Back to Journals » Vascular Health and Risk Management » Volume 20
Prevalence of Medication Associated with QTc Prolongation Used Among Critically Ill Patients
Authors Al-Azayzih A , Al-Qerem W , Al-Azzam S, Muflih S , Al-Husein BA , Kharaba Z, Kanaan RJ, Rahhal D
Received 27 September 2023
Accepted for publication 19 January 2024
Published 1 February 2024 Volume 2024:20 Pages 27—37
DOI https://doi.org/10.2147/VHRM.S438899
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Ahmad Al-Azayzih,1 Walid Al-Qerem,2 Sayer Al-Azzam,1 Suhaib Muflih,1 Belal A Al-Husein,1 Zelal Kharaba,3,4 Roaa J Kanaan,1 Dania Rahhal1
1Department of Clinical Pharmacy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, Jordan; 2Faculty of Pharmacy, Al-Zaytoonah University of Jordan, Amman, Jordan; 3College of Pharmacy, AL Ain University, Abu Dhabi, United Arab Emirates; 4Honorary Associate Lecturer, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK
Correspondence: Ahmad Al-Azayzih, Department of Clinical Pharmacy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, Jordan, Email [email protected]
Background: Acquired prolonged corrected QT (QTc) interval can lead to life-threatening Torsade de Pointes (TdP) arrhythmia. Multiple risk factors including medications, comorbidities, and electrolyte imbalances contribute significantly to acquired manifestations of the QTc prolongation. Critically ill patients are particularly more vulnerable to TdP due to complex medical conditions, aging, and polypharmacy.
Objective: This study aimed to assess the prevalence of TdP-associated medication prescribing, identify risk factors for QTc prolongation and TdP, and determine primary predictors of high TdP medication usage in critically ill patients in Jordan.
Methods: We conducted a retrospective cross-sectional analysis of electronic medical records for patients from King Abdullah University Hospital who were admitted to Intensive Care Unit (ICU) between (July 2012-July 2022). We collected data on patients’ demographics, clinical characteristics, comorbidities, laboratory results, and prescribed medications. Medications were categorized into three TdP risk levels according to CredibleMeds® assessment tool. Data were analyzed using descriptive statistics and a binary logistic regression model.
Results: Of the 13,300 patients (58.2% male, median age 62 years). Prescribing prevalence for medications with known TdP risk was 19%, possible risk (24.7%), conditional risk (21.6%), and confirmed conditional risk (8.3%). Common comorbidities included hypertension (40.9%), diabetes (33.3%), and cancer (15.4%). Drugs with known TdP risk included citalopram, amiodarone, clarithromycin, and ciprofloxacin. A binary regression model revealed that as age increased, the odds of TdP associated medication prescribing decreased (OR = 0.989, p < 0.001), while patients on more than five medications had higher odds (OR = 4.281, p < 0.001).
Conclusion: The study identified a notable prevalence of prescribing for medications with QTc prolongation/TdP risk in critically ill patients. Healthcare providers in the ICU should exercise caution to minimize the inadvertent prescription of TdP associated medications especially among older patients and those with polypharmacy.
Keywords: torsade de pointes, QTc interval, critically ill patients, Jordan, intensive care unit
Introduction
The prolonged QTc interval is a pathological cardiac condition detectable via electrocardiogram (ECG), characterized by an extended ventricular repolarization phase. This anomaly is associated with the potential development of a specific and life-threatening ventricular cardiac arrhythmia called Torsade’s de Pointes, which can result in critical scenarios, including sudden cardiac arrest.1 A prior comparative investigation conducted in the USA reported a substantial prevalence of QTc prolongation among acutely ill patients, with approximately 24% of these patients exhibiting this abnormality. Additionally, this study revealed that Torsade de Pointes accounted for 6% of in-hospital cardiac arrests.2 QTc prolongation and the subsequent development of Torsade de Points can arise from congenital or acquired conditions. The predominant contributory factor to acquired Torsade remains the administration of medications with diverse pharmacological effects that are known to prolong the QTc interval. Such medications include antiarrhythmic agents, antiemetics, antibiotics, and neuroleptic drugs.3 Other risk factors do encompass aging, female gender, thyroid gland disorders (hypothyroidism or hyperthyroidism), diabetes, electrolyte disturbances (hypokalemia and hypomagnesemia), and presence of cardiovascular conditions.4–6
Critically ill patients exhibit a heightened susceptibility to an elevated risk of developing QTc interval prolongation and concomitant occurrence of Torsade de Points flares compared to other segments of the patient population.7,8 Proarrhythmic complications encountered in critically ill patients typically stem from various factors beyond the administration of medications with recognized arrhythmogenic potential. These patients often contend with polypharmacy defined as the concurrent use of five or more medications,6 renal and hepatic disease conditions, electrolyte imbalances and other chronic comorbidities all of which are established higher incidence of proarrhythmic events.5 A study assessing critically ill patients in the Intensive Care Unit (ICU) reported that approximately 69% of these patients met the criteria for QTc interval monitoring as outlined by the American Heart Association (AHA) criteria. These criteria encompassed the use of medications known to carry a risk of QTc interval prolongation, bradyarrhythmia, and the presence of electrolyte disturbances specifically low levels of potassium and/or magnesium in the bloodstream.9
Geriatric patients with multiple comorbidities constitute a substantial proportion of ICU admissions. A previous investigation revealed that individuals aged 65 and above accounted for 45.7% of total ICU admissions.10 Aging itself is correlated with an increased incidence of cardiac arrhythmias, primarily attributed to physical and functional alterations in cardiac muscle and the electrical conduction system. These changes contribute to higher occurrence of age-related cardiac arrhythmias. Additionally, aging associated changes in the cardiac function play a role in promoting arrhythmias during the aging process.11
In response to the imperative need for safer medication usage and the prevention of QTc interval prolongation, which can lead to TdP events, CredibleMeds® (www.crediblemeds.org)12 has introduced an online tool to classify and assess drugs with potential to induce prolongation and TdP. This tool categorizes medications associated with TdP flare into three distinct groups: drugs with a known risk of TdP, drugs with a possible TdP risk, and drugs with a conditional TdP risk.
The primary objective of this study is to investigate the prevalence of prescribing of TdP associated medications among critically ill patients admitted to ICU. The secondary objective is to evaluate the associated factors that can increase the risk of QTc interval prolongation and TdP in this group. Finally, to identify the main predictors associated with higher level of TdP associated medication prescribing in these patients.
Methods
Study Design, Sample Characteristics, and Data Collection
A retrospective cross-sectional study was conducted at the Intensive Care Unit (ICU) of King Abdullah University Hospital (KAUH) in Jordan. All patients admitted to ICU at KAUH during the period of 10 years (from July 2012 – July 2022) were included in the study for further analysis. A total number of 13,300 electronic medical records were evaluated and assessed, respectively. Data was gathered by collecting patients’ medication records that have demographic data, clinical characteristics, past medical history, relevant biomedical lab results, and prescribed medications for all patients, co-existed comorbidities, and length of stay (LOS) duration in the ICU. Risk factors that are correlated positively with TdP occurrence were identified through evaluating patients’ age, gender, presence of structural or conduction related cardiac diseases, hypothyroidism, diabetes, liver and/or kidney impairment, and medication polypharmacy. Drugs associated with TdP and QTc prolongation risks were assessed and further categorized into three main categories according to the most updated CredibleMeds online QT-Drug List (August 20th, 2023).12 First, medications with “known risk category” which includes all medications with substantial body of evidence linking them to definite risk of causing QTc interval prolongation and risk of TdP when used as directed in the drug leaflet. Second, medications with “possible risk” category which includes all medications with substantial body of evidence linking them to possible risk of causing QTc prolongation but with inadequate evidence that such drugs, when utilized as directed in the drug leaflet, have a risk of TdP. Third, conditional risk category which include all medications with substantial body of evidence linking them to risk of causing QTc prolongation and developing TdP, but only under certain known circumstances (eg, use with concomitant QTc/TdP associated drug, use of excessive dose, their use could lead to hypokalemia and/or hypomagnesemia).
Statistical Analysis
Data were gathered and analyzed using both Microsoft Excel and SPSS (version 28). Descriptive statistics were employed to describe the demographics and other characteristics of the patients. Categorical variables were represented as frequencies (%), while continuous variables were expressed as medians (95% confidence interval). The primary focus was on determining the prevalence of drugs associated with TdP. Patients’ data were entered into a database to extract key variables, including the prevalence of TdP-inducing drugs, the distribution of TdP categories for each drug, and the examination of connections between various risk factors and different levels of medication-related TdP risk (encompassing known risk, possible risk, conditional risk, and confirmed conditional risk). A binary logistic regression model was constructed to determine factors linked to the usage of at least one drug with TdP risk. The independent variables were age, gender, length of stay, polypharmacy, and number of comorbidities. Statistical significance was determined at a p-value <0.05.
Ethical Approval
This study was approved by the Institutional Review Board (IRB) at Jordan University of Science and Technology (JUST) and affiliated King Abdullah University Hospital (KAUH). Approval Number was 56/162/2023. The patient’s medical records were assessed retrospectively and for study purposes only. Hence, the requirement for informed consent was waived by the Institutional Review Board (IRB) committee at JUST/KAUH.
Results
A total of 13,300 patients were included in the study (58.2% Male), with a median age of 62 (62–63) years old. The median length of stay for patients in the ICU was 4 (4–5) days. The median polypharmacy count was 6 (6–7) medications. Additionally, patients had a median of 1 (1–2) comorbidity (Table 1). A range of comorbidities are also documented in Table 1. The most occurrences were hypertension, diabetes, and cancer (40.9%, 33.3%, and 15.4% respectively). In contrast, the least common comorbidities were coronary artery diseases (0.3%), Parkinson (0.5%), anemia (0.7%).
 |
Table 1 Demographics and the Disease Profile of the Enrolled Patients |
Table 2 outlines the frequency distribution of various blood test parameters categorized as “Low”, “Normal”, and “High”. The majority of the patients had a normal value of ALP (93.1%), ESR (91.6%), total bilirubin (91.3%). In contrast, many patients had high values of neutrophils count, urea, and creatinine (46.5%, 33.6%, and 24.7% respectively).
 |
Table 2 Blood Test Parameters of Study Patients |
Crucial electrolytes are also presented in Table 2. Regarding magnesium, 85.6% of patients exhibit results within the normal range, while 10.6% display low levels, and 3.9% exhibit elevated levels. Similarly, the distribution of potassium illustrates that 81.4% of patients have levels considered normal, 8.5% have lower levels, and 10.1% have higher levels. Calcium levels also primarily appear to fall within the normal range, as 77.6% of patients show values within the expected range, 21.6% show lower values, and 0.8% show higher levels.
Table 3 provides an in-depth overview of medications categorized based on their potential risks related to TdP. These medications are grouped into “Known Risk of TdP”, “Possible Risk of TdP”, and “Conditional Risk of TdP”.
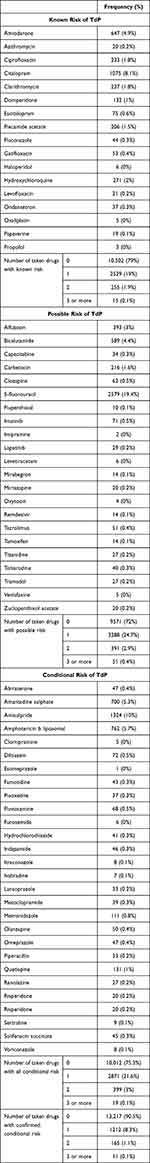 |
Table 3 Medications Categorizing Based on Their Potential Risks Related to TdP |
Regarding drugs with known risk of TdP, a subset of patients (ranging from 0.1% to 8.1%) are prescribed drugs such as amiodarone, ciprofloxacin, citalopram, and others that have a recognized risk of TdP. The number of drugs taken within this category varies, with the majority of patients (79%) taking no such drugs, while a smaller portion was on one (19%), two (1.9%), or three or more (0.1%) drugs with known TdP risk.
The “Possible Risk of TdP” category includes medications like 5-fluorouracil, alfuzosin, and bicalutamide, which could potentially be linked to TdP, although this association is not as firmly established. Seventy-two percent of the patients were not taking such drugs, while 24.7% were on one, 2.9% on two, and only 0.4% were on three or more.
Similarly, the “Conditional Risk of TdP” group features medications like abiraterone amantadine sulfate, amisulpride, and others, with varying levels of confirmed conditional risk, with the majority taking none (90.5%), followed by patients using one (8.3%), two (1.1%), and three or more (0.1%).
Table 4 provides a comprehensive overview of different risk factors and their associations with varying levels of medication risk concerning TdP. Drugs with known risk were more commonly prescribed to patients exhibiting polypharmacy (taking five or more medications), followed by patients with electrolyte disturbances, as well as those with impaired liver or kidney function (25.8%, 23.9%, and 23.6% respectively). Regarding drugs with possible risk, they were more frequently prescribed to patients with polypharmacy (36.1%), followed by diabetic patients (28.6%) and patients with electrolyte disturbances (27.3%). Drugs with confirmed conditional risk were more prescribed to patients with electrolyte imbalances, followed by those with polypharmacy, and then diabetic patients.
 |
Table 4 Medication Distribution According to Both Risk Factors and TdP Risk Category |
Overall, more than half of the patients with polypharmacy and those with electrolyte imbalances were prescribed at least one of the medications categorized as TdP risk drugs (including drugs with known risk, possible risk, and confirmed conditional risk).
A binary regression model was built to identify variables associated with taking at least one drug with TdP risk. Nagelkerke R Square was 0.23 indicating moderate model fit, and the results revealed that as the age increases the odds of taking at least one drug with TdP risk decreases (OR = 0.989, 95% Cl (0.987–0.991), p < 0.001). However, patients taking more than five medications demonstrated an elevated likelihood of using at least one drug with TdP risk (OR = 4.281, 95% Cl (3.935–4.658), p < 0.001).
Discussion
This study represents the inaugural effort to ascertain the prevalence of medications, prescribing characterized by definite and potential prolonged QT interval and TdP risks, along with the identification of risk factors contributing to the cumulative burden of TdP in critically ill patients from Jordan. The outcomes of our investigation offer invaluable insights into medication prescribing practices and potential vulnerabilities within this population.
The current study has unveiled a relatively high prevalence of medications prescribing among critically ill patients admitted to ICU encompassing varying levels of TdP risk. Specifically, the prevalence rates for the prescription of at least one drug with a known risk of TdP were 19%, possible risk (24.7%), conditional risk (21.6%), and confirmed conditional risk (8.3%). These prevalence figures exhibit notable disparities when compared to our previous study which was conducted on elderly outpatients and reported lower prevalence rates for drug prescribing with know and possible TdP risk equal to 14.9%, and 16.6%, respectively.13 These discrepancies in prevalence can be elucidated by the nature of the conditions prevailing in each respective healthcare setting. Critically ill patients are more susceptible to polypharmacy and administration of a greater number of medications. This phenomenon is primarily due to the presence of complex comorbidities, infections, and the imperative for intensive therapeutic management during their ICU confinement. Additionally, ICU patients frequently experience electrolyte imbalances, such as low potassium and magnesium levels, which are known triggers for TdP.14 Furthermore, the therapeutic objectives differ significantly between the two settings. While outpatient services concentrate on enhancing the quality of life and managing disease symptoms, the overarching goal in the ICU is to avert short-term mortality and enhance survival rates. Consequently, the prevention of medication-related issues may not be the foremost priority for ICU physicians during their patient care duties. Hypertension, diabetes, and cancer were the most common conditions found to exist among critically ill patients in this study. The history of hypertension and diabetes have been implicated as definite risk factors for QTc interval prolongation and instigating Torsade arrhythmia. Various reports have identified definite correlations between QTc interval prolongation and cardiovascular diseases and diabetes.15–18 An early study has found that prevalence of long QTc interval was higher in patients with type 2 diabetes and impaired insulin sensitivity was associated significantly with increased QTc interval among type 2 diabetic patients.16 On the other hand, a recent large scale epidemiological study has demonstrated that cancer patients had longer QTc interval compared to non-cancer healthy individuals.19 The same study attributed the reasons behind their note to the prescription pattern of antiemetic medications and pain killers linked to high risk of QTc prolongation in cancer patients.
In this study, citalopram, amiodarone, clarithromycin, and ciprofloxacin emerged as the most frequently prescribed medications with known risk of TdP. Notably, citalopram has been confirmed to prolong QTc interval even at lower doses particularly in geriatric patients with chronic comorbidities and polypharmacy. This finding positions citalopram as a less favorable treatment option when compared to other selective serotonin reuptake inhibitors within this patient population.20 It has been previously suggested that macrolides antibiotics, especially clarithromycin and erythromycin have the highest ability to induce QTc interval prolongation due to their intrinsic properties of inducing QTc prolongation and their strong inhibitory activity of the cytochrome P450 and metabolism inhibition of other drug linked to QTc interval prolongation.21
Exploring other risk factors known to prolong QTc interval and increase the likelihood for TdP development has revealed that more than half of the patients with polypharmacy and those with electrolyte imbalances, including hypokalemia and hypomagnesemia, were prescribed at least one of the medications categorized as TdP risk drugs. Since polypharmacy is often associated with individuals who have multiple chronic conditions, such as hypertension, diabetes, or heart disease.22 These conditions themselves can increase the risk of TdP independently, in addition to that, medications used to treat these comorbidities may further contribute to the TdP risk. Also, the existence of polypharmacy prescribing could carry greater number of serious drug–drug interactions, especially those boosting the pharmacokinetic availability of many drugs with potential TdP risk in the circulation leading to QTc interval prolongation yonder normal limits.23 Furthermore, polypharmacy may compromise the body’s ability to clear drugs from the system mainly at pharmacokinetic level suggesting more odds for clinically relevant drug–drug interactions.24
Limitation of the Study
This study is limited by its retrospective cross-sectional design. Causality relationship measurements were not feasible to assess in this study. The researchers were not able to address whether utilization of drugs with TdP risks was associated clinically with QTc interval prolongation. However, this study included a large sample size to address the prescribing practice among critically ill patients, especially for drugs with potential overlooked life threating consequences including TdP arrhythmia.
Conclusion
In summary, our study indicated a high prevalence of medication administration attributed to long QT prolongation/Torsade de Points risk among critically ill patients admitted to intensive care unit. This study showed urgent need to assess critically ill patients for their medications administered comprehensively to prevent unnecessary risk of developing life-threatening conditions, including Torsade de Pointes and sudden death.
Data Sharing Statement
Data used to conduct this research will be available from the corresponding author based on reasonable request.
Acknowledgment
This research obtained approval from the Deanship of Research and University Research Council at Jordan University of Science and Technology (JUST). Financial support was received from Deanship of Research at JUTS for conducting this research (Grant number: 20230430).
Disclosure
The authors declare no competing interest in this work.
References
1. Nelson S, Leung J, Alexander E, Susla GM. QTc prolongation in the intensive care unit: a review of offending agents. AACN Adv Crit Care. 2011;22(4):289–95; quiz 96–7. PubMed PMID: 22064575. doi:10.1097/NCI.0b013e31822db49d
2. Pickham D, Helfenbein E, Shinn JA, et al. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: results of the QT in Practice (QTIP) Study. Crit Care Med. 2012;40(2):394–399. PubMed PMID: 22001585. doi:10.1097/CCM.0b013e318232db4a
3. Li M, Ramos LG. Drug-induced QT prolongation and torsades de pointes. Pharm Ther. 2017;42(7):473–477. PubMed PMID: 28674475; PubMed Central PMCID: PMCPMC5481298. doi:10.1016/j.eupc.2005.05.010
4. Tisdale JE. Drug-induced QT interval prolongation and torsades de pointes: role of the pharmacist in risk assessment, prevention and management. Can Pharm. 2016. 149(3):139–152. doi:10.1177/1715163516641136. PubMed PMID: 27212965; PubMed Central PMCID: PMCPMC4860751.
5. Trinkley KE, Page RL, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;29(12):1719–1726. PubMed PMID: 24020938. doi:10.1185/03007995.2013.840568
6. Lima B, Razmjouei S, Bajwa MT, et al. Polypharmacy, gender disparities, and ethnic and racial predispositions in long QT syndrome: an in-depth review. Cureus. 2023;15(9):e46009. PubMed PMID: 37900391; PubMed Central PMCID: PMCPMC10600617. doi:10.7759/cureus.46009
7. Ding Y, Jeon R, Ran L, Pan W, Wang F, Li Q. New-onset QT prolongation is a novel predictor of mortality in critically ill patients. Crit Care. 2019;23(1):229. PubMed PMID: 31227008; PubMed Central PMCID: PMCPMC6588929. doi:10.1186/s13054-019-2514-6
8. Fernandes FM, Silva EP, Martins RR, Oliveira AG, Erdoes G. QTc interval prolongation in critically ill patients: prevalence, risk factors and associated medications. PLoS One. 2018;13(6):e0199028. PubMed PMID: 29898002; PubMed Central PMCID: PMCPMC5999273. doi:10.1371/journal.pone.0199028
9. Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572–576. PubMed PMID: 21040827. doi:10.1016/j.jelectrocard.2010.05.016
10. Fuchs L, Chronaki CE, Park S, et al. ICU admission characteristics and mortality rates among elderly and very elderly patients. Intensive Care Med. 2012;38(10):1654–1661. PubMed PMID: 22797350; PubMed Central PMCID: PMCPMC5718912. doi:10.1007/s00134-012-2629-6
11. Mirza M, Strunets A, Shen WK, Jahangir A. Mechanisms of arrhythmias and conduction disorders in older adults. Clin Geriatr Med. 2012;28(4):555–573. PubMed PMID: 23101571; PubMed Central PMCID: PMCPMC3610528. doi:10.1016/j.cger.2012.08.005
12. Woosley RLHC, Gallo T, Woosley D, Romero KA. QTdrugs list. 1457 E. Desert Garden Dr., Tucson, AZ 85718: AZCERT, Inc.; 2023. Available from: www.CredibleMeds.org.
13. Al-Azayzih A, Gharaibeh S, Jarab AS, Mukattash TL. Prevalence of torsades de pointes inducing drugs usage among elderly outpatients in North Jordan Hospitals. Saudi Pharm J. 2018;26(8):1146–1154. PubMed PMID: 30532635; PubMed Central PMCID: PMCPMC6260490. doi:10.1016/j.jsps.2018.07.002
14. Lee JW. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press. 2010;8(2):72–81. PubMed PMID: 21468200; PubMed Central PMCID: PMCPMC3043756. doi:10.5049/EBP.2010.8.2.72
15. Chugh SS, Reinier K, Singh T, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119(5):663–670. PubMed PMID: 19171855; PubMed Central PMCID: PMCPMC2734945. doi:10.1161/CIRCULATIONAHA.108.797035
16. Yang XH, Su JB, Zhang XL, et al. The relationship between insulin sensitivity and heart rate-corrected QT interval in patients with type 2 diabetes. Diabetol Metab Syndr. 2017;9(1):69. PubMed PMID: 28912840; PubMed Central PMCID: PMCPMC5594484. doi:10.1186/s13098-017-0268-3
17. Kaze AD, Yuyun MF, Erqou S, Fonarow GC, Echouffo-Tcheugui JB. Severe hypoglycemia and incidence of QT interval prolongation among adults with type 2 diabetes. J Clin Endocrinol Metab. 2022;107(7):e2743–e2750. PubMed PMID: 35396596; PubMed Central PMCID: PMCPMC9202715. doi:10.1210/clinem/dgac195
18. Akintunde AA, Oyedeji AT, Familoni OB, Ayodele OE, Opadijo OG. QT Interval prolongation and dispersion: epidemiology and clinical correlates in subjects with newly diagnosed systemic hypertension in Nigeria. J Cardiovasc Dis Res. 2012;3(4):290–295. PubMed PMID: 23233773; PubMed Central PMCID: PMCPMC3516009. doi:10.4103/0975-3583.102705
19. Kim P, Masha L, Olson A, et al. QT Prolongation in Cancer Patients. Front Cardiovasc Med. 2021;8:613625. PubMed PMID: 33718445; PubMed Central PMCID: PMCPMC7946823. doi:10.3389/fcvm.2021.613625
20. Alusik S, Paluch Z. [Citalopram and QT prolongation]. Vnitr Lek. 2018;63(12):952–956. PubMed PMID: 29334745. doi:10.36290/vnl.2017.174
21. Shaffer D, Singer S, Korvick J, Honig P. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis. 2002;35(2):197–200. PubMed PMID: 12087527. doi:10.1086/340861
22. Rieckert A, Trampisch US, Klaassen-Mielke R, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract. 2018;19(1):113. PubMed PMID: 30021528; PubMed Central PMCID: PMCPMC6052592. doi:10.1186/s12875-018-0795-5
23. Takeuchi H, Suzuki T, Remington G, Uchida H. Antipsychotic Polypharmacy and Corrected QT Interval: a Systematic Review. Can J Psychiatry. 2015;60(5):215–222. PubMed PMID: 26174525; PubMed Central PMCID: PMCPMC4484690. doi:10.1177/070674371506000503
24. Georgiev KD, Hvarchanova N, Stoychev E, Kanazirev B. Prevalence of polypharmacy and risk of potential drug-drug interactions among hospitalized patients with emphasis on the pharmacokinetics. Sci Prog. 2022;105(1):368504211070183. PubMed PMID: 35072561; PubMed Central PMCID: PMCPMC10358706. doi:10.1177/00368504211070183
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
