Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Prevalence of and Factors Associated with Reoccurrence of Opportunistic Infections Among Adult HIV/AIDS Patients Attending the ART Clinic at Public Health Facilities in Arba Minch Town, Southern Ethiopia
Authors Dembelu M , Woseneleh T
Received 7 July 2021
Accepted for publication 23 August 2021
Published 4 September 2021 Volume 2021:13 Pages 867—876
DOI https://doi.org/10.2147/HIV.S328362
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Maycas Dembelu, Teklu Woseneleh
Department of Nursing, College of Health Science, Mettu University, Mettu, Ethiopia
Correspondence: Maycas Dembelu
Department of Nursing, College of Health Science, Mettu University, Mettu, Ethiopia
Tel +251 936704900
Email [email protected]
Background: Opportunistic infections (OIs) in human immunodeficiency virus (HIV)-positive individuals are infections that are more frequent or more severe than normal because of HIV-mediated immunosuppression. When these OIs occur in acquired immune deficiency syndrome (AIDS) patients in the form of relapse or reinfection, they are said to be a reoccurrence of OI. This study will try to identify gaps in addressing the burden in the study area.
Methods: This cross-sectional study was conducted among 450 HIV/AIDS patients with previous OIs attending a public health facility in Arba Minch Town, Southern Ethiopia. This study was conducted from 5 April 2020 to 20 April 2020. Computer-generated simple random sampling was used to select the study participants. Analysis was performed using SPSS version 25 statistical software. Bivariate and multivariable logistic regression was used to identify factors associated with the reoccurrence of OIs. A P value of ≤ 0.05 was used to determine significant association. The results were reported as numerical figures, tables, and diagrams, based on the type of data.
Results: The mean ± standard deviation age of the 450 study participants was 34.3± 8.47 years. Eighty patients (17.8%) had chronic disease. In total, 119 HIV/AIDS patients (26.4%) were diagnosed with reoccurrence of OIs. Pulmonary tuberculosis was the major reoccurring OI. Age, rural residence, chronic disease, baseline anti-retroviral therapy (ART) adherence, current hemoglobin level, and current cell differentiation-4 (CD4) count were factors significantly associated with reoccurrence.
Conclusion: Although the magnitude of reoccurrence of OIs was lower than in previous studies, efforts have to be continued among stakeholders to tackle factors associated with the reoccurrence of OIs.
Keywords: reoccurrence, HIV/AIDS patients, prevalence, Arba Minch town
Introduction
Human immunodeficiency virus (HIV) infection, which leads to acquired immune deficiency syndrome (AIDS), is a worldwide pandemic. So far, 37.9 million people have been infected and 32 million lives have been lost.1,2 The burden of the infection is highest in Africa; of the total HIV/AIDS-positive people, two-thirds (25.7 million) live in Africa. Worldwide, one in three people seek health care for HIV/AIDS-related opportunistic infections (OIs).1 The rate of reoccurrence of OIs is increasing. For instance, in 2017 there were 160,684 recurrent tuberculosis (TB) cases, which represented an increase from the previous year, 2016, when there were around 150,000.3 Although there is no adequate information on the reoccurrence rate of OIs in Africa, there were 310,000 OI-related deaths among AIDS patients in the eastern and southern Africa region.4,5
OIs in HIV-positive individuals are infections that are more frequent or more severe because of HIV-mediated immunosuppression.6 When these infections occur in HIV/AIDS patients in the form of relapse or reinfection, they are said to be a reoccurrence of the OI.7 Among different types of OIs, pulmonary tuberculosis (TB) is the major reoccurring OI. TB reactivation in HIV-uninfected individuals is less than 10%, but the rate of reoccurrence is greater than 10% per year in HIV-positive individuals. Recurrent TB among HIV/AIDS patients is a major challenge for TB control programs, as it is associated with drug resistance and low cure rates.8
Visceral leishmaniasis (VL) in HIV/AIDS patients is another OI that leads to serious health problems. HIV patients who have VL are at increased risk of relapse, along with a decreased therapeutic response. The polyparasitic nature of VL, atypical manifestation, poor therapeutic outcome, and impaired access to health care for VL in HIV patients make the relapse in these patients faster than its natural history of occurrence in HIV-uninfected individuals.9 The reoccurrence of upper respiratory tract infections and some viral infections has also been challenging to manage since these infections can occur spontaneously or sometimes after treatment completion in HIV/AIDS patients. Opportunistic gastrointestinal infections, opportunistic central nervous system (CNS) infections, and opportunistic genital and skin infections also affect people living with HIV (PLHIV). These OIs reduce the immunity and survival of PLHIV.10–16
Different factors have been associated with the reoccurrence of OIs: older age, higher body mass index (BMI), incomplete treatment for OIs, not taking prescribed anti-retroviral therapy (ART) appropriately, cell differentiation-4 (CD4) count <200 cells/mm3, and clinical stage of HIV/AIDS are some of the factors that are significantly associated with the reoccurrence of OIs.8,17 Ethiopia is one of the countries in sub-Saharan Africa with a high burden of OIs. According to a hospital-based study conducted in northwestern Ethiopia, the prevalence of reoccurrence of OIs was 75%.18 This study will try to identify gaps in addressing the burden in the study area. It will also try to incorporate variables that previous studies failed to address regarding the reoccurrence of OIs among HIV/AIDS patients.
Methods and Materials
Study Design
A cross-sectional study design was applied on HIV/AIDS patients attending ART clinics.
Study Setting
This study was conducted in Arba Minch Town public health facilities. Arba Minch Town is the center of Gamo zone, South Western Ethiopia. Arba Minch Town has two subcities: Secha and Sikela. The town has four public health facilities, three of which provide ART services to HIV/AIDS patients, namely Arba Minch General Hospital, Secha Health Center, and Arba Minch Health Center. Currently, a universal “test and treat” treatment guideline is being implemented at a national level. The study was conducted from 5 April 2020 to 20 April 2020.
Study Population
The study population comprised all adult HIV/AIDS patients attending the ART clinic at public health facilities from 11 September 2013 to 15 September 2018 and who were previously diagnosed with and treated for OI.
Sample Size and Sampling Procedure
Sample size was calculated based on the single population proportion with a coefficient of reliability of 1.96, P=75%,18 margin of error of 4%; and considering a contingency for incomplete information, the final sample size was 494.
This study follows HIV/AIDS patients retrospectively who were enrolled into ART from September 2013 to September 2018. HIV/AIDS patients who were previously diagnosed and treated for OIs were included in the study. Starting from September 2013, there were 830 eligible HIV/AIDS patients; a sampling frame was prepared for these 830 patients. Sample size was allocated proportionally for each public health facility’s ART clinic based on patient flow. A computer-generated simple random sampling method was used to select the study participants.
Data Collection Procedure and Instruments
A data collection checklist was prepared from Ethiopian Federal Ministry of Health guidelines and individual patient cards. The checklist consists of socio-demographic variables: age, sex, residence, educational status, marital status, and occupational status; clinical and laboratory-related variables: BMI, World Health Organization (WHO) HIV/AIDS clinical stage, CD4 count, hemoglobin level, prophylaxis exposure, and chronic disease status; behavioral variable: ART adherence; and functional status-related variables. Baseline information was taken within 6 months of ART initiation, and current information was considered 6 months before reoccurrence or completion of the study. Time-varying CD4 count and hemoglobin level were measured every 6 months and at each visit, respectively; these and other clinical and laboratory-related variables were measured by appropriate diagnostic technology and registered in patients’ medical folders. To ascertain the diagnosis of reoccurring OIs, laboratory and clinical investigations were undertaken. For instance, GeneXpert® was used for diagnosing pulmonary TB, clinical investigations were carried out for diseases such as pneumonia and oral thrush, and clinical diagnosis, lumbar puncture, and cerebrospinal fluid (CSF) analyses were conducted for opportunistic CNS infections. Data were collected retrospectively, and the study end date was 9 March 2020. This study followed HIV/AIDS patients for 6.5 years. If any patient was diagnosed with any type of OI at any time during the study period, he/she would be considered as having an OI reoccurrence. Reoccurrence of OI was measured by looking at the individual patient’s medical record. Six BSc nurses were recruited to collect the data from three public health facilities.
Operational Definitions
- Reoccurrence: Diagnosing one or more OIs after treating the previous one.
- ART adherence:
Functional Status
- Working: Able to perform usual work in or out of the house.
- Ambulatory: Able to perform activities of daily living.
- Bedridden: Not able to perform activities of daily living.
Data Analysis and Quality Control
Data were coded and double entered into EpiData version 3.2, and validated accordingly. Then, they were exported to SPSS version 25. Based on related literature, some continuous variables were changed into categorical variables. Variables including baseline and current CD4 count, baseline and current hemoglobin, and baseline and current BMI were checked for assumption of observational independence; there was no multi-collinearity using the variance inflation factor (VIF) at a cut-off point of 10. The statistical significance and strength of associations between independent variables and an outcome variable were measured by bivariate logistic regression. Variables with a P-value of <0.25 and those of clinical significance were considered candidate variables for multivariable logistic regression. The model was built by the stepwise backward likelihood ratio method. Variables with a P-value of <0.05 were considered statistically significantly associated. The crude and adjusted odds ratios (COR and AOR), together with their corresponding 95% confidence intervals (CIs), were computed and interpreted accordingly.
To ensure quality of data, training was given for data collectors on procedures of the data collection process, objectives of the study, content of the data collection tool, and confidentiality of the data collection process. Advisors and the principal investigator supervised the data collection process for consistency and incompleteness of extracted data. In addition, data cleaning was carried out by sorting, frequency distribution, and cross-tabulation.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki, and the informed consent of patients was not required. This study was conducted as part of the fulfillment of a Master of Public Health (MPH) from Arba Minch University, but the institutional affiliation for both investigators is Mettu University. Ethical clearance was obtained from the institutional ethics review board of Arba Minch University (ref. no. IRB/189/12). Verbal approval was obtained from the public health facilities’ management and the ART focal person after explaining that there would be no exposition of data at an individual level and the confidentiality of data would be maintained. Owing to the retrospective review of HIV patients’ documents, we excluded anything that could expose their identity, particularly their name. In addition, to retrieve individual patients’ folders, we used only their medical record numbers. Furthermore, data collectors were ART clinic staff. By taking these measures, we ensured the confidentiality of our data collection process.
Results
Socio-Demographics
Data were collected from 450 HIV/AIDS patients, with a response rate of 91.1%. The mean ± standard deviation (SD) age of the respondents was 34.3±8.47 years (Table 1). Females accounted for more than half (58.4%) of the study participants. Thirty-nine percent of the study participants had attended primary education, around 57% were married, and 97 (21.6%) were government employees.
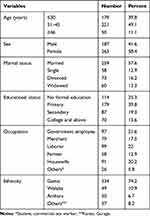 |
Table 1 Socio-Demographic Characteristics of HIV/AIDS Patients on ART at Public Health Facilities in Arba Minch Town, Ethiopia, 2020 |
Clinical and Laboratory Information
HIV/AIDS patients with one or more OIs at baseline were included in this study. At baseline, 429 HIV/AIDS patients (95.3%) were diagnosed with one OI at a time; the remaining 21 patients (4.7%) had more than one OI. Pulmonary TB, herpes zoster, and pneumonia were the main diseases occurring at baseline. Eighty patients (17.8%) had chronic disease (Figure 1). The baseline median (interquartile range) (IQR) CD4 count of these 450 patients was 322.5 (183.7–427) cells/mm3. The current median (IQR) CD4 count was 441.5 (210–630) cells/m3 (Figure 2). The baseline and current median (IQR) hemoglobin counts were 12.3 (10–13.9) and 12.9 (11–14) g/dL, respectively. All 450 patients were taking ART throughout the follow-up period. First line ART was the major prescribed drug, both at baseline and at follow-up. Among first line regimens, tenofovir (TDF), lamivudine (3TC), and efavirenz (EFV) was the most frequently prescribed drug combination, in 416 (92.4%) at baseline and 317 (70.4%) at follow-up. Isoniazid was the drug most commonly used for OI prophylaxis, in 167 patients (37.1%). ART side effects occurred in 63 patients (14%), with nausea occurring in 27 patients (42.8%).
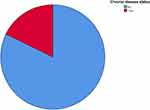 |
Figure 1 Chronic disease status of patients in the current study. |
 |
Figure 2 Distribution of patients’ CD4 count by WHO HIV clinical stage. |
At baseline, 244 (54.2%) were WHO HIV/AIDS clinical stage III, 143 (31.8%) were WHO HIV/AIDS clinical stage II, and the remaining 63 (14%) were WHO HIV/AIDS clinical stage IV patients. During the current visit, most patients, 289 (64.2%), were at WHO HIV/AIDS clinical stage I, followed by WHO HIV/AIDS clinical stage III, with 75 patients (16.7%) (Figure 3). OIs reoccurred in 119 patients (26.4%). (Table 2). Among the most frequently reoccurring OIs, pulmonary TB was the major one, occurring in 22 patients (17.5%). Wasting syndrome and Cryptococcus meningitis were the other most frequently occurring OIs, in 17 patients (14%) and 15 patients (12.6%), respectively.
 |
Table 2 Types of Opportunistic Infections Reoccurring Among HIV/AIDS Patients on ART at Public Health Facilities in Arba Minch Town, Ethiopia, 2020 |
 |
Figure 3 Current WHO clinical stage of patients in this study. |
Factors Associated with Reoccurrence of OIs
Variables that were candidates for multivariable analyses were selected based on P<0.25 and biological plausibility (Table 3). The variables included age, residence, baseline chronic disease, baseline WHO HIV/AIDS clinical stage, baseline and current ART adherence, baseline and current BMI, baseline and current hemoglobin, and baseline and current CD4 count. Patients who were aged 31–45 years were two times more likely to develop OIs compared to patients aged ≤30 years. Patients who resided in a rural area were 70% less likely to experience OI than their urban counterparts. Having a chronic disease is another factor for reoccurrence; the odds of reoccurrence of OIs was 3.25 times higher among those who had a chronic disease. The odds of reoccurrence was also 7.1 times higher among those who had fair adherence to ART compared to patients with good adherence to ART. The other factor associated with the outcome variable was current hemoglobin; patients who had a hemoglobin level of ≥10 g/dL were 71% less likely to develop OIs. Finally, in this study, patients with current CD4 counts of 200–350 and ≥351 cells/mm3 were 68% and 92% less likely to develop OIs, respectively.
Discussion
A cohort of 450 HIV-positive patients with baseline OI were followed for reoccurrence of OIs. The mean±SD age of the respondents was 34.3±8.47 years. Reoccurrence was measured every 6 months. In total, 119 patients (26.4%) developed OIs; pulmonary TB was the OI that occurred most frequently, followed by wasting syndrome. During the current visit, 289 patients (64.2%) were at WHO HIV/AIDS clinical stage I, 25 (5.6%) were at WHO HIV/AIDS clinical stage II, 75 (16.7%) were at WHO HIV/AIDS clinical stage III, and 61 (13.6%) were at WHO HIV/AIDS clinical stage IV.
In most studies, it was discussed that after treatment of the initial OI, reoccurrence of OI, particularly opportunistic TB infection, was identified. For instance, a study conducted in ART clinics in Rio de Janeiro, Brazil, showed that 8.9% of 1080 HIV patients with TB developed a reoccurrence of TB.8 In another study, the rate of reoccurrence of TB in patients on long-term ART was found to be 38%.19 The rate of reoccurrence of TB in the current study was 4.9%. Compared to the previous two studies, the magnitude was low. One of the reasons for this may be the small sample size in the current study compared to the other studies. Another reason is that the previous studies only sampled those who had had a previous TB infection as a study population, without considering other OIs.
A cross-sectional study conducted to determine the prevalence of OIs at Bach Mai Hospital, Hanoi, Vietnam, identified that 63.4% developed OIs.20 A study carried out in Abeokuta, Nigeria, showed that 34% of HIV/AIDS patients were infected with at least one OI.21 The magnitude of OI developing in HIV/AIDS patients in a study conducted in Southern Tigray, Ethiopia, was 55.3%.22 In a study by Alemu et al, the prevalence of opportunistic intestinal parasites was 28.2%.23 There was also a study conducted on pre-ART and ART HIV patients, where the overall prevalence of OIs was 33.6%. The rate of occurrence of OIs was higher among pre-ART patients, at 38%.24 In this study, the prevalence of OI reoccurrence was low. The gap between the current study and other literature on the prevalence of OIs could be explained by the reason that, in contrast to the current study, all of the previous research focused on the initial exposure to the OI, which increases the magnitude. Furthermore, taking both ART and OI prophylaxis reduces the chance of reoccurrence. In a retrospective analysis of reoccurrence of OIs among HIV/AIDS patients conducted in Northwest Ethiopia, the prevalence of OI reoccurrence was 75.7%, which is by far the highest level of morbidity in relation to local and global prevalence rates.18 The high prevalence in the above research compared to the current study may be due to the fact that the previous research followed both pre-ART and ART HIV/AIDS patients, which favors a high prevalence, whereas the current study considered only HIV/AIDS patients on ART.
A number of factors have been associated with the reoccurrence of OIs. This study tried to uncover different factors affecting the occurrence and reoccurrence of OIs. One of the factors associated with reoccurrence was the patient’s age. Being aged 30 years and above was associated with OI occurrence (AOR=2.141,95% CI (1.049–4.374), P=0.037). This finding is in line with several previous studies.17,20,25 So, this study showed a strong association between age and acquiring OIs. However, in contrast to this finding, a study by Majigo et al found that as the age of the patient increases, the risk of acquiring an OI will decrease.26 Although scientific evidence suggests that several physiological and biochemical protective factors deteriorate with increasing age, further research needs to be conducted to determine the exact effect of age on the development of OIs. In this study, residing in rural areas was associated with reoccurrence (AOR=0.301, 95% CI (0.118–0.768), P=0.012). Since this study mainly focuses on urban communities, HIV patients living in rural areas, being few in number, may affect the association. In addition, health extension programs may help these patients to protect themselves from infections.
The other factor associated with the reoccurrence of OIs in this study was the chronic disease status of the patient; having chronic disease was a significant factor for the development of OIs (AOR=3.251, 95% CI (1.497–7.060), P=0.003). This finding is further strengthened by a study on co-morbidities and multi-morbidities associated with HIV, in which HIV patients with chronic disease were more likely to develop OIs than their counterparts.17 Baseline ART adherence was another factor associated with the reoccurrence of OIs in this study (AOR=7.103, 95% CI (2.215–22.782), P=0.001). The study by Mellie and Mitike also supports this finding.18 In addition, a study by Solomon et al showed that patients who adhere to ART have a decreased chance of acquiring an OI, which directly improves the immune status of the patient.27 Current hemoglobin status is one of the clinical factors associated with the development of OIs (AOR=0.293, 95% CI (0.125-0.691), P=0.005). This finding is also supported by other studies, showing that the higher the hemoglobin level of a patient, the lower the chance of OI occurrence.12,18
This study also identified that current CD4 counts of 200–350 and above 350 cells/mm3, with (AOR=0.324, 95% CI (0.137–0.765), P=0.010) and (AOR=0.089, 95% CI (0.038–0.206), P=0.000), respectively, were inversely associated with the reoccurrence of OI. This finding is supported by a study by Golub et al, in which a CD4 count >200 cells/mm3 was associated with a significant decrease in reoccurrence rate.8 A current CD4 count <500 cells/mm3 and virological failure on ART were major factors associated with reoccurrence in a study conducted in Pune, Western India.19 These and other studies in the literature are in strong agreement that there is a significant relationship between patient CD4 count and opportunistic diseases.9,12,18,23,25
As a limitation, this study did not address the effect of HIV viral load on the reoccurrence of OIs. This is because none of the health institutions that participated in this study collected information on viral load, owing to a lack of reagents. In order to limit this effect, this study used CD4 count as an approximation for patients’ immunological status.
Conclusion
Data were collected retrospectively from 450 HIV/AIDS patients on ART to identify factors associated with the reoccurrence of OIs. Around 26.4% of patients developed OIs. Living in a rural residence, ART adherence, increase in CD4 count, and increase in hemoglobin level were protective against reoccurrence. However, age and having chronic illness favored the occurrence of infections. Based on the findings from this study, healthcare professionals at these public health institutions need to plan and provide care that protects HIV/AIDS patients against the reoccurrence of OI.
Acknowledgments
The authors would like to thank Arba Minch University, College of Medicine and Health Science, for giving them the chance to conduct this study. The authors would also like to thank both Arba Minch Town health facilities and the data collectors for their valuable support.
Funding
We received no funding for this work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. HIV/AIDS [fact sheet]. World Health Organization; UNAIDS; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/hiv-aids.
2. HIV-AIDS. World AIDS Day Special, 1 December 2019 [fact sheet]. World Health Organization Myanmar; 2019. Available from: https://www.who.int/docs/default-source/searo/myanmar/factsheet-hiv-aids-wad-2019.pdf?sfvrsn=ffebd469_0. Accessed August 29, 2021.
3. Global Tuberculosis Report. World Health Organization; 2018. Available from: https://apps.who.int/iris/handle/10665/274453. Accessed August 29, 2021.
4. HIV and AIDS in East and Southern Africa regional overview. Avert; 2018. Available from: https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/overview.
5. UNAIDS Data 2019. UNAIDS; 2019. Available from: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf. Accessed August 29, 2021.
6. Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H, The National Institutes of Health and the HIV Medicine Association of the Infectious Diseases Society of America, guidelines for the prevention and treatment of opportunistic infections in adults adolescent with HIV; 2019. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3407677/.
7. Naidoo K, Yende-Zuma N, Augustine S. A retrospective cohort study of body mass index and survival in HIV infected patients with and without TB co-infection. Infect Dis Poverty. 2018;7(1):35.
8. Golub JE, Durovni B, King BS, et al. Recurrent tuberculosis in HIV-infected patients in Rio de Janeiro, Brazil. Aids. 2008;22(18):2527–2533. doi:10.1097/QAD.0b013e328311ac4e
9. Cota GF, de Sousa MR, Rabello A, Jaffe CL. Predictors of visceral leishmaniasis relapse in HIV-infected patients: a systematic review. PLoS Negl Trop Dis. 2011;5(6):e1153. doi:10.1371/journal.pntd.0001153
10. Low A, Gavriilidis G, Larke N, et al. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2016;62(12):1595–1603. doi:10.1093/cid/ciw125
11. Buchacz K, Lau B, Jing Y, et al. Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000-2010. J Infect Dis. 2016;214(6):862–872. doi:10.1093/infdis/jiw085
12. Dana Weissberg FM, Kambugu A, Fehr J, et al. Ten years of antiretroviral therapy: incidences, patterns and risk factors of opportunistic infections in an urban Ugandan cohort. PLoS One. 2018;13(11). doi:10.1371/journal.pone.0206796
13. Hughes CB, Dickson RC, Krishna M, et al. HCV recurrence in HIV-infected patients after liver transplant. J Int Assoc Physicians AIDS Care. 2010;9(2):87–93. doi:10.1177/1545109710362592
14. Olum R, Baluku JB, Okidi R, Andia-Biraro I, Bongomin F. Prevalence of HIV-associated esophageal candidiasis in sub-Saharan Africa: a systematic review and meta-analysis. Trop Med Health. 2020;48(1):1–10.
15. Bowen LN, Smith B, Reich D, Quezado M, Nath A. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol. 2016;12(11):662–674.
16. Chelidze K, Thomas C, Chang AY, Freeman EE. HIV-related skin disease in the era of antiretroviral therapy: recognition and management. Am J Clin Dermatol. 2019;20(3):423–442. doi:10.1007/s40257-019-00422-0
17. Maggi P, Santoro CR, Nofri M, et al. Clusterization of co-morbidities and multi-morbidities among persons living with HIV: a cross-sectional study. BMC Infect Dis. 2019;19(1):555. doi:10.1186/s12879-019-4184-z
18. Mellie H, Mitike G. Time to recurrence of any opportunistic infection after treatment of it among people living with HIV Infection in Debre Markos, Northwest Ethiopia: retrospective cohort study. J AIDS Clin Res. 2014;03:07. doi:10.4172/2155-6113.1000226
19. Dravid A, Natarajan K, Medisetty M, et al. Incidence of tuberculosis among HIV infected individuals on long term antiretroviral therapy in private healthcare sector in Pune, Western India. BMC Infect Dis. 2019;19(1):714. doi:10.1186/s12879-019-4361-0
20. Dang LVP, Nguyen QH, Ishizaki A, et al. Prevalence of opportunistic infections and associated factors in HIV-Infected men who have sex with men on antiretroviral therapy in Bach Mai Hospital, Hanoi, Vietnam: a case-control study. Am J Men’s Health. 2020;14(3):1557988320926743. doi:10.1177/1557988320926743
21. Amoo JK, Akindele AA, Amoo AOJ, et al. Prevalence of enteric parasitic infections among people living with HIV in Abeokuta, Nigeria. Pan Afr Med J. 2018;30:66. doi:10.11604/pamj.2018.30.66.13160
22. Weldearegawi TZ, Gerensea H, Berihu H, Gidey G, Welearegay MZ. The magnitude of opportunistic infections and associated factors in HIV-infected adults on antiretroviral therapy in southern zone Tigray, Ethiopia: a cross-sectional study. Pan Afr Med J. 2020;35. doi:10.11604/pamj.2020.35.126.17839
23. Alemu G, Alelign D, Abossie A. Prevalence of opportunistic intestinal parasites and associated factors among HIV patients while receiving ART at Arba Minch Hospital in Southern Ethiopia: a cross-sectional study. Ethiop J Health Sci. 2018;28(2):147–156. doi:10.4314/ejhs.v28i2.6
24. Dereje N, Moges K, Nigatu Y, Holland R. Prevalence and predictors of opportunistic infections among HIV positive Adults On Antiretroviral therapy (On-ART) versus pre-ART In Addis Ababa, Ethiopia: a comparative cross-sectional study. HIV/AIDS. 2019;11:229–237. doi:10.2147/HIV.S218213
25. Tanuma J, Lee KH, Haneuse S, et al. Incidence of AIDS-defining opportunistic infections and mortality during antiretroviral therapy in a cohort of Adult HIV-Infected Individuals in Hanoi, 2007-2014. PLoS One. 2016;11(3):e0150781. doi:10.1371/journal.pone.0150781
26. Majigo M, Somi G, Joachim A, et al. Prevalence and incidence rate of tuberculosis among HIV-infected patients enrolled in HIV care, treatment, and support program in mainland Tanzania. Trop Med Health. 2020;48:1. doi:10.1186/s41182-020-00264-1
27. Solomon FB, Angore BN, Koyra HC, Tufa EG, Berheto TM, Admasu M. Spectrum of opportunistic infections and associated factors among people living with HIV/AIDS in the era of highly active anti-retroviral treatment in Dawro Zone hospital: a retrospective study. BMC Res Notes. 2018;11(1):604. doi:10.1186/s13104-018-3707-9
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

