Back to Journals » Integrated Blood Pressure Control » Volume 16
Prevalence of and Factors Associated with Hypertension Among Adults on Dolutegravir-Based Antiretroviral Therapy in Uganda: A Cross Sectional Study
Authors Kasoma Mutebi R, Weil Semulimi A , Mukisa J, Namusobya M, Namirembe JC, Nalugga EA, Batte C, Mukunya D, Kirenga B, Kalyesubula R , Byakika-Kibwika P
Received 15 January 2023
Accepted for publication 7 April 2023
Published 19 April 2023 Volume 2023:16 Pages 11—21
DOI https://doi.org/10.2147/IBPC.S403023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Ronald Kasoma Mutebi,1– 3 Andrew Weil Semulimi,1,4 John Mukisa,5 Martha Namusobya,1 Joy Christine Namirembe,3 Esther Alice Nalugga,1 Charles Batte,1 David Mukunya,6 Bruce Kirenga,1,7 Robert Kalyesubula,4 Pauline Byakika-Kibwika7
1Lung Institute, Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda; 2Clinical Epidemiology Unit, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda; 3Department of Medicine, Mengo Hospital, Kampala, Uganda; 4Department of Physiology, School of Biomedical Sciences, College of Health Sciences, Makerere University, Kampala, Uganda; 5Department of Immunology and Molecular Biology, School of Biomedical Sciences, College of Health Sciences, Kampala, Uganda; 6Department of Community and Public Health, Faculty of Health Sciences, Busitema University, Mbale, Uganda; 7Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda
Correspondence: Ronald Kasoma Mutebi, Department of Medicine, Mengo Hospital, PO BOX 7161, Kampala, Uganda, Email [email protected]
Introduction: Dolutegravir-based anti-retroviral therapy (ART) regimens were rolled out as first line HIV treatment in Uganda due to their tolerability, efficacy and high resistance barrier to human immunodeficiency virus (HIV). They have however been associated with weight gain, dyslipidemia and hyperglycemia which are cardiometabolic risk factors of hypertension. We assessed the prevalence and factors associated with hypertension among adults on dolutegravir regimens.
Methods: We conducted a cross-sectional study on 430 systematically sampled adults on dolutegravir-based ART for ≥ 6 months. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or history of use of antihypertensive agents.
Results: The prevalence of hypertension was 27.2% (117 of 430 participants) [95% CI: 23.2– 31.6]. Majority were female (70.7%), the median age 42 [34, 50] years, with body mass index (BMI) ≥ 25 kg/m3 (59.6%) and median duration on DTG-based regimens of 28 [15, 33] months. Being male [aPR: 1.496, 95% CI: 1.122– 1.994, P = 0.006], age ≥ 45 years [aPR: 4.23, 95% CI: 2.206– 8.108, P < 0.001] and 35– 44 years [aPR: 2.455, 95% CI: 1.216– 4.947, P < 0.012] as compared with age < 35 years, BMI ≥ 25 kg/m3 [aPR: 1.489, 95% CI: 1.072– 2.067, P = 0.017] as compared with BMI < 25 kg/m3, duration on dolutegravir-based ART [aPR: 1.008, 95% CI: 1.001– 1.015, P = 0.037], family history of hypertension [aPR: 1.457, 95% CI: 1.064– 1.995, P = 0.019] and history of heart disease [aPR: 1.73, 95% CI: 1.205– 2.484, P = 0.003] were associated with hypertension.
Conclusion: One in every four people with HIV (PWH) on dolutegravir-based ART has hypertension. We recommend the integration of hypertension management in the HIV treatment package and policies to improve existing supply chains for low cost and high-quality hypertension medications.
Keywords: hypertension, dolutegravir, HIV
Introduction
Globally, 36 million adults live with HIV (PWH). Sub-Saharan Africa (SSA) alone accounts for more than 60% of this global HIV burden. In 2020, there were an estimated 1.7 million new infections in SSA which accounted for almost 70% of the global total of new HIV infections. With the improved strategy against HIV such as the test and treat strategy, more than 90% of PWH in Uganda have access to ART.1 As survival rates improve due to ART, long-term HIV treatment has been associated with emerging challenges such as cardiovascular diseases, majorly hypertension.2
Among PWH, the burden of hypertension varies in different regions of the world.2–5 However, SSA carries the biggest part of this dual HIV–hypertension burden in addition to epidemic poverty.5,6 Many risk factors such as older age, black ethnicity, male gender, modifiable factors such as obesity, smoking and alcohol use have been attributed to hypertension among PWH.6 Other HIV-related factors such as some ART agents, longer effects of ART and duration of HIV infection have also been reported to be associated with hypertension.7
Despite its recommendation by the World Health Organization (WHO) for first-line ART regimens, dolutegravir, an integrase inhibitor, has been associated with serious adverse drug effects such as obesity and hyperglycemia.8–13 These are cardiometabolic risk factors of hypertension hence posing an increased risk of hypertension among PWH on dolutegravir.
Dolutegravir-based regimens have widely been rolled out in various HIV clinics as a first-line treatment option for HIV since 2018 in Uganda and other countries in SSA.14,15 This implies that the population of PWH on dolutegravir is large as many were switched from other regimens such as efavirenz-based regimens to dolutegravir. This was not only due to superiority, low potential for drug interaction and high genetic barrier resistance against HIV of dolutegravir regimens over other regimens such as efavirenz but also their tolerability in various communities.15,16
With paucity of data on the prevalence of hypertension among PWH on dolutegravir, our study aimed to determined prevalence of and factors associated with hypertension among adults on dolutegravir-based ART in Uganda.
Methods
Study Design and Setting
We conducted a cross-sectional hospital-based study between October and December 2021 among adults on dolutegravir-based ART at the counseling and home care department (CHCD) in Mengo Hospital. Mengo Hospital is a Kampala-based, private not for profit missionary hospital located in Rubaga division. CHCD is a specialized HIV outpatient clinic which offers free HIV/AIDS testing, treatment and monitoring, and with more than 7000 of 8800 PWH on dolutegravir-based ART. It consists of a team of various cadres among whom are clinicians, nurses and laboratory staff who have received training in HIV management and care. CHCD receives supplies and medicines from the government of Uganda like all other HIV clinics hence there is a steady supply. It mostly serves clients from Rubaga division and other surrounding villages.
Study Population
We included all adults (≥ 18 years) PWH on dolutegravir-based ART regimens who were receiving routine HIV care with no previous history of hypertension prior to start of a dolutegravir-based ART regimen and had provided a written informed consent. We excluded critically ill and pregnant mothers due to the hospital referral per protocol to specialized departments.
Using the Kish Leslie formula with a hypothesized prevalence of hypertension at 50% for maximum sample size calculation, level of significance at 5%, desired level of power at 80% and 10% non-response rate, a sample of 430 participants was obtained.17 Every fourth patient in the register for the clinic day was enroled following systematic sampling.
Data Collection Procedure and Study Procedure
Trained research assistants administered the pretested questionnaires and performed the physical measurements. We used the questionnaire to collect data on sociodemographic, behavioral, and clinical characteristics. Sociodemographic and behavioral factors included age, sex, marital status, education status, employment status, history of smoking, alcohol use since start of dolutegravir-based ART and physical activity. Clinical factors included current WHO stage, self-report of family history of DM and history since dolutegravir start of diabetes mellitus (DM) or cardiovascular disease (CVD). Using a data abstraction tool, we also collected data on recent viral load, duration on DTG and the current ART regimen from both the electronic database and also from the physical clients’ files.
Blood pressure was measured using a validated electric powered automated blood pressure monitor (SECA blood pressure monitor) 10 minutes after rest while the patient was seated in a quiet office and 5 minutes between each measurement. The measurements were done on the right arm using an appropriate cuff size of an OMRON device. Height and weight were measured twice using SECA 769 digital column scale in meters and kilograms, respectively while patient was standing in an upright position without shoes, parallel to the digital column and perpendicular to the weight scale. We recorded an average of each two readings into the database for analysis. Participants on dolutegravir-based ART scheduled for a clinic visit day were requested to fast overnight until after having a blood sample taken by the health worker at the CHCD in order to measure their fasting blood sugar (FBS). The blood sample was collected from the non-dominant arm or on patient’s preference. FBS and lipid profile tests were performed using EMATE glucometer (units: mmol/l) on site and COBAS e311 (units: mmol/l) in Mulago Hospital biochemistry laboratory, respectively.
Hypertension was defined based on the 2020 International Society of Hypertension Global Hypertension Practice Guidelines as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg and/or self-report of use of anti-hypertensive medication.18
Body mass index: Body mass index was calculated as division of the average weight in kilograms against the square of height in meters. BMI < 25 kg/m3 and ≥ 25 kg/m3 were classified as normal and overweight/obese, respectively.
Statistical Analysis
All filled data collection forms were checked for completion, double data entry using EpiData 3.0 and was cleaned in Excel. Analysis was done in STATA version 14.0. Continuous variables were summarized as medians (interquartile range) while categorical variables as proportions. The prevalence was calculated as the proportion of hypertension among participants. Data are presented in tables. At bivariable and multivariable analysis, a generalized linear model regression of the Poisson family with a log link (modified Poisson) using robust standard errors was performed since the prevalence of hypertension was more than 10%.19 Variables with a P-value of ≤ 0.2 at bivariable analysis and those known in literature such as sex and body mass index were run in the multivariable regression to determine the association of various predictor variables with hypertension. Collinearity, interaction and confounding were assessed during the analysis.
Results
Description of the Participants
Of the 430 participants, the majority were female (n = 301, 70.7%), 41.1% were aged ≥ 45 years, 86 (20%) reported to have ever used alcohol, 8 (1.9%) reported to have used tobacco, 255 (59.6%) had a BMI ≥ 25 kg/m3 while 103 (23.9%) and 158 (36.7%) had a family history of diabetes and hypertension, respectively. Nearly all the participants (423, 98.4%) have their HIV viral load suppressed, 416 (96.7%) on tenofovir/lamivudine/dolutegravir and 287 (67.4%) had been on dolutegravir-based ART regimen for more than 24 months with a median duration of 28 [15, 33] months (Table 1).
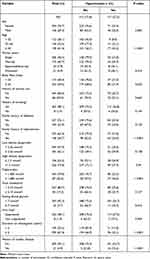 |
Table 1 Hypertension and Its Associated Factors Among the Study Participants |
Prevalence of Hypertension
From the study, the prevalence of hypertension was 27.2% (117 of 430 participants) with a 95% confidence interval (CI) of 23.2–31.6% (Figure 1). Among the hypertensive participants, the median systolic and diastolic pressure readings were 142 [130, 152] mmHg and 90 [84, 97] mmHg, respectively whereas those among the normotensive participants were 115 [107, 122] mmHg and 71 [66, 79] mmHg, respectively. The prevalence was higher among males at 36.5% [95% CI: 28.5–45.4] as compared with females at 23.4% [95% CI: 18.9–28.5], those with body mass index ≥ 25 kg/m3 at 31.4% [95% CI: 26.0–37.4] as compared with those with < 25 kg/m3 at 21.4% [95% CI: 15.9–28.2] and those who had been on dolutegravir-based ART for ≥ 2 years at 33.1% [95% CI: 27.9–38.8] as compared with those < 2 years at 15% [95% CI: 9.9–22] (Table 1).
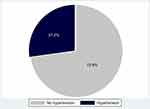 |
Figure 1 Prevalence of hypertension among the study participants. |
The majority of the participants with hypertension were aged ≥ 45 years, had elevated low- and high-density lipoproteins, elevated triglycerides and fasting blood glucose. From the sub-group analysis, 100 (23.3%) and 96 (22.3%) had stage 1 and 2 hypertension, respectively, based on the American Heart Association Guidelines 2020 (Table 2).
 |
Table 2 Hypertension Based on AHS Guidelines 2020 (Sub-Analysis) |
Factors Associated with Hypertension
At multivariate analysis, the final model only included factors that were significant (P < 0.05). At multivariate analysis, being male [aPR: 1.496, 95% CI: 1.122–1.994, P = 0.006], age of 35–44 years [aPR: 2.455, 95% CI: 1.219–4.947, P = 0.012], age ≥ 45 years [aPR: 4.23, 95% CI: 2.206–8.108, P < 0.001], body mass index ≥ 25 kg/m3 [aPR: 1.489, 95% CI: 1.072–2.068, P = 0.017], duration on dolutegravir-based ART [aPR: 1.008, 95% CI: 1.001–1.0152, P = 0.037], family history of hypertension [aPR: 1.457, 95% CI: 1.065–1.995, P = 0.019] and history of heart disease [aPR: 1.73, 95% CI: 1.205–2.484, P = 0.003] were significantly associated with hypertension (Table 3).
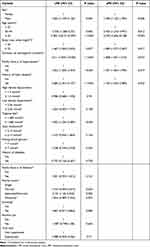 |
Table 3 Bivariate and Multivariate Analyses of the Factors Associated with Hypertension Among the Study Participants |
Discussion
In this cross-sectional study, the majority of the study participants had their HIV under control as evidenced by the magnitude of those with a suppressed viral load at 98.4%. The prevalence of hypertension among study participants on dolutegravir-based ART was 27.2% (95% CI: 23.2–31.6). It is fairly similar to that of Uganda’s nationwide NCD risk factor survey data conducted in 2014.20 The prevalence in the study was different from the sub-analysis prevalence of stage 2 hypertension (Table 2) because participants who had a normal blood pressure though on antihypertensive agents were included, hence a higher study prevalence.
Despite the fact that females dominated the study, the prevalence was higher among males. It was also higher in those with a family history of hypertension and those with a BMI of 25 kg/m3 and above.
Being male was associated with hypertension among the study participants. This finding is similar to what was found in other studies among PWH.21,22 This is not surprising as estrogen is believed to have a positive impact on the vasodilation of the vessels hence subsequent control of hypertension.23 This implies that menopause is a major blow in every aging woman’s life since estrogen lowers.23
Body mass index ≥ 25 kg/m3 was significantly associated with hypertension. These findings are similar to other studies conducted in various countries such as Kenya, Tanzania, South Africa, Tunisia and China.24–27 This is not surprising as nearly 60% of the participants had a body mass index ≥ 25 kg/m3. Overweight and obesity are traditionally defined by accumulation of body fat above 20% expressed by a BMI between 25–29.9 kg/m3 and ≥ 30 kg/m3, respectively.28 Obesity has been listed among a sole risk factor of hypertension and other illnesses such as diabetes mellitus.28,29 Despite the unclear dolutegravir–obesity mechanism, various studies have reported association of overweight, obesity and dolutegravir.12,30 This implies that prevention programs focusing on weight reduction and healthy diet are key in reducing overweight and obesity among PWH on dolutegravir.
Duration on dolutegravir-based ART was significantly associated with hypertension among the study participants. Others studies have reported an association of a longer duration ART with hypertension.4,5 In another meta-analysis of HIV patients, it was revealed that longer duration of ART exposure was associated with an increased risk of hypertension.31 The mechanism of how a longer duration on ART leads to hypertension is unclear. However, various hypotheses have been attributed to weight gain that could result from the specific regimens such as those with dolutegravir coupled with unhealthy lifestyle habits such as physical inactivity and unchecked diet (excess carbohydrates).32 Another hypothesis considers how the long-term exposure to ART leads to persistent activation of the immune system. Within the vascular system, high levels of elevated biomarkers such as interleukin 6 (IL-6) lead to vascular changes hence loss of elasticity that is key in vasodilation to maintain the vascular blood pressure.33 It is not surprising that even from the sub-analysis, duration on dolutegravir above 24 months were associated with hypertension (Figure 2).
 |
Figure 2 A graph of the multivariable analysis, DTG, dolutegravir. |
We also found that hypertension was associated with increasing age. Various changes occur with increasing age. They include loss of elastin, collagen deposition and protein glycation in the vessel walls. These lead to thickening and stiffening of the vessels hence diastolic and systolic hypertension.34–36 With the improved survivorship due to ART, a bigger population of PWH are aging which makes them candidates for hypertension. Various studies have shown how increasing age is associated with hypertension.21,24,27,37,38
History of cardiac disease was also significantly associated with hypertension. Similar studies of patients on other ART regimens have too been reported to be associated with hypertension.39
There was no significant association between hypertension and triglycerides, low density lipoproteins, high density lipoproteins, total cholesterol, history of smoking, alcohol use, ART regimen, marital status, education status, employment status and baseline CD4. This could be explained by the study design being cross sectional and the presence of selection bias as we only selected those on dolutegravir.
In a sub-analysis done with hypertension based on the American Heart Association definition, 196 (45.6%) participants were classified to have stage 1 or stage 2 hypertension. It also reveals that 66 (15.4%) had elevated blood pressure (systolic blood pressure 120–129 mmHg and diastolic blood pressure < 80 mmHg).40 The above prevalence is different from the 27.2% obtained in the study. This is because patients on antihypertensives are not classified as hypertensive as per the study’s definition.
Our study had some strengths. It was conducted in a large HIV treatment center from one region. The majority of the participants were stable on ART as evidenced by the suppressed viral load. This implies that it likely represents the general population on ART due to a nationwide scale up of the PWH on dolutegravir in Mengo Hospital. There were potential limitations in our study. Being a cross-sectional study, our study was limited by the inability to assess the temporal relationships. Other limitations include: missing data during abstraction, a possibility of recall bias during interviews, lack of control group hence compromised internal validity, selection bias as all participants were recruited from hospital and also some patients not reporting their true fasting status. Measurements of hypertension 10 minutes apart contrary to the conventional 6 hours apart may give misleading results and hence overestimate or underestimate the true prevalence.
Conclusions
In summary, our study findings reveal that one in every four adults on dolutegravir-based ART is hypertensive. This similar to Uganda’s general population hypertension prevalence. We recommend integration and strengthening of screening and management of hypertension among PWH on ART. Future research for cost-effective interventions such as routine screening on every visit in order to reduce the burden of hypertension among PWH is needed.
Patient and Public Involvement
Patients did not participate in any aspect of the research study such as design, methods, interpretation of findings or contribute to the writing of this paper.
Data Sharing Statement
The data from this study will be available on request to the corresponding author.
Ethics
The study was approved by Makerere University School of Medicine Research Ethics Committee (SOMREC) with approval number: MAK-SOMREC-2021-178. Mengo Hospital provided the administrative authorization. Informed consent was obtained from all eligible participants using an informed consent form by their signature or thumb–print while others were signed by a witness for those who could not read and write. This study complies with the Declaration of Helsinki.
Consent for Publication
All authors consented to have the manuscript with the attached documents published.
Acknowledgments
Study participants, Mengo Hospital administration and the CHCD staff, clinical epidemiology unit (CEU), supervisors; Professor Joan Kalyango (CEU director), Dr Achilles Katamba, Dr William Lumu, Edith Namulema and the MakNCD training programme for mentoring and funding the research and school.
Funding
The study was supported by the Makerere University Non-Communicable Diseases (MAKNCD) Research Training Program: supported by the Fogarty International Centre of the National Institutes of Health under Award Number D43TW011401.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Disclosure
There was no conflict of interest as declared by the authors.
References
1. UNAIDS. Uganda, UNAIDS; 2022. Available from: https://www.unaids.org/en/regionscountries/countries/uganda.
2. Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens. 2017;11(8):530–540. doi:10.1016/j.jash.2017.06.004
3. Gonah L, Moodley I, Hlongwana K. Prevalence of diabetes mellitus and hypertension in people living with human immunodeficiency virus on antiretroviral therapy in Gweru district, Zimbabwe. Afr J Prim Health Care Fam Med. 2020;12(1):e1–e6. doi:10.4102/phcfm.v12i1.2473
4. Lukas K, Markos E, Belayneh F, Habte A. The magnitude of hypertension and associated factors among clients on highly active antiretroviral treatment in Southern Ethiopia, 2020: a hospital-based cross-sectional study. PLoS One. 2021;16(10):e0258576. doi:10.1371/journal.pone.0258576
5. Kagaruki GB, Mayige MT, Ngadaya ES, et al. Magnitude and risk factors of non-communicable diseases among people living with HIV in Tanzania: a cross sectional study from Mbeya and Dar es Salaam regions. BMC Public Health. 2014;14:904. doi:10.1186/1471-2458-14-904
6. Mogaka JN, Sharma M, Temu T, et al. Prevalence and factors associated with hypertension among adults with and without HIV in Western Kenya. PLoS One. 2022;17(1):e0262400. doi:10.1371/journal.pone.0262400
7. Lackland DT, Keil JE, Gazes PC, Hames CG, Tyroler HA. Outcomes of black and white hypertensive individuals after 30 years of follow-up. Clin Exp Hypertens. 1995;17(7):1091–1105. doi:10.3109/10641969509033654
8. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267–1274. doi:10.1093/cid/ciz407
9. Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis. 2020;33(1):10–19. doi:10.1097/QCO.0000000000000616
10. Goldberg RN, Kania AT, Michienzi SA-O, Patel M, Badowski MA-O. Weight gain in incarcerated individuals living with HIV after switching to integrase strand inhibitor-based therapy. J Int Assoc Provid AIDS Care. 2021;20:2325958221996860. doi:10.1177/2325958221996860
11. Lake JE, Wu K, Bares SH, et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis. 2020;71(9):e471–e477. doi:10.1093/cid/ciaa177
12. Menard A, Meddeb L, Tissot-DuPont H, et al. Dolutegravir and weight gain: an unexpected bothering side effect? Aids. 2017;31(10):1499–1500. doi:10.1097/QAD.0000000000001495
13. Norwood J, Turner M, Bofill C, et al. Weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr. 2017;76(5):527. doi:10.1097/QAI.0000000000001525
14. Rolle C-P, Berhe M, Singh T, et al. Dolutegravir/lamivudine as a first-line regimen in a test-and-treat setting for newly diagnosed people living with HIV. AIDS. 2021;35(12):1957–1965. doi:10.1097/qad.0000000000002979
15. World Health Organization. WHO recommends dolutegravir as preferred HIV treatment option in all populations; 2019. Available from: https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations.
16. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Available from: https://www.who.int/publications/i/item/9789240031593.
17. Kish L. Survey Sampling. R package version; 2011.
18. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026
19. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi:10.1093/aje/kwh090
20. Guwatudde D, Mutungi G, Wesonga R, et al. The epidemiology of hypertension in Uganda: findings from the national non-communicable diseases risk factor survey. PLoS One. 2015;10(9):e0138991. doi:10.1371/journal.pone.0138991
21. Mbuthia GW, Magutah K, McGarvey ST. The prevalence and associated factors of hypertension among HIV patients. Int J Hypertens. 2021;2021:5544916. doi:10.1155/2021/5544916
22. Everett B, Zajacova A. Gender differences in hypertension and hypertension awareness among young adults. Biodemography Soc Biol. 2015;61(1):1–17. doi:10.1080/19485565.2014.929488
23. Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53(3):688–708. doi:10.1016/s0008-6363(01)00527-2
24. Bosu WK, Reilly ST, Aheto JMK, Zucchelli E. Hypertension in older adults in Africa: a systematic review and meta-analysis. PLoS One. 2019;14(4):e0214934. doi:10.1371/journal.pone.0214934
25. Ondimu DO, Kikuvi GM, Otieno WN. Risk factors for hypertension among young adults (18–35) years attending in Tenwek Mission Hospital, Bomet County, Kenya in 2018. Pan Afr Med J. 2019;33:210. doi:10.11604/pamj.2019.33.210.18407
26. Akpa OM, Made F, Ojo A, et al. Regional patterns and association between obesity and hypertension in Africa: evidence from the H3Africa CHAIR Study. Hypertension. 2020;75(5):1167–1178. doi:10.1161/hypertensionaha.119.14147
27. Shen Y, Chang C, Zhang J, Jiang Y, Ni B, Wang Y. Prevalence and risk factors associated with hypertension and prehypertension in a working population at high altitude in China: a cross-sectional study. Environ Health Prev Med. 2017;22(1):19. doi:10.1186/s12199-017-0634-7
28. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi:10.1016/j.metabol.2018.09.005
29. Vecchié A, Dallegri F, Carbone F, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6–17. doi:10.1016/j.ejim.2017.10.020
30. Lamorde M, Atwiine M, Owarwo NC, et al. Dolutegravir-associated hyperglycaemia in patients with HIV. Lancet HIV. 2020;7(7):e461–2. doi:10.1016/S2352-3018(20)30042-4
31. Nduka CU, Stranges S, Sarki AM, Kimani PK, Uthman OA. Evidence of increased blood pressure and hypertension risk among people living with HIV on antiretroviral therapy: a systematic review with meta-analysis. J Hum Hypertens. 2016;30(6):355–362. doi:10.1038/jhh.2015.97
32. Sundström J, Lind L, Lampa E, et al. Weight gain and blood pressure. J Hypertens. 2020;38(3):387–394. doi:10.1097/hjh.0000000000002298
33. Masenga SK, Hamooya BM, Nzala S, et al. Patho-immune mechanisms of hypertension in HIV: a systematic and thematic review. Curr Hypertens Rep. 2019;21(7):1–19. doi:10.1007/s11906-019-0956-5
34. Jia G, Aroor AR, Jia C, Sowers JR. Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta Mol Basis Dis. 2019;1865(7):1802–1809. doi:10.1016/j.bbadis.2018.08.008
35. Tesauro M, Mauriello A, Rovella V, et al. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med. 2017;281(5):471–482. doi:10.1111/joim.12605
36. Trache A, Massett MP, Woodman CR. Vascular smooth muscle stiffness and its role in aging. Curr Top Membr. 2020;86:217–253. doi:10.1016/bs.ctm.2020.08.008
37. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–292. doi:10.1161/hypertensionaha.119.14240
38. Chang WT, Chen JS, Tsai MH, Tsai WC, Juang JN, Liu PY. Interplay of aging and hypertension in cardiac remodeling: a mathematical geometric model. PLoS One. 2016;11(12):e0168071. doi:10.1371/journal.pone.0168071
39. Muhammad S, Sani MU, Okeahialam BN. Cardiovascular disease risk factors among HIV-infected Nigerians receiving highly active antiretroviral therapy. Niger Med J. 2013;54(3):185–190. doi:10.4103/0300-1652.114591
40. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160–164. doi:10.1016/j.tcm.2019.05.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
