (Obeticholic Acid) for Patients with a Rare Chronic Liver Disease. US Food and Drug Administration; 2018.
67. Siddiqui MS, Van Natta ML, Connelly MA, et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol. 2020;72(1):25–33. doi:10.1016/j.jhep.2019.10.006
68. Singh G, Correa R. Fibrate medications. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Available from https://www.ncbi.nlm.nih.gov/books/NBK547756/.
69. Kersten S, Stienstra R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie. 2017;136:75–84. doi:10.1016/j.biochi.2016.12.019
70. Harada K, Isse K, Kamihira T, et al. Th1 cytokine-induced downregulation of PPARγ in human biliary cells relates to cholangitis in primary biliary cirrhosis. Hepatology. 2005;41:1329–1338. doi:10.1002/hep.20705
71. Nozaki Y, Harada K, Sanzen T, et al. PPARγ ligand attenuates portal inflammation in the MRL-lpr mouse: a new strategy to restrain cholangiopathy in primary biliary cirrhosis. Med Mol Morphol. 2013;46:153–159. doi:10.1007/s00795-013-0017-0
72. Hellman L, Zumoff B, Kessler G, Kara E, Rubin IL, Rosenfeld RS. Reduction of cholesterol and lipids in man by ethyl P-chlorophenoxyisobutyrate. Ann Intern Med. 1963;59:477–494. doi:10.7326/0003-4819-59-4-477
73. Iwasaki S, Tsuda K, Ueta H, et al. Bezafibrate may have a beneficial effect in pre-cirrhotic primary biliary cirrhosis. Hepatol Res. 1999;16:12–18. doi:10.1016/S1386-6346(99)00033-9
74. Iwasaki S, Ohira H, Nishiguchi S, et al. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: a prospective, multicenter study. Hepatol Res. 2008;38:557–564. doi:10.1111/j.1872-034X.2007.00305.x
75. Nakai S, Masaki T, Kurokohchi K, Deguchi A, Nishioka M. Combination therapy of bezafibrate and ursodeoxycholic acid in primary biliary cirrhosis: a preliminary study. Am J Gastroenterol. 2000;95:326–327. doi:10.1111/j.1572-0241.2000.01667.x
76. Kurihara T, Niimi A, Maeda A, Shigemoto M, Yamashita K. Bezafibrate in the treatment of primary biliary cirrhosis: comparison with ursodeoxycholic acid. Am J Gastroenterol. 2000;95:2990–2992. doi:10.1111/j.1572-0241.2000.03220.x
77. Kurihara T, Maeda A, Shigemoto M, Yamashita K, Hashimoto E. Investigation into the efficacy of bezafibrate against primary biliary cirrhosis, with histological references from cases receiving long term monotherapy. Am J Gastroenterol. 2002;97:212–214. doi:10.1111/j.1572-0241.2002.05413.x
78. Ohira H, Sato Y, Ueno T, Sata M. Fenofibrate treatment in patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2147–2149. doi:10.1111/j.1572-0241.2002.05944.x
79. Ohmoto K, Yoshioka N, Yamamoto S. Long-term effect of bezafibrate on parameters of hepatic fibrosis in primary biliary cirrhosis. J Gastroenterol. 2006;41:502–503. doi:10.1007/s00535-006-1778-1
80. Hosonuma K, Sato K, Yamazaki Y, et al. A prospective randomized controlled study of long-term combination therapy using ursodeoxycholic acid and bezafibrate in patients with primary biliary cirrhosis and dyslipidemia. Am J Gastroenterol. 2015;110:423–431. doi:10.1038/ajg.2015.20
81. Levy C, Peter JA, Nelson DR, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther. 2011;33:235–242. doi:10.1111/j.1365-2036.2010.04512.x
82. Lens S, Leoz M, Nazal L, Bruguera M, Pares A. Bezafibrate normalizes alkaline phosphatase in primary biliary cirrhosis patients with incomplete response to ursodeoxycholic acid. Liver Int. 2014;34:197–203. doi:10.1111/liv.12290
83. Corpechot C, Chazouilleres O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378:2171–2181. doi:10.1056/NEJMoa1714519
84. Tanaka A, Hirohara J, Nakano T, et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2021;75(3):565–571. doi:10.1016/j.jhep.2021.04.010
85. Honda A, Tanaka A, Kaneko T, et al. Bezafibrate improves GLOBE and UK-PBC scores and long-term outcomes in patients with primary biliary cholangitis. Hepatology. 2019;70(6):2035–2046. doi:10.1002/hep.30552
86. Hegade VS, Khanna A, Walker LJ, Wong LL, Dyson JK, Jones DEJ. Long-term fenofibrate treatment in primary biliary cholangitis improves biochemistry but not the UK-PBC risk score. Dig Dis Sci. 2016;61(10):3037–3044. doi:10.1007/s10620-016-4250-y
87. Liberopoulos EN, Florentin M, Elisaf MS, Mikhailidis DP, Tsianos E. Fenofibrate in primary biliary cirrhosis: a pilot study. Open Cardiovasc Med J. 2010;4:120–126. doi:10.2174/1874192401004010120
88. Ding D, Guo G, Liu Y, et al. Efficacy and safety of fenofibrate addition therapy in patients with cirrhotic primary biliary cholangitis with incomplete response to ursodeoxycholic acid. Hepatol Commun. 2022;6(12):3487–3495. doi:10.1002/hep4.2103
89. Guoyun X, Dawei D, Ning L, et al. Efficacy and safety of fenofibrate add-on therapy in patients with primary biliary cholangitis refractory to ursodeoxycholic acid: a retrospective study and updated meta-analysis. Front Pharmacol. 2022;13:948362. doi:10.3389/fphar.2022.948362
90. Wang L, Sun K, Tian A, et al. Fenofibrate improves GLOBE and UK-PBC scores and histological features in primary biliary cholangitis [published online ahead of print, 2021 May 5]. Minerva Med. 2021. doi:10.23736/S0026-4806.21.07316-X
91. Duan W, Ou X, Wang X, et al. Efficacy and safety of fenofibrate add-on therapy for patients with primary biliary cholangitis and a suboptimal response to UDCA. Rev Esp Enferm Dig. 2018;110(9):557–563. doi:10.17235/reed.2018.5533/2018
92. Shen N, Pan J, Miao H, Zhang H, Xing L, Yu X. Fibrates for the treatment of pruritus in primary biliary cholangitis: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(7):7697–7705. doi:10.21037/apm-21-1304
93. Reig A, Sesé P, Parés A. Effects of bezafibrate on outcome and pruritus in primary biliary cholangitis with suboptimal ursodeoxycholic acid response. Am J Gastroenterol. 2018;113(1):49–55. doi:10.1038/ajg.2017.287
94. Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the study of liver diseases. Hepatology. 2019;69(1):394–419. doi:10.1002/hep.30145
95. Xia X, Jung D, Webb P, et al. Liver X receptor β and peroxisome proliferator-activated receptor δ regulate cholesterol transport in murine cholangiocytes. Hepatology. 2012;56:2288–2296. doi:10.1002/hep.25919
96. Mukundan L, Odegaard JI, Morel CR, et al. PPAR-δ senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266–1272. doi:10.1038/nm.2048
97. Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106(10):1559–1569. doi:10.1161/CIRCRESAHA.110.216523
98. Jones D, Boudes PF, Swain MG, et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716–726. doi:10.1016/S2468-1253(17)30246-7
99. Mayo M, Bowlus C, Galambos M, et al. Seladelpar for the treatment of primary biliary cholangitis: experience with 25 cirrhotic patients.
100. Hirschfield GM, Kowdley KV, Shiffman ML, et al. ENHANCE: safety and efficacy of seladelpar in patients with primary biliary cholangitis—a phase 3 international, randomized, placebo-controlled study [AASLD abstract LO11]. Hepatology. 2020;72(suppl 1):1253–1266. doi:10.1002/hep.31110
101. Harrison S, Nadege GT, Khazanchi A, et al. A 52-week multi-center double-blind randomized Phase2 study of seladelpar, a potent and selective peroxisome proliferator-activated receptor delta (PPAR-delta) agonist in patients with nonalcoholic steatohepatitis (NASH). Hepatology. 2020;24:154.
102. FDA Lifts All Clinical Holds on Seladelpar. Available from: https://www.globenewswire.com/news-release/2020/07/23/2066548/0/en/FDA-Lifts-All-Clinical-Holds-on-Seladelpar.html.
103. Kremer AE, Mayo MJ, Hirschfield G, et al. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2022;42(1):112–123. doi:10.1111/liv.15039
104. Hansen B, Watkins E, Yang K, et al. Seladelpar treatment of patients with Primary Biliary Cholangitis (PBC) for 2 years improves the globe pbc score and predicts improved transplant-free survival. Digest Dis Week. 2022;162:S1122–S1123.
105. Westerouen Van Meeteren MJ, Drenth JPH, Tjwa ET. Elafibranor: a potential drug for the treatment of nonalcoholic steatohepatitis (NASH). Expert Opin Investig Drugs. 2020;29(2):117–123. doi:10.1080/13543784.2020.1668375
106. Schattenberg JM, Pares A, Kowdley KV, et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol. 2021;74(6):1344–1354. doi:10.1016/j.jhep.2021.01.013
107. Jain MR, Giri SR, Bhoi B, et al. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018;38:1084–1094. doi:10.1111/liv.13634
108. Vuppalanchi R, Caldwell SH, Pyrsopoulos N. Results of a phase 2, prospective, multicenter, randomized, double-blind, placebo-controlled study to evaluate safety, tolerability, and efficacy of saroglitazar magnesium in patients with primary biliary cholangitis (EPICS). Gastroenterol Hepatol (N Y). 2021;17(2 Suppl 3):8.
109. Vuppalanchi R, González-Huezo MS, Payan-Olivas R, et al. A multicenter, open-label, single-arm study to evaluate the efficacy and safety of saroglitazar in patients with primary biliary cholangitis. Clin Transl Gastroenterol. 2021;12(4):e00327. doi:10.14309/ctg.0000000000000327
110. Liang S, Kisseleva T, Brenner DA. The role of NADPH oxidases (NOXs) in liver fibrosis and the activation of myofibroblasts. Front Physiol. 2016;7:17. doi:10.3389/fphys.2016.00017
111. Carmona-Cuenca I, Roncero C, Sancho P, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49(6):965–976. doi:10.1016/j.jhep.2008.07.021
112. Zheng H, Xu N, Zhang Z, Wang F, Xiao J, Ji X. Setanaxib (GKT137831) ameliorates doxorubicin-induced cardiotoxicity by inhibiting the NOX1/NOX4/reactive oxygen species/MAPK pathway. Front Pharmacol. 2022;13:823975. doi:10.3389/fphar.2022.823975
113. Dalekos GN, Invernizzi P, Nevens F, et al. Efficacy of GKT831 in patients with primary biliary cholangitis and inadequate response to ursodeoxycholic acid: interim efficacy results of a phase 2 clinical trial. J Hepatol. 2019;70:e1–e2. doi:10.1016/S0618-8278(19)30002-7
114. Schramm C, Wedemeyer H, Mason A, et al. Farnesoid X receptor agonist tropifexor attenuates cholestasis in a randomised trial in patients with primary biliary cholangitis. JHEP Rep. 2022;4(11):100544. doi:10.1016/j.jhepr.2022.100544
115. Laffitte B, Young K, Joseph S, et al. A novel, highly potent, non-bile acid FXR agonist for the treatment of NASH and cholestasis. Hepatol Int. 2016;10:S97.
116. Neuschwander-Tetri BA. Targeting the FXR nuclear receptor to treat liver disease. Gastroenterology. 2015;148:704–706. doi:10.1053/j.gastro.2015.02.037
117. Patel K, Harrison SA, Elkhashab M, et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72(1):58–71. doi:10.1002/hep.31205
118. Trauner M, Gulamhusein A, Hameed B, et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology. 2019;70(3):788–801. doi:10.1002/hep.30509
119. Kowdley KV, Minuk GY, Pagadala MR. The nonsteroidal farnesoid X receptor (FXR) agonist Cilofexor improves liver biochemistry in patients with primary biliary cholangitis (PBC): a phase 2, randomized, placebo-controlled trial. Hepatology. 2019;70:31a–2.
120. Kowdley KV, Bonder A, Heneghan MA, et al. Final data of the phase 2a intrepid study with EDP-305, a non-bile acid farnesoid x receptor (FXR) agonist. Hepatology. 2020;72(1 SUPPL):746A–747A.
121. Wang LX, Frey MR, Kohli R. The role of FGF19 and MALRD1 in enterohepatic bile acid signaling. Front Endocrinol (Lausanne). 2022;12. doi:10.3389/fendo.2021.799648
122. Potthoff MJ, Boney-Montoya J, Choi M, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011;13(6):729–738. doi:10.1016/j.cmet.2011.03.019
123. Wu X, Ge H, Lemon B, et al. FGF19-induced hepatocyte proliferation is mediated through FGFR4 activation. J Biol Chem. 2010;285(8):5165–5170. doi:10.1074/jbc.M109.068783
124. Harrison SA, Neff G, Guy CD, et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2021;160(1):219–231.e1. doi:10.1053/j.gastro.2020.08.004
125. Hirschfield GM, Chazouillères O, Drenth JP, et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol. 2019;70(3):483–493. doi:10.1016/j.jhep.2018.10.035
126. Mayo MJ, Wigg AJ, Leggett BA, et al. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun. 2018;2(9):1037–1050. doi:10.1002/hep4.1209
127. Edsbacker S, Andersson P, Lindberg C, Paulson J, Rytfeldt A, Thalen A. Liver metabolism of budesonide in rat, mouse, and man. Comparative aspects. Drug Metab Dispos. 1987;15:403–411.
128. Wagner M, Fickert P. Drug therapies for chronic cholestatic liver diseases. Annu Rev Pharmacol Toxicol. 2020;60:503–527. doi:10.1146/annurev-pharmtox-010818-021059
129. Hirschfield GM, Kupcinkas L, Ott P, et al. Budesonide add-on therapy in PBC patients with an incomplete response to UDCA: phase 3 trial.
130. Hempfling W, Grunhage F, Dilger K, Reichel C, Beuers U, Sauerbruch T. Pharmacokinetics and pharmacodynamic action of budesonide in early- and late-stage primary biliary cirrhosis. Hepatology. 2003;38(1):196–202. doi:10.1053/jhep.2003.50266
131. Mells GF, Pells G, Newton JL, et al; UK-PBC Consortium. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology. 2013;58(1):273–283. doi:10.1002/hep.26365
132. Kuo A, Kuo A, Bowlus CL. Management of symptom complexes in primary biliary cholangitis. Curr Opin Gastroenterol. 2016;32(03):204–209. doi:10.1097/MOG.0000000000000254
133. Talwalkar JA, Souto E, Jorgensen RA, Lindor KD. Natural history of pruritus in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2003;1(4):297–302. doi:10.1016/S1542-3565(03)00134-4
134. Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41(10):751–790. doi:10.2165/00003088-200241100-00005
135. Kremer AE, Oude Elferink RP, Beuers U. Pathophysiology and current management of pruritus in liver disease. Clin Res Hepatol Gastroenterol. 2011;35(2):89–97. doi:10.1016/j.clinre.2010.10.007
136. Levy C, Kendrick S, Bowlus CL, et al. GLIMMER: a randomized phase 2b dose-ranging trial of linerixibat in primary biliary cholangitis patients with pruritus [published online ahead of print, 2022 Nov 4]. Clin Gastroenterol Hepatol. 2022;(22):S1542–3565. doi:10.1016/j.cgh.2022.10.032
137. Mayo MJ, Pockros PJ, Jones D, et al. A randomized, controlled, phase 2 study of maralixibat in the treatment of itching associated with primary biliary cholangitis. Hepatol Commun. 2019;3(3):365–381. doi:10.1002/hep4.1305
138. Gordon SC, Trudeau S, Regev A, et al. Baricitinib and primary biliary cholangitis. J Transl Autoimmun. 2021;4:100107. doi:10.1016/j.jtauto.2021.100107
139. de Graaf KL, Lapeyre G, Guilhot F, et al. NI-0801, an anti-chemokine (C-X-C motif) ligand 10 antibody, in patients with primary biliary cholangitis and an incomplete response to ursodeoxycholic acid. Hepatol Commun. 2018;2:492–503. doi:10.1002/hep4.1170
140. Bowlus CL, Yang GX, Liu CH, et al. Therapeutic trials of biologics in primary biliary cholangitis: an open label study of Abatacept and review of the literature. J Autoimmun. 2019;101:26–34. doi:10.1016/j.jaut.2019.04.005
141. Hirschfield GM, Gershwin ME, Strauss R, et al. Ustekinumab for patients with primary biliary cholangitis who have an inadequate response to ursodeoxycholic acid: a proof-of-concept study. Hepatology. 2016;64:189–191. doi:10.1002/hep.28359
142. Tsuda M, Moritoki Y, Lian Z-X. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology. 2012;55:512–521. doi:10.1002/hep.24748
143. Myers RP, Swain MG, Lee SS, Shaheen AA, Burak KW. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am J Gastroenterol. 2013;108:933–941. doi:10.1038/ajg.2013.51
144. Wang L, Li J, Liu H, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28(Suppl. 1):85–92. doi:10.1111/jgh.12029
145. Wang L, Han Q, Chen H, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23(20):2482–2489. doi:10.1089/scd.2013.0500
146. Mason AL, Farr GH, Xu L, Hubscher SG, Neuberger JM. Pilot studies of single and combination antiretroviral therapy in patients with primary biliary cirrhosis. Am J Gastroenterol. 2004;99(12):2348–2355. doi:10.1111/j.1572-0241.2004.40741.x
147. Lytvyak E, Niazi M, Pai R, et al. Combination antiretroviral therapy improves recurrent primary biliary cholangitis following liver transplantation. Liver Int. 2021;41(8):1879–1883. doi:10.1111/liv.14964
148. Kilanczyk E, Banales JM, Wunsch E, et al. S-adenosyl-L-methionine (SAMe) halts the autoimmune response in patients with primary biliary cholangitis (PBC) via antioxidant and S-glutathionylation processes in cholangiocytes. Biochim Biophys Acta Mol Basis Dis. 2020;1866(11):165895. doi:10.1016/j.bbadis.2020.165895
149. Wunsch E, Raszeja-Wyszomirska J, Barbier O, Milkiewicz M, Krawczyk M, Milkiewicz P. Effect of S-adenosyl-L-methionine on liver biochemistry and quality of life in patients with primary biliary cholangitis treated with ursodeoxycholic acid. A prospective, open label pilot study. J Gastrointestin Liver Dis. 2018;27(3):273–279. doi:10.15403/jgld.2014.1121.273.icz
150. Moriya K, Asada K, Suzuki S, et al. Benefit of glucosyl Hesperidin in patients with primary biliary cholangitis: a multicenter, open-label, randomized control study. Medicine. 2022;101(48):e32127. doi:10.1097/MD.0000000000032127
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
