Back to Journals » Journal of Blood Medicine » Volume 6
Prevalence of HIV-related thrombocytopenia among clients at Mbarara Regional Referral Hospital, Mbarara, southwestern Uganda
Authors Taremwa IM , Muyindike W, Muwanguzi E , Boum II Y, Natukunda B
Received 13 January 2015
Accepted for publication 19 February 2015
Published 10 April 2015 Volume 2015:6 Pages 109—113
DOI https://doi.org/10.2147/JBM.S80857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Video abstract presented by Ivan Taremwa
Views: 1115
Ivan M Taremwa,1 Winnie R Muyindike,2 Enoch Muwanguzi,1 Yap Boum II,1,3 Bernard Natukunda1
1Department of Medical Laboratory Sciences, Faculty of Medicine, Mbarara University of Science and Technology, 2Immune Suppression Syndrome Clinic, Mbarara Regional Referral Hospital, 3Epicentre Mbarara Research Centre, Mbarara, Uganda
Aims/objectives: We aimed to determine the prevalence and correlates of thrombocytopenia among people living with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) and to assess occurrence of antiplatelet antibodies, among thrombocytopenic HIV clients at Mbarara Regional Referral Hospital, southwestern Uganda.
Materials and methods: This was a retrospective review of hematologic results at enrollment to HIV care from 2005 to 2013. The prevalence and correlates of thrombocytopenia were estimated based on the Immune Suppressed Syndrome (ISS) Clinic electronic database. A cross-sectional study determined the occurrence of antiplatelet antibodies, using the monoclonal antibody-specific immobilization of platelet antigens (MAIPA) technique.
Results: We reviewed 15,030 client records. The median age was 35.0 (range 18–78; interquartile range [IQR] 28–42) years, and there were 63.2% (n=9,500) females. The overall prevalence of thrombocytopenia was 17.4% (95% confidence interval [CI]: 16.8%–18.0%). The prevalence of thrombocytopenia was 17.8% (95% CI: 17.1%–18.4%) among antiretroviral therapy (ART)-naïve clients (n=2,675) and was 13.0% (95% CI: 0.3%–21.9%) for clients who were on ART (n=6). The study found a significant association between thrombocytopenia and other cytopenias, CD4 counts, ART, and deteriorating HIV stage (P<0.05). Two of the 40 participants (5.0%) had antiplatelet antibodies.
Conclusion: This study has showed a high prevalence of HIV-related thrombocytopenia. Antiplatelet antibodies were found in 5.0% of HIV-infected thrombocytopenic participants. Our study shows a significant association of thrombocytopenia burden in a high-HIV study population (Southwest Uganda); therefore, there is need to monitor platelet counts and initiate platelet transfusion in our blood banking practices, to avert possible risks of bleeding.
Keywords: antiplatelet antibodies, cytopenia, AIDS
Introduction
Deranged hematological parameters, including thrombocytopenia, are features of human immunodeficiency virus (HIV) infection.1 Thrombocytopenia, defined by platelet cell count of less than 150×109/L,2,3 occurs in about 4%–24% of HIV-infected cases.4,5 Mechanisms for thrombocytopenia, such as platelet involvement in immune responses, cytopathic effect of antiretroviral therapy (ART) regimens, and antigenic mimicry, are key in a setting of HIV.6,7 Thrombocytopenia has been linked to adverse sequelae and is regarded as an independent predictor of morbidity and mortality among the HIV-infected group, owing to increased risk of bleeding, which may occur in the mucous membranes, skin, soft tissue, and intracranial sites.8,9 The associated bleeding may cause death if it involves critical sites.10 To avert the risk of bleeding, platelet transfusion may be indicated; however, in Uganda, component transfusion is not readily available,11 which limits success of clinical interventions in an immunologically compromised population. Although cytopenias have been widely reported in HIV infection, there is little data regarding prevalence, correlates, and etiologic association of HIV-related thrombocytopenia in Uganda. This study sought to determine HIV-related thrombocytopenia in a high-HIV/acquired immune deficiency syndrome (AIDS) study population (Southwest Uganda). The findings from this study will form a basis for management of complications that arise from thrombocytopenia among HIV clients in this setting.
Materials and methods
Study participants
These were HIV-positive adult males and females who had been enrolled for care at the Immune Suppressed Syndrome (ISS) Clinic in Mbarara, Uganda. The hematologic results were retrieved from the ISS database. We sought informed consent of patients with thrombocytopenia as found in their previous full blood count (FBC) results, and investigated the presence of antiplatelet antibodies.
Sample collection
Blood was drawn, with minimal stasis, from the antecubital vein. For each sample, 3 mL of blood was collected into a plain Vacutainer, allowed to clot, and centrifuged, to obtain hemolysis-free serum that was kept frozen at −80°C at the Epicentre Mbarara Research Centre, Mbarara, Uganda.
Laboratory analysis
We performed indirect monoclonal antibody-specific immobilization of platelet antigens (MAIPA) for 40 serum samples from thrombocytopenic HIV clients, to screen and identify antiplatelet antibodies. Antibody screening was done using platelets from a pool of six group O donors selected for their platelet genotype (Advanced Practical Diagnostics BVBA, Turnhout, Belgium); these were incubated with serum, and mouse monoclonal antibodies specific for platelet glycoproteins Ia/IIa, Ib/IX, IIb/IIIa, and anti β-2-microglobulin. Lysates were cleared by centrifugation and transferred to microplate wells precoated with goat anti-mouse immunoglobulin G (IgG). The bound complex was detected using goat peroxidase-coupled anti-human IgG and revealed by peroxidase substrate O-phenylenediamine. The reaction was stopped using sulfuric acid, and absorbance was read at 492 nm. All positive antibody screens were identified, using a standard six-cell genotyped panel, by similar or additional methods. A participant was regarded to have antiplatelet antibodies if one or more platelet antiglycoproteins were identified.
Statistical analysis
Retrospective data from the ISS database and data from laboratory analyses were entered into a statistical software package of EXCEL® 5.0 (Microsoft Corp, Redmond, WA, USA) and transferred to STATA 12 (StataCorp LP, College Station, TX, USA) to carry out data management and analysis, respectively. Descriptive statistics (median and interquartile range [IQR]) were used. Univariate and multivariate analyses using logistic regression were done to establish predictor variables. Proportions for categorical variables were compared using the chi-square test. The P-value was considered to be statistically significant when less than 0.05.
Ethical considerations
We sought clients’ informed consent, and the study was approved by the Institutional Review Committee of Mbarara University of Science and Technology, and the Uganda National Council for Science and Technology.
Results
Records for 15,030 HIV clients at enrollment into care at the Mbarara Regional Referral Hospital ISS clinic were used. The study group comprised 9,500 females. Participants had a median age of 35.0 (range 18–78; IQR 28–42) years.
Out of 15,030 participants, 2,617 had thrombocytopenia, giving an overall prevalence of 17.4% (95% confidence interval [CI]: 16.8%–18.0%). The prevalence of thrombocytopenia among HIV clients who were ART-naïve (n=2,675) was 17.8% (95% CI: 17.1%–18.4%), while that for clients who were on ART for up to 6 months (n=6) was 13.0% (95% CI: 0.3%–21.9%).
We used univariate and multivariate analyses to determine association of thrombocytopenia with clinical stage, CD4 count, ART, anemia, and leucopenia, which we found significant (P<0.05) (Tables 1 and 2).
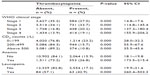  | Table 1 Correlation of thrombocytopenia with different participants’ variables |
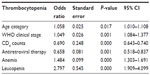  | Table 2 Multivariate analysis of thrombocytopenia among participants |
Of the 40 thrombocytopenic samples tested for antiplatelet antibodies, two (5.0%) had glycoprotein IIb/IIIa and IIa/Ia.
Discussion
In this study, the overall prevalence of HIV-related thrombocytopenia was 17.4% (95% CI: 16.8%–18.0%); this is comparable to the 20% reported in Iran12 and 12.7% in Ethiopia.13 This is attributed to HIV-induced hemopoiesis dysfunction and platelet immunologic involvement, which aggravates their destruction.8 The prevalence of thrombocytopenia among HIV-infected, ART-naïve clients was 17.8% (95% CI: 17.1%–18.4%); this is similar to the 16.1% reported in Nigeria14 and 13.5% in Rwanda15 but higher than the 8.3% reported in Kampala, Uganda16 and the 5.9% reported in Ethiopia.17 The high prevalence of thrombocytopenia in this group is probably attributed to already established mechanisms of HIV-related thrombocytopenia.18,19 This study showed a positive effect of regimens containing the nucleoside analog reverse-transcriptase inhibitor, zidovudine (AZT), which is similar to other reports;17 thus exclusive use of prophylactic co-trimoxazole among the ART-naïve HIV clients may be an independent predictor of accelerated thrombocytopenia. Since we detected antiglycoproteins in one of the ART-naïve participants, it is likely that such antibodies cause thrombocytopenia.20
The prevalence of thrombocytopenia among HIV-infected clients who were on ART for up to 6 months was 13.0% (95% CI: 0.3%–21.9%); this is much higher than the 4.1% reported in Ethiopia.21 This is probably due to diminished use of AZT regimens, in our study population, due to associated anemia.22 We analyzed for ART combinations as a predictor of thrombocytopenia, and from this, we elucidate reduced thrombocytopenia in participants on AZT regimens. The observed prevalence may as well be attributed to factors linked to induced cytopenias that vary according to the participant’s profile, the etiologic basis, and ART option.23 The occurrence of antiglycoprotein in one of the participants in this group shows antiplatelet antibody involvement in the etiology.8
Using univariate and multivariate analyses to relate the independent predictors of thrombocytopenia, diminished CD4 counts, other cytopenias, and enrollment on ART showed significant association with the occurrence of thrombocytopenia in our study participants (P<0.05), which is consistent with findings reported by other studies in the region.16,21 This may be due to defective hemopoiesis and the effect of ART, as previously reported.21,24 The study found a significant association of thrombocytopenia with other cytopenias in all HIV stages (refer to Table 3). This may be due to defective bone marrow, accelerated platelet destruction, and to AZT- or stavudine-containing regimens.25
There was an increasing risk of thrombocytopenia with HIV infection progression, and this is similar to findings from other studies.17,26 This may be because thrombocytopenia is greater in advanced HIV infection.15
The antiplatelet antibody prevalence of 5.0% is comparable with the 9.4% found among HIV Chilean population27 but lower than the 17.0% in the Italian population.28 The higher anti-human platelet antigens (HPAs) against IIb/IIIa than Ia/IIa is due to their close association with the chronic thrombocytopenic states associated with HIV.29 Anti-HPA-5, which targets Ib/IX, was missing in our study participants, as these are linked to thrombocytopenia due to neonatal alloimmunization.30 Antiplatelet antibodies were seen in mild and moderate thrombocytopenic clients, which implies that severe thrombocytopenia is not associated with the production of antiplatelet antibodies, as already reported.28
Limitation of the study
The key limitation of study was the small sample size of participants enrolled for antiplatelet antibody assay owing to time and financial constraints.
Recommendations
The authors recommend a larger study to be carried out and that as a basis of critical care and management, immune thrombocytopenia should be considered in our study population.
Conclusion
This study has observed a high prevalence of thrombocytopenia, especially in the ART-naïve population, and the occurrence of antiglycoproteins in our sample population. These results indicate that there is a need to monitor platelet counts and to initiate platelet component transfusion among HIV thrombocytopenic clients, in our current blood banking practice.
Acknowledgments
The authors wish to thank the study participants and the ISS Clinic team. We are grateful to the Epicentre Mbarara Research Centre, the Medical Education Partnership Initiative – Medical Education Services for All Ugandans (MEPI-MESAU) consortium, and the Uganda Research Student Support Fund (URSSF) for their support. We are grateful to Dr Mark Siedner and Michael Kanyesigye for their contributions to this study. This work was made possible by Medical Education for Equitable Services to All Ugandans a Medical Education Partnership Initiative (5R24TW008886) from the Office of Global AIDS Coordinator and the U. S. Department of Health and Human Services, Health Resources and Services Administration and National Institutes of Health.
Author contributions
IMT participated in study conception and design; data acquisition, analysis, and interpretation; and manuscript drafting and revision. WRM participated in study conception; data acquisition, analysis, and interpretation; and manuscript drafting. EM participated in study conception and design; data acquisition, analysis, and interpretation; and manuscript drafting. YB participated in study conception and design; data acquisition, analysis, and interpretation; and critically revised the manuscript. BN participated in study conception and design; data acquisition, analysis and interpretation; and critically revised the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Cosby CD. Hematological disorders associated with human immunodeficiency virus and AIDS. J Infus Nurs. 2007;30(1):22–32. | |
Giles. C. The platelet count and mean platelet volume. Br J Haematol. 1981;48(1):31–37. | |
Dacie JV, Lewis SM. Practical Haematology. 7th ed. Edinburgh: Elsevier Churchill Livingstone; 1991. | |
Sloand EM, Klein HG, Banks SM, Vareldzis B, Merritt S, Pierce P. Epidemiology of thrombocytopenia in HIV infection. Eur J Haematol. 1992;48(3):168–172. | |
Liebman H. Other immune thrombocytopenias. Semin Hematol. 2007;44(4 Suppl 5):S24–S34. | |
Ballem PJ. Belzberg A, Devine DV, et al. Kinetic studies of the mechanism of thrombocytopenia in patients with human immunodeficiency virus infection. N Eng J Med. 1992;327(25):1779–1784. | |
McDonald EJ. and Butler A. Immune thrombocytopaenia in adults: a single-centre retrospective review of patients presenting over 7 years. N Z Med J. 2010;123(1312):18–25. | |
Coyle TE. Hematologic complications of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Med Clin North Am. 1997;81(2):449–470. | |
Johannessen A, Naman E, Ngowi BJ, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. | |
Racoosin JA, Kessler CM. Bleeding episodes in HIV-positive patients taking HIV protease inhibitors: a case series. Haemophilia. 1999;5(4):266–269. | |
Natukunda B. Post-Transfusion and Maternal Red Blood Cell Alloimmunization in Uganda [doctoral thesis]. Leiden: Leiden University; 2013. | |
Alaei K, Alaei A, Mansoori D. Thrombocytopenia in HIV-infected patients, Islamic Republic of Iran. East Mediterr Health J. 2000;8(6):758–764. | |
Addis Z, Yitayew G, Tachebele B. Prevalence of some hematological abnormalities among HIV positive patients on their first visit to a tertiary health institution in Ethiopia; A cross sectional study. International Blood Research and Reviews. 2014;2(6):270–278. | |
Akinbami A, Olajumoke O, Titilope A, et al. Hematologic abnormalities in treatment-naive HIV patients. Infect Dis (Auckl). 2010;3:45–49. | |
Munyazesa E, Emile I, Mutimura E, et al. Assessment of haematological parameters in HIV-infected and uninfected Rwandan women: a cross-sectional study. BMJ Open. 2012;2(6):e001600. | |
Kyeyune R, Saathoff E, Ezeamama AE, Löscher T, Fawzi W, Guwatudde D. Prevalence and correlates of cytopenias in HIV-infected adults initiating highly active antiretroviral therapy in Uganda. BMC Infect Dis. 2014;14:496. | |
Wondimeneh Y, Muluye D, Ferede G. Prevalence and associated factors of thrombocytopenia among HAART-naive HIV-positive patients at Gondar University Hospital, Northwest Ethiopia. BMC Res Notes. 2014;7:5. | |
Li Z, Nardi MA, Karpatkin S. Role of molecular mimicry to HIV-1 peptides in HIV-1-related immunologic thrombocytopenia. Blood. 2005;106(2):572–576. | |
Moses A Nelson J, Bagby GC Jr. The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91(5):1479–1495. | |
Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009;46(1 Suppl 2):S2–S14. | |
Enawgaw B, Alem M, Addis Z, Melku M. Determination of hematological and immunological parameters among HIV positive patients taking highly active antiretroviral treatment and treatment naive in the antiretroviral therapy clinic of Gondar University Hospital, Gondar, Northwest Ethiopia: a comparative cross-sectional study. BMC Hematol. 2014;14:8. | |
Montaner JS, Le T, Fanning M, et al. The effect of zidovudine on platelet count in HIV-infected individuals. J Acquir Immune Defic Syndr. 1990;3(6):565–570. | |
Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results From the Multistate Adult and Adolescent Spectrum of HIV Disease Surveillance Project. Blood. 1998;91(1):301–308. | |
Berhane K, Karim R, Cohen MH, et al. Impact of highly active antiretroviral therapy on anemia and relationship between anemia and survival in a large cohort of HIV-infected women: Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2004;37(2):1245–1252. | |
Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(1):29–33. | |
Ferede G, Wondimeneh Y. Prevalence and related factors of anemia in HAART-naive HIV positive patients at Gondar University Hospital, Northwest Ethiopia. BMC Hematol. 2013;13:8. | |
Palomo I, Alarcón M, Sepulveda C, Pereira J, Espinola R, Pierangeli S. Prevalence of antiphospholipid and antiplatelet antibodies in human immunodeficiency virus (HIV)-infected Chilean patients. J Clin Lab Anal. 2003;17(6):209–215. | |
Riccio A, Natale D, Fratellanza G, et al. The lack of correlation between the positivity of anti-platelet antibodies and the presence of thrombocytopenia in course of HIV infection. Boll Soc Ital Biol Sper. 1993;69(3):203–208. | |
Aster RH, Newman PJ. HPA-1a/b(PlA1/A2, Zwa/b): the odyssey of an alloantigen system. Immunohematology. 2007;23(1):2–8. | |
Kaplan C, Morel-Kopp MC, Kroll H, et al. HPA-5b (Br(a)) neonatal alloimmune thrombocytopenia: clinical and immunological analysis of 39 cases. Br J Haematol. 1991;78(3):425–429. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

