Back to Journals » Cancer Management and Research » Volume 13
Prevalence of Fatigue and Associated Factors Among Cancer Patients Attending Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia
Authors Nugusse T, Lemlem SB , Deressa J , Kisa S
Received 18 November 2020
Accepted for publication 21 January 2021
Published 24 February 2021 Volume 2021:13 Pages 1909—1916
DOI https://doi.org/10.2147/CMAR.S291298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Teka Nugusse,1 Semarya Berhe Lemlem,2 Jembere Deressa,2 Sezer Kisa3
1Department of Nursing, Ayder Specialized Referral Hospital, Mekelle, Ethiopia; 2Department of Midwifery, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Nursing and Health Promotion, Oslo Metropolitan University, Oslo, Norway
Correspondence: Jembere Deressa
Department of Midwifery, College of Health Sciences, Addis Ababa University, P.O. Box 9086, Addis Ababa, Ethiopia
Tel +251 912782147
Email [email protected]
Background: Fatigue is a subjective and distressing symptom in cancer patients and has profound effects on daily life. The rates of fatigue during treatment are reported to be 25– 90%. Its causes are secondary to their treatment course, cancer itself and associated factors.
Purpose: To assess the prevalence of fatigue and associated factors among cancer patients at Tikur Anbessa Specialized Hospital in Addis Ababa, Ethiopia, 2019.
Patients and Methods: A cross-sectional study design was conducted on cancer patients undergoing treatment in Tikur Anbessa Specialized Hospital. A sample of 278 was selected using systematic random sampling technique and Brief Fatigue Inventory questionnaire was used for data collection. The data were entered into EPI data version 3.1 and transferred to SPSS version 24 for analysis. Bivariate and multivariable logistic regression were conducted to summarize the data. The significant statistical test was determined at 95% confidence interval and at p< 0.05.
Results: The mean age of the participants was 44.9 ± 14 years. The prevalence of fatigue identified by this study was 208 (74.8%). Age, stage of cancer, presence of infection, type of cancer, and type of treatment had shown a significant association with fatigue [AOR = 3.15, 95% CI: (1.35– 7.34)], [AOR = 0.02, 95% CI: (0.003– 0.172)], [AOR = 4.15, 95% CI: (1.06– 16.07)], [AOR = 5.19, 95% CI: (1.59– 16.90)], [AOR = 0.18, 95% CI: (0.07– 0.462)] respectively.
Conclusion: The prevalence of fatigue in cancer patients in this study was high. Risk factors were age of the patients, stage of cancer, presence of infection, cervical cancer and radiation therapy.
Keywords: cancer, fatigue, chemotherapy, specialized hospital, Ethiopia
Introduction
Cancer now ranks as the leading cause of death globally and it has a great impact on affected people.1 The number of worldwide cases of cancer is projected to increase 65% from 12.7 million in 2008 to 21 million in 2030.2 Similarly, Cancer (CA) is an increasing public health burden on sub-Saharan Africa at large. In Ethiopia, hospital records show that there are more than 150,000 cancer cases per year and currently cancer accounts for 4% of all deaths.3
According to the European Association for Palliative Care (EAPC) fatigue is defined as “a subjective feeling of tiredness, weakness or lack of energy”.4 The National Comprehensive Cancer Network (NCCN) defines fatigue as “a persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning”.5–7 In contrast to the tiredness sometimes felt by a healthy individual, fatigue in cancer patients is perceived as being of greater magnitude, disproportionate to activity or exertion, and not relieved by rest.8
Fatigue in cancer patients is seven times more prevalent than fatigue in the normal population and it is different from normal fatigue due to overexertion or lack of sleep.9 In addition to the direct impact of cancer, various treatment modalities, particularly chemotherapy and radiation, are known to cause fatigue for many patients for an extended period of time.10
Globally, an estimated 50–90% of cancer patients experience the difficulties of fatigue, the latter number being for patients subjected to chemotherapy and radiotherapy.11 Fatigue is now understood to be the most common symptom associated with cancer and its treatment and is an underestimated symptom in cancer patients.10,12–14 The causes are multifactorial such as cancer itself, cancer treatment, malnutrition, opioids, anxiety medication effect, etc.15,16
With the continued development of cancer diagnostic and therapeutic technologies, patient survival duration has been significantly extended. However, fatigue related to treatment for cancer can have a major negative impact on quality of life by altering a person’s ability to engage in meaningful personal work and social activities. As a result, improvements in the quality of life of cancer patients have fallen and it can be treatable if provided proper care.10,17–19 Fatigue occurs most often after surgery, chemotherapy, radiotherapy and cancer patients report it as the major obstacle to normal functioning and a good quality of life.12,14,20 Differences in the incidence and severity of fatigue have been noted by age, gender, stage of disease, and functional ability and these variances should be interpreted with caution because the effects of different types of disease and treatment protocols on the incidence and severity of fatigue are unknown.21
Fatigue in cancer patients has been reported in 90% of patients treated with radiation, and 80% of those under chemotherapy treatment.10,22 It affects the physical, social, cognitive, and emotional life of the patients and their families; however, it remains an under-recognized and undertreated problem in cancer survivors.6,23 Fatigue management strategies tend to focus on both non-pharmacological and pharmacological in which non-pharmacological treatment accounts for patient/family education and counselling, physical activities, behavioral intervention, psycho stimulants, and a care for contributing factors such as pain, emotional distress, sleep disturbance, nausea/vomiting, lack of appetite and anemia.24–27 Unlike other symptom treatments fatigue requires a general supportive care developed by the NCCN and the oncology of nursing society.25 Nevertheless, knowledge about this condition remains fragmentary and scarce.28
Health care professionals focus on symptoms, such as pain, nausea, and vomiting. These may be the reason why the patients stop their treatment schedule and refuse to go for next follow-up due to fatigue.10,29 Cancer is still a challenge for low- and middle-income countries. This is due to limited information in Ethiopia related to the prevalence of fatigue in cancer-diagnosed patients and its associated factors at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, 2019.
Patients and Methods
Study Area and Period
The study was conducted at the oncology clinic Tikur Anbessa Specialized Hospital, College of Health Sciences, Addis Ababa University, Ethiopia. The study was conducted from February 1 to March 30, 2019 at the oncology unit of TASH, College of Health Sciences, Addis Ababa University, Ethiopia.
Study Design and Population
An institutional based cross-sectional study was conducted among all adult cancer patients attending the oncology unit.
Inclusion and Exclusion Criteria
Patients diagnosed with cancer and undergoing treatment and aged >18 years were included and patients who suffer from mental or cognitive disorders and not willing to participate were excluded.
Sample Size Determination
The sample size was calculated using single population proportion formula, assuming p = 0.5 n = 384.
Since flow of patients during data collection period is less than 10,000 then correction formulas will be applied. NF = n/1 + n/N = 384/1 + 384/750 = 253
NF = desired sample size
n = the calculated sample size N = total population.
After adding 10% non-response rate the final sample size was 278.
Sampling Technique
Tikur Anbessa Specialized Hospital (TASH) is the only public hospital treating cancer patients in the country. The oncology unit of TASH patients who fulfilled the criteria was identified from their charts. A one-year record check of adults receiving chemotherapy cancer treatment traced 9,000 cases in the oncology unit. 750 patients had monthly follow-up treatments and then 278 patients were sampled within the study period by simple random sampling and/or systematic sampling technique for every two patients (chemotherapy cancer treatment) and were enrolled in the study.
Data Collection Tools and Procedures
A standard Brief Fatigue Inventory (BFI) questionnaire was used to collect the required data in the form of an exit interview. The data collection tool has two parts, the first contained patient socio-demographic information and the second part contained a BFI scale questionnaire. The original English version of the BFI was first translated into Amharic by two independent translators and then back translated into English by another independent translator who had not seen the original English version. All the translators are language experts who are fluent in English. Next, the English back-translated versions were compared with the originals. In case a back-translated item did not agree with the original, the first two translators performed a second translation and the second two translators performed second back-translations. It is a brief questionnaire, which consists of ten items, using a numerical rating scale of 0–10 and was checked for validity and reliability. Three nurses with a Bachelor of Science degree in nursing and one with previous experience of supervision were recruited to administer the interviews.
Data Quality Control
Training was given to interviewers about techniques and the concept of the questionnaire before data collection. Moreover, the instrument was pretested on 5% of the total sample size and pretested subjects were excluded from the actual data collection. During the process of data collection, the supervisor was overseeing the overall activity and checking the completeness and consistency of the data.
Data Processing and Analysis
The collected data were cleaned, checked, coded and entered into Epi data, version 3.1, software and exported to SPSS, version 24, for analysis. Frequency distribution and percentage of different variables were computed to describe and summarize the basic social demographic characteristics of the respondents. Binary logistic regression was run to determine the COR and variables with p < 0.25 was run on a multivariate logistic regression model. The statistical significance of the variable was declared at p < 0.05 and odds ratio at 95% confidence interval.
Results
Socio-Demographic Characteristics
A total of 278 patients diagnosed with cancer, composed of 124 males and 154 females, participated in this observational study. The mean age of the participant was 44.9 ± SD 14 years. Table 1 shows that, of the total study participants, 55% live in an urban area and 45% in a rural area.
 |
Table 1 Socio-Demographic Characteristics of the Cancer Patients in TASH, Addis Ababa, Ethiopia, 2019 (n = 278) |
Clinical Characteristics
The study participants were diagnosed with all types of cancer and were undergoing treatment. All of the participants received treatment in the form of chemotherapy, radiation and surgery as indicated in Table 2 and about 23.0% had infections.
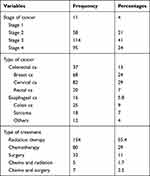 |
Table 2 Clinical Characteristics of Cancer Diagnosed Patients in TASH, Addis Ababa, Ethiopia, 2019 (n = 278) |
Prevalence of Fatigue and Treatment Modality
Figure 1 shows the relationship between prevalence of fatigue and treatment modality among cancer diagnosed patients in TASH, Addis Ababa, Ethiopia.
 |
Figure 1 Bar chart showing relationships between prevalence of fatigue and treatment modality among cancer diagnosed patients in TASH, Addis Ababa, Ethiopia, 2019 (n = 278). |
Interference with Daily Routine Activities
Fatigue interferes with the normal activities of cancer-diagnosed patients in TASH, as presented in Figure 2.
 |
Figure 2 Fatigue interference with normal activities of cancer-diagnosed patients in TASH, Addis Ababa, Ethiopia, 2019 (n = 278). |
Associated Factors with Fatigue in Cancer Diagnosed Patients
During bivariate logistic regression analysis; variables with significance level <0.25 were considered in multivariable logistic regression. Those independent variables with a p value of less than 0.25 in bivariate analysis (age, level of education, address, type of cancer, stage of cancer, type of treatment and presence of infection) were entered into multivariable logistic regression analysis. By controlling confounding factors the following five variables (age, stage of cancer, presence of infection, type of cancer and type of treatment) had shown a significant association, with p<0.05.
Accordingly, patients whose age was from 41–60 years were 3 times more likely to have fatigue than those from age 20–40 years [AOR = 3.2, 95% CI (1.35–7.34), p = 0.008]. The odds of developing fatigue among patients who had stage I cancer was 98.0%, stage II cancer 89.0% and stage III cancer was 84.0% less likely to have fatigue than patients with stage IV cancer [AOR = 0.02, 95% CI (0.003–0.172), p = 0.001], [AOR = 0.109, 95% CI (0.030–0.397), p = 0.001], [AOR = 0.2, 95% CI (0.048–0.535), p = 0.003], respectively. Furthermore, the odds of fatigue were 4 times more likely in cancer patients who had the presence of an infection than cancer `patients with no infection [AOR = 4.2, 95% CI (1.064–16.069), p = 0.002]. On the other hand, the odds of fatigue were 5 times greater in breast cancer patients, about 8 times greater in cervical cancer patients and 8 times greater in colon cancer patients than for patients with colorectal cancer [AOR = 5.2, 95% CI (1.590–16.9), p = 0.006], [AOR = 8.3, 95% CI (2.234–30.610)], [AOR = 7.6, 95% CI (1.317–44.262) p = 0.023] respectively.
Finally, those who got treatment chemotherapy (82.0%), surgery (87.4%) and chemo and radiation (97.8%) were less likely than patients who who underwent radiation therapy to suffer from fatigue [AOR = 0.2, 95% CI (0.068–0.462), p = 0.001], [AOR = 0.13, 95% CI (0.041–0.388), p = 0.001], [AOR = 0.021, 95% CI (0.002–0.287), p = 0.004], respectively, as indicated in Table 3.
 |
Table 3 Multivariable Logistic Regression Analysis Among Cancer Patients Undergoing Treatment in TASH, Addis Ababa, Ethiopia, 2019 (n = 278) |
Discussion
This study identified the prevalence of fatigue and associated factors among cancer patients on 278 study participants and fatigue is the most widespread observable fact in individuals with cancer who receive radiation therapy, chemotherapy and the most long-lasting impact felt during and after treatment. The prevalence of fatigue was 74.8% in this study and this finding is consistent with the studies conducted in the Netherlands and Alabama.21,25 However, this result is higher than the results reported in Norway, Italy, Germany and Canada22,28 and lower than the study results found in Texas, Poland, Jordan, and India.30–34 This discrepancy could be due to the difference in socioeconomic status and health care delivery system. This study showed that fatigue in patients who had received radiation therapy is similar to the prevalence of fatigue among patients receiving radiotherapy reported in the UK but lower than the result found in Rasht teaching hospital.31
This study showed that patients whose age was 41–60 years were 3 times more likely to have fatigue than those from age 20–40 years [AOR = 3.2, 95% CI (1.352–7.336), p = 0.008]. This is consistent with the studies conducted in the Netherlands and North Carolina.30
Patients who had stage III cancer were significantly associated with the prevalence of fatigue [AOR=0.2, 95% CI (0.048–0.535), p=0.003] and this finding is in line with the study results from China, Sweden, and a USA hospital.10,12 This study indicated that a higher stage of cancer shows high scores of fatigue, which is comparable with meta-analysis conducted in women breast cancer survivors with stage III cancer and treated with chemotherapy who were at a higher risk for severe fatigue than survivors with stage I cancer.31 The present study showed the prevalence of fatigue among types of cancer such as cervical and breast cancer is consistent with the study result reported in India that the most prevalent fatigue was cervical cancer (28.6%), followed by breast cancer.32 Clinical factors like infection have been seen as causative elements in fatigue and that was identified in this study and it is consistent with the study results reported from the Netherlands of increased risk for severe fatigue following cancer treatment.33
The present study shows that treating with chemotherapy, surgery and chemoradiation has the highest prevalence of fatigue, which is in line with the study results from India, Texas and the USA that after a course of chemotherapy treatment patients rated their level of fatigue as moderate to severe.34–36
Conclusion
The prevalence of fatigue in this study is high. Age, stage of cancer, presence of infection, type of cancer and type of treatment had shown a significant association with fatigue. However, in this study sex of the patients, address, marital status, level of education, occupation, monthly income, medical payment, and type of admission had no significant association with fatigue. Yet, it is difficult to conclude that those variables are not completely important.
Fatigue profoundly affects patients’ abilities to perform activities associated with daily living and limits their personal and social roles within their family and community, resulting in a significant decrement in overall quality of life.
Ethics Approval and Consent to Participate
Ethical approval of the study was obtained from the Institutional Review Board (IRB) of the College of Health Sciences, Addis Ababa University with Protocol number: 010/19/SNM. Prior to administering the interview, the objectives of the study were clearly explained to the participants and written consent was obtained. Participants were informed that their participation was voluntary and that they could withdraw from the study at any time if they wished to do so and this would not affect any service that they will get from the institution. All the information given by the respondents has been used for research purpose only. Participants’ privacy and confidentiality of the information were maintained by the Declaration of Helsinki.
Acknowledgments
The authors acknowledge Addis Ababa University for this paper, which was uploaded to the Addis Ababa University repository as a thesis in June 2019,37 the College of Health Science and Tikur Anbessa Specialized Hospital management bodies, the study participants and the research assistants.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Olsen M Cancer in Sub-Saharan Africa: The need for new paradigms in global health. 2015.
2. Wu H-S, Harden JK. Symptom burden and quality of life in survivorship: a review of the literature. Cancer Nurs. 2015;38(1):E29–E54. doi:10.1097/NCC.0000000000000135
3. Woldu M, Legese D, Abamecha F, Berha A. The prevalence of cancer and its associated risk factors among patients visiting oncology unit, Tikur Anbessa Specialized Hospital, Addis Ababa-Ethiopia. J Cancer Sci Ther. 2017;9:414–421.
4. Aapro M, Scotte F, Bouillet T, Currow D, Vigano A. A practical approach to fatigue management in colorectal cancer. Clin Colorectal Cancer. 2017;16(4):275–285. doi:10.1016/j.clcc.2016.04.010
5. Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. The Lancet. 2003;362(9384):640–650. doi:10.1016/S0140-6736(03)14186-4
6. Ross DD, Alexander CS. Management of common symptoms in terminally Ill Patients: part I. Am Fam Physician. 2001;64:5.
7. Moradian S, Krzyzanowska MK, Maguire R, et al. Usability evaluation of a mobile phone–based system for remote monitoring and management of chemotherapy-related side effects in cancer patients: mixed-methods study. JMIR Cancer. 2018;4(2):e10932. doi:10.2196/10932
8. van Weert E, Hoekstra-Weebers J, Otter R, Postema K, Sanderman R, van der Schans C. Cancer-related fatigue: predictors and effects of rehabilitation. The Oncologist. 2006;11(2):184–196.
9. Davis MP, Khoshknabi D, Yue GH. Management of fatigue in cancer patients. Curr Pain Headache Rep. 2006;10(4):260–269. doi:10.1007/s11916-006-0030-2
10. Cella D, Davis K, Breitbart W, Curt G, Coalition F. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19(14):3385–3391. doi:10.1200/JCO.2001.19.14.3385
11. Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer‐related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98(9):1786–1801. doi:10.1002/cncr.11742
12. Huang X, Zhang Q, Kang X, Song Y, Zhao W. Factors associated with cancer-related fatigue in breast cancer patients undergoing endocrine therapy in an urban setting: a cross-sectional study. BMC Cancer. 2010;10(1):453. doi:10.1186/1471-2407-10-453
13. Spratt DE, Sakae M, Riaz N, et al. Time course and predictors for cancer-related fatigue in a series of oropharyngeal cancer patients treated with chemoradiation therapy. The Oncologist. 2012;17(4):569–576. doi:10.1634/theoncologist.2011-0437
14. van Weert E, Hoekstra-Weebers J, Otter R, Postema K, Sanderman R, van der Schansa C. Symptom management and supportive care. The Oncologist. 2006;11:184–196.
15. Opanga Y, Kaduka L, Muniu E, et al. Double burden of malnutrition among cancer outpatients in Nairobi, Kenya: challenges and opportunities for action. Afr Health Agenda Int J. 2018;1:2.
16. Berger A. Treating fatigue in cancer patients. The Oncologist. 2003;8(Supplement 1):10–14. doi:10.1634/theoncologist.8-suppl_1-10
17. Tian L, Lin L, Li HL, et al. Prevalence and associated factors of cancer-related fatigue among cancer patients in eastern China. The Oncologist. 2016;21(11):1349–1354. doi:10.1634/theoncologist.2015-0537
18. O’Higgins C, Brady B, O’Connor B, Walsh D, Reilly R. The pathophysiology of cancer-related fatigue: current controversies. Supportive Care Cancer. 2018;1–12.
19. Mohandas H, Jaganathan SK, Mani MP, Ayyar M, Thevi GR. Cancer-related fatigue treatment: an overview. J Cancer Res Ther. 2017;13(6):916. doi:10.4103/jcrt.JCRT_50_17
20. Theobald DE. Cancer pain, fatigue, distress, and insomnia in cancer patients. Clin Cornerstone. 2004;6(1):S15–S21. doi:10.1016/S1098-3597(05)80003-1
21. Schwartz A, Nail L, Chen R, et al. Fatigue patterns observed in patients receiving chemotherapy and radiotherapy. Cancer Invest. 2000;18(1):11–19. doi:10.3109/07357900009023057
22. Watson T, Mock V. Exercise as an intervention for cancer-related fatigue. Phys Ther. 2004;84(8):736–743. doi:10.1093/ptj/84.8.736
23. Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs. 2014;18:38–58. doi:10.1188/14.CJON.S3.38-58
24. Campos M, Hassan B, Riechelmann R, Del Giglio A. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22(6):1273–1279. doi:10.1093/annonc/mdq458
25. Wang XS, Woodruff JF. Cancer-related and treatment-related fatigue. Gynecol Oncol. 2015;136(3):446–452. doi:10.1016/j.ygyno.2014.10.013
26. Pettit SD, Kirch R. Do current approaches to assessing therapy related adverse events align with the needs of long-term cancer patients and survivors? Cardio-Oncology. 2018;4(1):5. doi:10.1186/s40959-018-0031-4
27. Borneman T. Assessment and management of cancer-related fatigue. J Hospice Palliative Nurs. 2013;15(2):77–86. doi:10.1097/NJH.0b013e318286dc19
28. Iop A, Manfredi A, Bonura S. Fatigue in cancer patients receiving chemotherapy: an analysis of published studies. Ann Oncol. 2004;15(5):712–720. doi:10.1093/annonc/mdh102
29. Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Supportive Care Cancer. 2002;10(5):389–398. doi:10.1007/s005200100293
30. Butt Z, Rao AV, Lai J-S, Abernethy AP, Rosenbloom SK, Cella D. Age-associated differences in fatigue among patients with cancer. J Pain Symptom Manage. 2010;40(2):217–223. doi:10.1016/j.jpainsymman.2009.12.016
31. Sharifi Rizi M, Adib M, Kazem Nejad Leili E. The assessment of fatigue and its related factors in patients with cancer. J Holistic Nurs Midwifery. 2017;27(2):75–83. doi:10.18869/acadpub.hnmj.27.2.75
32. Banipal RPS SH, Singh B. Assessment of cancer-related fatigue among cancer patients receiving various therapies: a cross-sectional observational study. Indian J Palliat Care. 2017;23(2):207. doi:10.4103/IJPC.IJPC_135_16
33. Bhyat FLH. Fatigue in cancer patients receiving radical anti-cancer treatments. Int J Appl. 2014;4:4.
34. Ebede CCJY, Escalante CP. Cancer-related fatigue in cancer survivorship. Med Clinics. 2017;101(6):1085–1097. doi:10.1016/j.mcna.2017.06.007
35. Yeo TPCS. Cancer-related fatigue: impact on patient quality of life and management approaches. Nursing. 2015;5:65–76.
36. Muhbes FJAH. Assessment of fatigue and its as-sociated factors in breast cancer patients under treatment. Int J Clin Pharmacol Toxicol. 2012;1(1):9–14. doi:10.19070/2167-910X-120002
37. Nugusse TG, Lemlem SB, Deressa JT, Kisa S. A research thesis in Addis Ababa University for master of science degree oncology nursing. Thesis Dissertation. 2019.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
