Back to Journals » Clinical Ophthalmology » Volume 14
Prevalence, Pattern and Risk Factors of Retinal Diseases Among an Elderly Population in Nepal: The Bhaktapur Retina Study
Authors Thapa R, Khanal S, Tan HS, Thapa SS, van Rens GHMB
Received 19 May 2020
Accepted for publication 9 July 2020
Published 24 July 2020 Volume 2020:14 Pages 2109—2118
DOI https://doi.org/10.2147/OPTH.S262131
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Raba Thapa,1 Shankar Khanal,2 Hendra Stevie Tan,3 Suman Shumsher Thapa,1 Gerardus Hermanus Maria Bartholomeus van Rens3
1Tilganga Institute of Ophthalmology, Kathmandu, Nepal; 2Central Department of Statistics, Tribhuvan University, Kirtipur, Nepal; 3Department of Ophthalmology, Amsterdam University Medical Center, Vrije University Amsterdam, Amsterdam, the Netherlands
Correspondence: Raba Thapa
Tilganga Institute of Ophthalmology, Bagmati Bridge, Kathmandu, Nepal
Tel +977-1-4493775
Fax +977-1-4474937
Email [email protected]
Introduction: Retinal diseases are an emerging cause of visual impairment in the developing world. The aim of this study was to explore the prevalence, pattern, and risk factors of retinal diseases in Nepal.
Methods: This is a population-based, cross-sectional study conducted from 2013 to 2015. The sample size was 2100 subjects age 60 years and above from 30 clusters of Bhaktapur district, Nepal. Detailed history, visual acuity, and anterior and posterior segment examinations were performed. Blood sugar and blood pressure were measured.
Results: Complete information was available for 1860 (88.57%) subjects. Mean age was 69.64± 7.31 years, ranging from 60 to 95 years. The prevalence of any retinal disorder was 52.37% (95% confidence interval (CI): 50.07– 54.66%). The prevalence of retinal disorders increased with ageing: 51.26% between 60 and 69 years and 53.05% among those age 80 years and above. Age-related macular degeneration (AMD) was the most common retinal disease (35.43%), followed by hypertensive retinopathy (4.35%), epiretinal membrane (ERM) (3.66%), branch retinal vein occlusion (BRVO) (2.90%), and diabetic retinopathy (DR) (2.15%). Other rare retinal disorders included myopic fundus (0.86%), chorioretinal scar (0.54%), retinal holes (0.32%), retinitis pigmentosa (0.32%), retinal detachment (0.16%), and coloboma (0.11%). In multivariate logistic regression analysis, those with prior cataract surgery (odds ratio (OR), 1.71; 95% CI: 1.32– 2.22, p < 0.001) and systemic hypertension (OR, 1.21; 95% CI: 1.001– 1.47, p = 0.049) had significantly increased retinal disorders.
Conclusion: Prevalence of retinal disorder was 52.37% at age 60 years and above. AMD, hypertensive retinopathy, ERM, BRVO, and DR were the most common retinal disorders. Retinal disorders increased with ageing. Retinal disorders were found associated with hypertension and prior cataract surgery. Timely screening, control of blood sugar and high blood pressure, and regular eye check-ups could help to save vision from retinal diseases.
Keywords: retinal diseases, age-related macular degeneration, hypertensive retinopathy, diabetic retinopathy, retinal vein occlusion, epiretinal membrane
Summary
Retinal diseases are an emerging cause of visual impairment in the developing world. Population-based studies have reported that the prevalence of retinal disorders ranges from 5.35% to 21.02% at age 40 years and above. This study aims to explore the prevalence, pattern and risk factors of retinal diseases in Nepal. This is a population-based, cross-sectional study conducted from 2013 to 2015. The sample size was 2100 subjects age 60 years and above from 30 clusters of Bhaktapur district, Nepal. Detailed history, visual acuity, and anterior and posterior segment examinations were performed. Complete information was available for 1860 (88.57%) subjects. Mean age was 69.64±7.31 years, ranging from 60 to 95 years. The prevalence of any retinal disorder was 52.37% (95% Confidence Interval (CI); 50.07–54.66%). The prevalence of retinal disorders increased with ageing. Age-related macular degeneration (AMD) was the most common retinal disease (35.43%), followed by hypertensive retinopathy (4.35%), epiretinal membrane (ERM) (3.66%), branch retinal vein occlusion (BRVO) (2.90%), diabetic retinopathy (DR) (2.15%), myopic fundus (0.86%), chorioretinal scar (0.54%), retinal holes (0.32%), retinitis pigmentosa (0.32%), retinal detachment (0.16%), and coloboma (0.11%). In multivariate logistic regression analysis, those with prior cataract surgery (Odds Ratio (OR), 1.71; 95% CI: 1.32–2.22, p < 0.001) and systemic hypertension (OR, 1.21; 95% CI: 1.001–1.47, p = 0.049) were associated with increased retinal disorders. Emphasis on screening, control of blood sugar and high blood pressure, and regular eye check-ups could help to save vision from retinal diseases.
Introduction
Retinal diseases are an important cause of ocular morbidity and visual impairment globally.1 Population-based studies have reported prevalence of retinal disorders ranging from 5.35% to 21.02% at age 40 years and above.2–4 In developed countries, retinal diseases are the most common cause of irreversible blindness. In the developing world, retinal diseases are the second most common cause of blindness after cataract. Age-related macular degeneration (AMD) is the most common cause of irreversible blindness among the elderly age group, and diabetic retinopathy (DR) is the most common cause of blindness among the working age group in developed countries.5–8 Retinal diseases are also an emerging cause of blindness in the developing world. Increased life expectancy, changing life styles, and systemic diseases like diabetes mellitus and hypertension are contributing factors. In Southeast Asian countries, retinal problems are one of the leading causes of visual impairment. India and China have large populations with diabetes, and DR is an emerging cause of visual impairment in these countries.9–13 In population- and hospital-based studies, AMD, hypertensive retinopathy, DR, and retinal vein occlusion (RVO) are the most common retinal problems among the vitreoretinal disorders in the region.2,4,14-16
In Nepal, retinal diseases were the third leading cause of blindness in a national population-based survey conducted in 1981.17 The Rapid Assessment of Avoidable Blindness (RAAB) survey conducted in 2010 showed posterior segment diseases are the second major cause of blindness.18 Other population-based studies conducted in Nepal also showed similar results, and retinal diseases have been the most common cause of visual impairment among people who underwent cataract surgery.19–21 Life expectancy is increasing over the last decades in Nepal.22,23 There is good control for communicable diseases, but non-communicable diseases like diabetes and cardiovascular diseases are major causes of morbidity and mortality.24 Diabetes is epidemic, especially in middle-aged and elderly populations in urban areas.25 Studies have reported diabetes rates of 4–8% in semi-urban areas.4,26 Systemic hypertension is also increasing as a major health problem in Nepal.27,28 Retinal diseases are major challenges in Nepal, so timely interventions are essential to prevent blindness.
Ageing, diabetes, hypertension, smoking, high myopia and post cataract surgery status are important risk factors for many retinal problems,2–4,6–8,17,18,20,21 but retinal problems can be largely asymptomatic until advanced stages in many cases, so timely precautions, early detection, and prompt treatment are necessary to prevent irreversible blindness. Identification of major retinal problems and their risk factors could facilitate regular eye check-ups and other preventive measures.
There are only a few published studies on the prevalence and risk factors of retinal diseases in Nepal.4,14 The Bhaktapur Retina Study (BRS) aims to explore the prevalence, pattern and risk factors of retinal diseases among subjects age 60 years and above residing in Bhaktapur district, Nepal.
Materials and Methods
Study Population
The Bhaktapur Retina Study is a population-based, cross-sectional study conducted on an age group 60 years and above to explore the prevalence, pattern and risk factors of retinal disorders in the Bhaktapur district of Nepal, a district adjoining Kathmandu, the capital city of Nepal. According to the 2001 census, the population of Bhaktapur district was 298,704, and 48,223 were above the age of 40 years.22 The required sample size for this study was estimated to be 2100 subjects after assuming 7.05% prevalence for retinal disorders in individuals 60 to 69 years, a relative precision of 25%, 85% compliance, and a design effect of 2. The 7.05% prevalence of retinal disorders was derived from the occurrence of retinal disorders in the Bhaktapur Glaucoma Study (BGS).4 The study population was drawn from the BGS sample conducted from 2007 to 2010, where a WHO 30-cluster sampling method was used.29 Details of the study methods have also been described in other companion papers.30–33
In brief, from these 30 clusters, a house-to-house enumeration was done, and a name list was prepared. From this name list, 4800 subjects above the age of 40 years were selected using EPI-INFO software, version 3.5.1.34 From these 4800 subjects, only those above 60 years of age were re-invited to participate for an eye-examination in the BRS; 62% of the original BGS subjects above the age of 60 years participated again in the BRS; among the remaining subjects, 15% had passed away, 5% had moved to other places, and 18% of the subjects were unable to visit the study site. In order to meet the desired sample size of 2100, the rest (38%) were selected from adjoining clusters as a cross-sectional survey. Two female community health workers visited the subjects at their homes and invited them to participate in the study. All subjects attended the community eye centre located in the Bhaktapur district and underwent a detailed history and ocular examination. The study was conducted from August 2013 to December 2015.
A structured questionnaire was developed to assess the prevalence and risk factors for retinal disorders. Mid-level ophthalmic personnel were involved in the interview, and two ophthalmologists examined the study subjects. Fifty cases were pre-tested. No respondents reported difficulties in answering the questionnaire, and there were no significant variations in anterior and posterior segment examination findings.
Patient Examination
All patients provided a detailed ocular and medical history. Detailed visual acuity, anterior segment and dilated posterior segment examinations including measurement of intraocular pressure were done.
Two retina specialists performed standardized eye examinations on the patients. Those study participants needing further evaluation with macular OCT and fundus fluorescein angiography were referred to a tertiary eye hospital.
DR was graded using Early Treatment Diabetic Retinopathy Study (ETDRS) criteria.35 Briefly, DR was graded as non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). Subjects were categorized as having DR if they had any form of NPDR or PDR in at least in one eye, irrespective of stage.
AMD was graded according to the classification developed by the International Age-related Maculopathy (ARM) Epidemiological Study Group by the retina specialist at each clinical examination.36 Briefly, ARM is a degenerative disorder in persons ≥50 years of age characterized by the presence of any of the following abnormalities in the macula: soft drusen ≥63 microns, hyperpigmentation and/or hypopigmentation of the retinal pigment epithelium (RPE), RPE detachments and associated neurosensory detachment, (peri) retinal hemorrhage, geographic atrophy of the RPE, or (peri) retinal fibrous scarring in the absence of other retinal (vascular) disorders. All stages of AMD were included in this study.
Hypertensive retinopathy was graded according to Modified Scheie Classification.37 Briefly, grade 0: no changes; grade 1: barely detectable arterial narrowing; grade 2: obvious arterial narrowing with focal irregularities; grade 3: grade 2 plus retinal hemorrhage and/or exudates; grade 4: grade 3 plus disc swelling.
Participants were diagnosed as having retinal diseases if they had any retinal problems in one eye or both eyes.
Assessment and Definitions of Risk Factors
A detailed history was obtained using a standardized questionnaire. All subjects underwent blood examination for non-fasting blood sugar levels and measurements of blood pressure. Height and weight were recorded using standard techniques. Age, gender, literacy, occupation, and presence of systemic problems like diabetes mellitus, hypertension, use of tobacco, and alcohol were elicited from the self-reported history.
When the subjects were able to read and write in the national language, they were categorized as literate as defined by the Government of Nepal.
The predominant profession was considered as the occupation. Those involved in farming were listed as working in agriculture. The rest of the other professions such as office work, business, health professionals were grouped under “other occupations.”
Venous blood samples were taken for assessment of non-fasting blood sugar. The diagnosis of diabetes mellitus was based on either the use of diabetic medications or a random blood sugar level of 200 mg/dl or greater.29,38 Blood pressure (BP) was measured on all subjects. Subjects were categorized as hypertensive if systolic blood pressure was 140 mmHg or more, if diastolic blood pressure was 90 mmHg or more, or if they used antihypertensive medications.
The study was approved by the Tilganga Institute of Ophthalmology, Institutional Review Committee (TIO-IRC) on 28 June 2013 (TIO-IRC approval no. 1/2013). The study was conducted in accordance with the Declaration of Helsinki. Informed consent was written in the vernacular and was read to those unable to read. Subjects were asked to sign the consent form prior to enrollment in the study, and thumb impressions were taken for those unable to sign.
Statistical Analysis
Descriptive statistical measures such as mean ± Standard Deviation (SD) for continuous variables, and percentages were computed for categorical variables. Association between two independent categorical variables was assessed by using Chi-square or Fisher’s exact tests wherever applicable. The associations of continuous variables with two independent groups were analyzed by using independent t-tests. The effects of different independent variables on retinal diseases were examined by univariate and multiple logistic regression analysis. All the results were considered significant if the p-value was <0.05. Statistical analysis was performed using STATA 13.0, College Station, Texas, USA.
Results
Complete information was available for 1860 (88.57%) of the subjects in the study. Age and gender were compared between the responder and the non-responder groups. There was no significant difference in age or gender between the two groups (Table 1).
 |
Table 1 Comparison of Responders and Non-Responders in the Study Population |
The age ranged from 60 to 95 years with a mean±SD age of 69.64±7.31 years. Mean±SD age of men was 69.98±7.37 years, and women were 69.36±7.26 years. Half of the study subjects (51.08%) were between 60 and 69 years of age, whereas 11.45% were of 80 years and above. There were more females in the study (1039; 55.86%) than males. Among the total, 1433 (77.04%) persons were illiterate, and 1351 (72.63%) were farmers by occupation.
The demographic characteristics and prevalence of retinal disorders in the study population are shown in Table 2. The prevalence of retinal disorder was 52.37% (95% Confidence Interval (CI); 50.07–54.66%) of overall study subjects. The prevalence of unilateral retinal disorders was 18.9% (95% CI: 17.2–20.8%), while bilateral retinal disorders was 33.5% (95% CI: 31.2–35.6%)
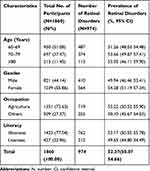 |
Table 2 Demographic Distribution of Retinal Disorders in the Study Population |
The prevalence of retinal disorders was 51.26% between age 60–69 years, 53.66% between age 70–79 years, and 53.05 years for age 80 years and above. The prevalence of retinal disorders was 49.94% for males and 54.28% for females. Among those with agricultural occupations, 53.22% had a retinal disorder, while among the other occupations it was 50.10%. Illiterates had more retinal disorders (53.17%) than literates (49.65%). (Table 2).
Table 3 shows the prevalence and pattern of various retinal disorders. Among the retinal disorders, the most common retinal problem was AMD, affecting 35.43% of subjects. Overall, hypertensive retinopathy (HTN retinopathy) was found in 4.35% study subjects. Among those with HTN retinopathy, grade 1 HTN retinopathy was found in 2.85% of study subjects, grade 2 HTN retinopathy in 1.02%, grade 3 HTN retinopathy in 0.48%, and no subjects with grade 4 HTN retinopathy. Epiretinal membrane (ERM) was found in 3.66%, BRVO in 2.95%, and DR in 2.15% of study subjects. Posterior vitreous detachment (PVD) was found in 4.78% of study subjects (Table 3).
 |
Table 3 Pattern of Retinal Disorders in the Study Population |
Table 4 shows the risk of retinal disorders with various demographic, systemic and ocular factors in univariate regression analysis. Retinal disorders were significantly higher among those who had undergone cataract surgery (Odds Ratio (OR), (CI): 1.69, 1.30–2.21; p value <0.001). Females as compared to males (OR, CI: 1.19, 0.99–1.43), hypertension as compared to non-hypertensive cases (OR, CI: 1.20, 0.99–1.45), and those who consume alcohol as compared to those who do not consume alcohol (OR, CI: 1.18, 0.98–1.42) had higher risk of retinal diseases, but each OR was of borderline significance. Remaining other factors such as age, literacy, smoking, diabetes, Body Mass Index (BMI) and occupation were not statistically associated with retinal disease (Table 4).
 |
Table 4 Risk Factors for Retinal Disorders in Univariate Logistic Regression Analysis |
Considering all ten variables as candidate variables for multiple logistic regression through a stepwise selection procedure, final multiple logistic regression analysis came up with two significant variables in association with retinal disorders, namely those who had undergone cataract surgery as compared to phakic cases (OR, 1.71; 95% CI: 1.32–2.22, p < 0.001) and those with hypertension as compared to non-hypertensive cases (OR, 1.21; 95% CI: 1.001–1.47, p = 0.049) (Table 5).
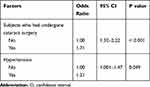 |
Table 5 Risk Factors for Retinal Disorders in Multivariate Logistic Regression Analysis |
Discussion
The study findings of the Bhaktapur Retina Study could be representative for the neighbouring most densely populated part of Nepal, the Kathmandu Valley, due to similar demographic and socioeconomic conditions. The findings of the study could help to estimate the burden and underlying risk factors for retinal disorders for further blindness intervention programs.
The overall prevalence of retinal disorder was 52.37% of study subjects age 60 years and above. The prevalence of retinal disorder was 51.26% between 60 and 69 years, 53.66% between 70 and 79 years, and 53.05% at age 80 years and above. There was a slight increase of retinal disorders with ageing, but the increase was not statistically significant. In a study conducted five years ago in the same area, the prevalence of retinal disorders was 7.7% in subjects age 60 years and above. The prevalence of retinal disorders was 7.05% between 60 and 69 years, 12.42% between 70 and 79 years, 15.60% between 80 and 89 years, and 28.57% at age 90 years and above.4 Their lower prevalence of retinal disorders could be due to underestimation of retinal problems, as their study was focussed primarily on glaucoma, so subtle retinal disorders may have been missed. The BRS focused on retinal diseases, and all patients were examined by fellowship-trained retina specialists. This could have led to detection of more retinal disorders in our study. The proportion of elderly population was higher in BRS. The more retinal disorders among elderly population could be another possible cause for higher prevalence of retinal disorders in BRS relative to BGS.
The prevalence of retinal diseases was 15.5% in the 60–69 years age group, and 21% in those 70 years and above in a study conducted in neighbouring India.2 The risk of retinal disorders was 2.8 times higher among the group 60–69 years old and 3.8 times higher among the age group 71 years and older as compared to the group 40–49 years of age. Retinal disease increased with ageing, consistent with our study. In a population-based study conducted in Tehran, the prevalence of retinal diseases was 32.74% in the age group 60–69 years and 46.08% among those age 70 years and older. In their series, retinal diseases were significantly associated with ageing.3 The prevalence of retinal disorder was slightly higher in females in our study (54.28%), but the prevalence of retinal diseases was not significantly associated with gender. This is consistent with other studies.2–4
AMD was the most common retinal problem (35.43%) affecting our study population. AMD was also the most common retinal problem in the previous population-based studies conducted in Nepal and elsewhere, consistent with our study.2–4,14 The Beaver Dam Eye Study reported a prevalence of dry AMD in 26.2% and wet AMD in 5.5%; altogether, AMD comprised 31.7% of the age group 75 years and older.39 The Los Angeles Latino Eye Study reported the presence of any soft drusen in 30.5% between ages 60–69 years, 40.4% between ages 70–79 years, and 58.1% at 80 years and above.40 The Rotterdam Eye Study reported drusen of 63 µm and larger in at least one eye in 40.8% in the 55–64 years age group and 52.6% at ages 85 years and older.41 A study conducted in India reported a prevalence of AMD of 31.05% between the age of 56–65 years and 54.79% between 66 and 75 years.42 The prevalence of AMD in our series was comparable to the above studies from Asian countries and with the Western developed world.
Hypertensive retinopathy was the second most common retinal problem (4.35%) among the study subjects in our series. Grade 1, grade 2 and grade 3 hypertensive retinopathy comprised 2.85%, 1.02%, and 0.48% of the study subjects, respectively. Although grade 1 and grade 2 hypertensive retinopathy are not vision threatening conditions, timely precautions and treatment of hypertension are required to prevent future progression. Our findings were consistent with other studies from Nepal and Poland where HTN retinopathy was a major retinal problem.14,43
In our study, a macular ERM was found in 3.66% of study subjects. A study conducted in China reported the presence of ERM in 8% of those age 60–69 years, 9.9% between age 70–79 years, and 20.6% among those age 80 years and above.44 Another study conducted in Japan reported ERM in 5.08% between age 55–64 years, 8.87% between age 65–74 years, and 9.75% at age over 75 years.45 A study conducted among the Indians residing in Singapore reported ERM in 12.1% in the 60–69 year age range and 27.7% in the 70–80 years age range.46 The lower prevalence in our series could probably be due to underestimation of ERMs, as optical coherence tomography (OCT) was not done routinely in our subjects, and diagnosis was based on clinical evaluation only.
In our study, diabetes was present in 9.1% of the study population, and DR was found in 2.15% of the study subjects. Our finding was comparable to the population prevalence in the Blue Mountain Eye Study, which reported a prevalence of 2.4% in the 60–69 years age range, 2.7% between 70 and 79 years, and 2.3% at age 80 years and above. A population-based study conducted in Tehran reported a prevalence of DR in 5.83% between 60 and 69 years, and 4.77% of those age 70 years and older.3 Our prevalence of DR is lower than their series. The population prevalence of DR ranged from 0.5% to 1.41% in some studies conducted in China and India in relatively younger age groups.2,47,48 This difference in prevalence could be due to differences between the populations.
RVO was found in 3.1% of study subjects, of which BRVO comprised 2.9%. A study conducted in high altitude regions of Nepal reported RVO as the third most common retinal disorder, comprising 7.1%, of which 4.9% were BRVO.14 A study conducted in Japan reported the prevalence of RVO in 3.2%, 2.3% and 4.6% of study subjects 60–69 years, 70–79 years and 80 years and above, respectively. BRVO was found in 2.7%, 2.3% and 4.6% of study subjects 60–69 years, 70–79 years, and 80 years and above, respectively. Our findings are consistent with this study.49 RVO was a major retinal problem in other studies conducted in Nepal and elsewhere.2–4 Our finding is consistent with these studies. Patient with RVO needs regular follow up and treatment of underlying risk factors.
Other retinal disorders present in our study population were macular hole, retinal detachment, lattice degeneration, peripheral retinal tear and holes, retinitis pigmentosa, macular and chorioretinal scars and chorioretinal coloboma. Those retinal lesions found in our study population were consistent with other studies.2–4,43
In multivariate logistic regression analysis, the risk of developing retinal disorders for those who had undergone prior cataract surgery was 1.7 times more than those who were phakic (OR, 1.71, p <0.001) and was 1.2 times higher in patients with hypertension (OR, 1.21, p=0.049) as compared to non-hypertensive patients. The relationship between retinal disorders and those who had cataract surgery is also found in other studies.50–52 Besides actual higher prevalence, more detection of retinal lesions because of clear media may be possible among those who had prior cataract surgery. Besides hypertensive retinopathy, studies have reported hypertension as a risk factor for major retinal diseases such as RVO, DR, and AMD. Our findings are consistent with the other studies.2–4,53
We found an overall high prevalence of retinal disorders in this elderly population. This warrants additional awareness campaigns, screening of retinal diseases among the high-risk groups, and referral of vision threatening cases for timely treatment to prevent avoidable blindness.
The major strength of the study is the large sample size of an elderly population. The study focussed primarily on retinal diseases, and all study subjects were examined by fellowship-trained retina specialists for accurate diagnosis of retinal disorders. The limitation of the study is that we were not able to assess serum lipid panels or glycosylated haemoglobin. These could have been useful for further risk factor assessment of retinal diseases. Macular OCT was not available for routine use at the study site. Those participants who require macular OCT and fundus fluorescein angiography were referred for further evaluation.
Conclusion
The prevalence of any retinal disorders was high (52.37%) among those age 60 years and above in the Bhaktapur district of Nepal. Age-related macular degeneration (35.43%) was the most common retinal problem, followed by various grades of hypertensive retinopathy (4.35%), epiretinal membrane (3.66%), branch retinal vein occlusion (2.95%), and diabetic retinopathy (2.15%). Retinal disorders increased with ageing. Those having prior cataract surgery (p<0.001) and systemic hypertension (p=0.049) had significantly higher retinal disorders. These study findings highlight the need for screening of retinal diseases in high-risk subjects, control of systemic hypertension and blood sugar, and regular eye check-ups for early detection of vision threatening retinal diseases to save the vision among the elderly population.
Consent for Publication
Written informed consent was taken from study participants prior to enrollment in the study and for publication of the study findings.
Abbreviations
AMD, Age-related macular degeneration; ARM, Age-related maculopathy; BRVO, Branch retinal vein occlusion; BRS, Bhaktapur Retina Study; BGS, Bhaktapur Glaucoma Study; BCVA, Best corrected visual acuity; BP, Blood pressure; BMI, Body mass index; CI, Confidence Interval; CRVO, Central retinal vein occlusion; DR, Diabetic retinopathy; ERM, Epiretinal membrane; ETDRS, Early Treatment of Diabetic Retinopathy Study; HTN, Hypertension; NPDR, Non-proliferative diabetic retinopathy; N, Number; OCT, Optical coherence tomography; OR, Odds Ratio; PDR, Proliferative diabetic retinopathy; PVD, Posterior vitreous detachment; RAAB, Rapid assessment of avoidable blindness; RPE, Retinal pigment epithelium; SD, Standard deviation; TIO, Tilganga Institute of Ophthalmology; WHO, World Health Organization.
Data Sharing Statement
Data available for the study are included in this study.
Ethics Approval and Informed Consent
The study was approved by the Tilganga Institute of Ophthalmology, Kathmandu, Nepal Institutional Review Committee (TIO-IRC) on 28 June 2013 (TIO-IRC approval number; 1/2013). The study was conducted in accordance with the Declaration of Helsinki. The objectives, risk and benefits of the study were fully explained to the study participants. Study participants confidentiality was ensured. The participants were informed about their rights to withdraw from the study at any time without any prejudice. Informed consent was written in the vernacular and was read to those unable to read. Subjects were asked to sign the consent form prior to enrollment in the study, and thumb impressions were taken for those unable to sign.
Acknowledgments
We acknowledge Bhaktapur Municipality, Tilganga Institute of Ophthalmology and Vrije University Medical Center for support to conduct this study. We acknowledge Prof. Dr. Paul S. Bernstein, MD, PhD from the John A. Moran Eye Center, University of Utah, USA for editing the manuscript. We also acknowledge all of the participants for their cooperation in the study.
Author Contributions
RT prepared the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851.
2. Nirmalan PK, Katz J, Robin A, et al. Prevalence of vitreoretinal disorders in a rural population of southern India. Arch Ophthalmol. 2004;122:581–586. doi:10.1001/archopht.122.4.581
3. Hatef E, Fotouhi A, Hadhemi H, Mohammad K, Jalali KH. Prevalence of retinal diseases and their pattern in Tehran. The Tehran eye study. Retina. 2008;28:755–762. doi:10.1097/IAE.0b013e3181613463
4. Thapa SS, Thapa R, Paudyal I, et al. Prevalence and pattern of vitreo-retinal disorders in Nepal: the Bhaktapur Glaucoma Study. BMC Ophthalmol. 2013;13:9. doi:10.1186/1471-2415-13-9
5. Klaver CCW, Wolfs RCW, Vingerling JR, Hofman A, de Jong PTVM. Age specific prevalence and causes of blindness and visual impairment in an older population. The Rotterdam Study. Arch Ophthalmol. 1998;116(5):653–658. doi:10.1001/archopht.116.5.653
6. Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age related maculopathy in Australia. The blue mountains eye study. Ophthalmology. 1995;102:1450–1460. doi:10.1016/S0161-6420(95)30846-9
7. Eye Diseases prevalence Research Group. Prevalence of age related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi:10.1001/archopht.122.4.564
8. The eye diseases prevalence research group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. doi:10.1001/archopht.122.4.552
9. West S, Sommer A. Prevention of the blindness and priorities for the future. Bull World Health Organ. 2001;79:244–248.
10. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi:10.2337/diacare.27.5.1047
11. Dandona L, Dandona R, Naduvilath TJ, et al. Is current eye-care-policy focus almost exclusively on cataract adequate to deal with blindness in India? Lancet. 1998;351:1312–1316. doi:10.1016/S0140-6736(97)09509-3
12. Iwase A, Araie M, Tomidokoro A, Yamamota T, Shimizu H, Kitazawa Y; Tajimi Study Group. Prevalence and causes of low vision and blindness in a Japanese adult population. Tajimi study group. Ophthalmology. 2006;113:1354–1362. doi:10.1016/j.ophtha.2006.04.022
13. Liang YB, Friedman DS, Wong TY, et al. The prevalence and causes of low vision and blindness in a rural Chinese adult population. The Handan Eye Study. Ophthalmology. 2008;115:1965–1972. doi:10.1016/j.ophtha.2008.05.030
14. Thapa R, Paudyal G, Crandall A, Tabin G. Vitreo-retinal disorders at high altitude in Nepal. Nepal J Ophthalmol. 2013;5(8):57–62. doi:10.3126/nepjoph.v5i1.7823
15. Rai BB, Morley MG, Bernstein PS, Maddess T. Pattern of vitreo-retinal diseases at the national referral hospital in Bhutan: a retrospective, hospital-based study. BMC Ophthalmol. 2020;20:51. doi:10.1186/s12886-020-01335-x
16. Chauhan A, Chaudhary KP, Rajput GC. Pattern of retinal diseases in hilly terrain of Himachal Pradesh, India. Int Eye Sci. 2014;14(12):2114–2118.
17. Brilliant GE, Pokhrel RP, Grasset NC, et al. The Epidemiology of Blindness in Nepal: Report of the 1981 Nepal Blindness Survey. Chelsea, MI: The Sewa Foundation; 1988.
18. Rapid assessment of avoidable blindness survey. The epidemiology of blindness in Nepal. Nepal Netra Jyoti Sangh 2012; 1–72.
19. Sapkota YD, Pokharel GP, Nirmalan PK, Dulal S, Maharjan IM, Prakash K. Prevalence of blindness and cataract surgery in Gandaki zone, Nepal. Br J Ophthalmol. 2006;90:411–416. doi:10.1136/bjo.2005.082503
20. Thapa SS, Berg RVD, Khanal S, et al. Prevalence of visual impairment, cataract surgery, and awareness of cataract and glaucoma in Bhaktapur District of Nepal. BMC Ophthalmol. 2011;11:2. doi:10.1186/1471-2415-11-2
21. Thapa SS, Khanal S, Paudyal I, et al. Outcome of cataract surgery: a population-based developing world study in the Bhaktapur District, Nepal. Clin Exp Ophthalmol. 2011;39(9):851–857. doi:10.1111/j.1442-9071.2011.02576.x
22. Central Bureau of Statistics: Population Census 2001. National Report. National Planning Commission Secretariat: Kathmandu, Nepal, 2002.
23. Central Bureau of Statistics: Population Census 2011. Government of Nepal, National Planning Commission Secretariat: Kathmandu, Nepal, 2011.
24. Government of Nepal, Ministry of Health, Department of Health Service, Annual Report 2017–18.
25. Singh DL, Bhattarai MD. High prevalence of diabetes and impaired fasting glycaemia in urban Nepal. Diabet Med. 2003;20:170–171. doi:10.1046/j.1464-5491.2003.00829_4.x
26. Paudyal G, Shrestha MK, Meyer JJ, Thapa R, Gurung R, Ruit S. Prevalence of diabetic retinopathy following a community screening for diabetes. Nepal Med Coll J. 2008;10(3):160–163.
27. Vaidya A, Pathak RP, Pandey MR. Prevalence of hypertension in Nepalese community triples in 25 years: a repeat cross-sectional study in rural Kathmandu. Indian Heart J. 2012;64:128–131. doi:10.1016/S0019-4832(12)60045-5
28. Chataut J, Adhikari RK, Sinha NP. Prevalence and risk factors for hypertension in adults living in central development region of Nepal. Kathmandu Univ Med J. 2011;33(1):13–18.
29. Thapa SS, Rana PP, Twayana SN, et al. Rationale, methods and baseline demographics of the Bhaktapur glaucoma study. Clin Exp Ophthalmol. 2011;39:126–134. doi:10.1111/j.1442-9071.2010.02429.x
30. Thapa R, Bajimaya S, Paudyal G, et al. Prevalence and risk factors for diabetic retinopathy among elderly population with diabetes in Nepal: the Bhaktapur retina study. Clin Ophthalmol. 2018;12:561–568. doi:10.2147/OPTH.S157560
31. Thapa R, Bajimaya S, Paudyal G, et al. Prevalence of risk factors for retinal vein occlusion among elderly population in Nepal: the Bhaktapur retina study. BMC Ophthalmol. 2017;17:162. doi:10.1186/s12886-017-0552-x
32. Thapa R, Bajimaya S, Paudyal G, et al. Prevalence of risk factors for age-related macular degeneration in Nepal: the Bhaktapur retina study. Clin Ophthalmol. 2017;11:963–972. doi:10.2147/OPTH.S132338
33. Thapa R, Bajimaya S, Paudyal G, et al. Population awareness of diabetic eye disease and age- related macular degeneration in Nepal: the Bhaktapur Retina Study. BMC Ophthalmol. 2015;15:188. doi:10.1186/s12886-015-0175-z
34. Bennett S, Woods T, Liyanage WM, Smith DL. A simplified general method for cluster sample surveys in developing countries. World Health Stat Q. 1991;44:98–106.
35. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy: ETDRS report 9. Ophthalmology. 1981;98:766–785.
36. Bird AC, Bressler NM, Bressler SB; International ARM epidemiological study group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39:367–374. doi:10.1016/S0039-6257(05)80092-X
37. Schubert HD. Ocular manifestations of systemic hypertension. Curr Opin Ophthalmol. 1998;9:69–72. doi:10.1097/00055735-199812000-00012
38. Report of World Health Organization/International Diabetes Federation Consultation: definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. World Health Organization 2006; 1–50.
39. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Bever Dam eye study. Ophthalmology. 1992;99(6):933–943. doi:10.1016/S0161-6420(92)31871-8
40. Varma R, Fraser-Bell S, Tan S, Klein R, Azen SP; Los Angelos Latino Eye Study Group. Prevalence of age related macular degeneration in Latinos. The Los Angelos Latino eye study. Ophthalmology. 2004;111(7):1288–1297. doi:10.1016/j.ophtha.2004.01.023
41. Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology. 1995;102(2):205–210. doi:10.1016/S0161-6420(95)31034-2
42. Sharma R, Mehta K, Bhatti JS, et al. Prevalence and predictors of age-related macular degeneration in the population of Punjab: North Indian age related macular degeneration epidemiology and molecular genetic study. Int J Health Sci Res. 2018;8(10):1–8.
43. Nowak MS, Jurowski P. The prevalence and pattern of vitreoretinal diseases in a sample: the population of older adults in the city of Lodz, Poland. Klin Oczna. 2018;119:3.
44. Duan XR, Ltang YB, Friedman DS, et al. Prevalence and associations of epiretinal membrane in a rural Chinese adult population: the Handan eye study. Invest Ophthalmol Vis Sci. 2009;50(5):2018–2023. doi:10.1167/iovs.08-2624
45. Kawasaki R, Wang JJ, Sato H, et al. Prevalence and association of epiretinal membrane in an adult Japanese population: the Funagata eye study. Eye. 2009;23:1045–1051. doi:10.1038/eye.2008.238
46. Kob V, Cheung CY, Wong WL, et al. Prevalence and risk factors of epiretinal membrane in Asian Indians. Invest Ophthalmol Vis Sci. 2012;53:1018–1022. doi:10.1167/iovs.11-8557
47. Sunita M, Singh AK, Rogye A, et al. Prevalence of diabetic retinopathy in urban slums: the Aditya Jyot Diabetic Retinopathy in urban Mumbai slum report no. 2. Ophthalmic Epidemiol. 2017;24(5):303–310. doi:10.1080/09286586.2017.1290258
48. Song P, Jinyue Y, Chan YE, Theodoratau E, Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health. 2018;8(1):1–16.
49. Yasuda M, Kiyobara Y, Arakawa S, et al. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: the Hisayama study. Invest Ophthalmol Vis Sci. 2010;51:3205–3209. doi:10.1167/iovs.09-4453
50. Sen P, Bhargava A, Vijaya L, George R. Prevalence of idiopathic macular hole in adult rural and urban South Indian population. Clin Exp Ophthalmol. 2008;36(3):257–260. doi:10.1111/j.1442-9071.2008.01715.x
51. Cho H, Madu A. Etiology and treatment of the inflammatory causes of cystoid macular edema. J Inflamm Res. 2009;2:37–43. doi:10.2147/JIR.S5706
52. Berrod JP, Sautiere B, Rozot P, Raspiller A. Retinal detachment after cataract surgery. Int Ophthalmol. 1996–1997;20(6):301–308.
53. Wang S, Xu L, Jonas JB, et al. Major eye diseases and risk factors associated with systemic hypertension in an adult Chinese population. Ophthalmology. 2009;116:2373–2380. doi:10.1016/j.ophtha.2009.05.041
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
