Back to Journals » Patient Preference and Adherence » Volume 17
Prevalence and Safety of Prescribing PPIs with Clopidogrel in Palestine
Authors Abukhalil AD , Al Sheikh T, Muallem S, Al-Shami N , Naseef HA
Received 15 January 2023
Accepted for publication 15 March 2023
Published 20 March 2023 Volume 2023:17 Pages 749—759
DOI https://doi.org/10.2147/PPA.S404139
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Johnny Chen
Abdallah Damin Abukhalil, Tala Al Sheikh,* Sandra Muallem,* Ni’meh Al-Shami, Hani A Naseef
Pharmacy Department, Faculty of Pharmacy, Nursing and Health Professions, Birzeit University, Birzeit, West Bank, Palestine
*These authors contributed equally to this work
Correspondence: Abdallah Damin Abukhalil; Ni’meh Al-Shami, Pharmacy Department, Faculty of Pharmacy, Nursing and Health Professions, Birzeit University, Birzeit, West Bank, Palestine, Tel/Fax +970-2-2982017, Email [email protected]; [email protected]
Background: Proton pump inhibitors (PPIs) are commonly prescribed medications that are thought to increase the risk of cardiovascular events because they reduce the effectiveness of clopidogrel via shared hepatic pathways.
Objective: This study examined the prevalence of concomitant prescribing of clopidogrel/PPI among patients diagnosed with acute coronary syndrome and the adverse cardiovascular event associated with this interaction.
Methods: A retrospective cohort study was conducted by retrieving patient data from the Nat Health Insurance claims processor database in Palestine. Adults diagnosed with Acute Coronary Syndrome (ACS) from 2019 through 2021 who were prescribed clopidogrel or clopidogrel in combination with a PPI were included in the study. Endpoints were adverse cardiac events, including readmission for revascularization during the first year of treatment.
Results: The study included 443 patients; the prevalence of prescribing concomitant clopidogrel with a PPI was 74.7%, whereas 49.2% were prescribed interacting PPI (omeprazole, esomeprazole, and lansoprazole). 59 (13.3%) of participants experienced a cardiovascular event within 1 year of starting therapy, including 27 (12.4%) patients who had a cardiovascular event while taking an interacting PPI. No significant association was found between PPI administration and increased CV event risk in patients receiving concomitant clopidogrel and PPIs therapy (p = 0.579).
Conclusion: In this study, we observed a high prevalence of prescribing a PPI in combination with clopidogrel, regardless of the FDA recommendations. No significant increase in cardiovascular events was observed in patients receiving concomitant clopidogrel and PPI therapy.
Keywords: clopidogrel, PPI, proton pump inhibitors, cardiovascular disease, cardiovascular event, drug-drug interaction, PPIs interactions, clopidogrel PPI combinations
Introduction
Proton pump inhibitors (PPIs) and clopidogrel are commonly prescribed medications that share a common metabolic pathway. Clopidogrel is an antiplatelet medication indicated for many cardiovascular disorders, including acute coronary syndrome (ACS), stable ischemic heart disease, and percutaneous coronary intervention to reduce the risk of ischemic cardiac events.1,2
Clinical guidelines recommend antiplatelet therapy for the secondary prevention of atherosclerotic cardiovascular disease (ASCVD).3 Clopidogrel is a prodrug that is biotransformed in the liver via cytochrome P450 enzymes, including CYP2C19. Clopidogrel’s active metabolite binds irreversibly to platelet ADP receptor P2Y12, preventing ADP-induced platelet aggregation.4–6
PPIs are anti-acid secretory medications used for the prophylaxis or treatment of many gastrointestinal disorders. Clinical guidelines recommend PPIs for patients who require dual antiplatelet therapy (clopidogrel and aspirin) to reduce the risk of duodenal-gastric bleeding complications by reducing gastric acid secretions.5,7–9
PPIs are inhibitors of the isoenzyme CYP2C19 and are co-substrates for CYP3A4 (omeprazole being predominantly metabolized by this pathway, followed by esomeprazole and lansoprazole).10,11 The concomitant use of PPIs and clopidogrel may diminish clopidogrel activity in preventing platelet aggregation by inhibiting major enzymes that activate the conversion of clopidogrel into its active metabolite.5 This interaction could be clinically significant, increasing the risk for cardiovascular events such as myocardial infarction (MI), recurrent ACS, rehospitalization, stroke, angina, coronary artery bypass graft (CABG), or even death.12
In 2009 the food and drug administration (FDA) requested a product labeling change that recommends avoiding omeprazole and esomeprazole with clopidogrel due to the significance of this interaction.8 Nevertheless, in 2012, the American College of Cardiology Foundation and the American Heart Association announced not to ban the use of PPI and clopidogrel in appropriate clinical settings.13 Literature has conflicting data on the clinical significance of this interaction.14 In a recent meta-analysis, concomitant PPI and clopidogrel treatments increased the risk of major cardiovascular events.15 A study conducted in 2017 evaluated the effect of six PPIs on the antiplatelet effect of clopidogrel and showed no significant interaction between PPIs and clopidogrel in healthy patients and that the interaction may only be applicable in specific clinical settings.6
The severity of this interaction may vary across different PPIs; for example, a significant interaction is associated with omeprazole, whereas pantoprazole is less likely to interact with clopidogrel and should be used in situations where both clopidogrel and PPI are indicated; pantoprazole did not increase the rate of ischemic stroke among clopidogrel users.16,17 Furthermore, Histamine 2 receptor antagonists (H2RAs) are proposed as a safer alternative to PPIs.18
A high prevalence of prescribing PPIs among cardiovascular patients receiving antiplatelet therapy has been reported by many studies; furthermore, inappropriate prescribing of PPIs has been documented.19–22 However, no studies have been conducted in Palestine to determine physician adherence to the FDA recommendations when prescribing PPIs and clopidogrel and the clinical interaction associated with this combination. In this study, we utilized data from the Nat Health Insurance claim processor to examine the prescriber’s adherence to FDA recommendations and evaluate whether each of the four PPIs (omeprazole, esomeprazole, pantoprazole, and lansoprazole) is associated with an increased risk of cardiovascular events when used concomitantly with clopidogrel.
Materials and Methods
Study Design
A retrospective cohort observational study reviewed three years of data from January 1, 2019, to December 31, 2021. The data were retrieved from Nat Health, a major health insurance claims processor in Palestine. This study included all patients aged ≥ 18 years who were initially prescribed clopidogrel 75 alone or combined with any PPI. The study endpoints were an adverse cardiovascular event, defined as any inpatient hospitalization for acute MI, angina, CABG, or recurrent ACS during the first year of therapy. In addition, the study excluded patients with no follow-up history, missing prescription refills, or missing medical information (Figure 1).
 |
Figure 1 Study Flow Chart. |
A data collection form was designed to collect patient information and was divided into four sections. The first section included patients’ ID, age range, and gender, and the second section included patient medication history (clopidogrel, aspirin, PPIs, and H2-receptor antagonist (H2RA) and duration of treatment, and the third section consisted of patient comorbidities as classified the Charlson Comorbidity Index (CCI). These comorbidities included: a history of myocardial infarction (MI), congestive heart failure (CHF), stroke, dementia, chronic obstructive pulmonary disease (COPD), peptic ulcer disease (PUD), diabetes mellitus (DM), liver disease, chronic kidney disease (CKD), cancer and hemiplegia. Finally, Section four included hospital admissions for adverse cardiovascular outcomes during the study period based on the International Classification of Diseases (ICD-9) diagnosis code. All data were collected in an Excel spreadsheet. During the study period, patients were assessed for adverse cardiac events while taking clopidogrel with or without PPI concurrently. Furthermore, the prevalence of clopidogrel prescriptions combined with PPIs was evaluated.
Statistical Analysis
Statistical Package for the Social Sciences version 22 was used to analyze the data with 95% confidence intervals (CIs) and a 5% accepted margin of error. Recoding of data was done to re-categorize variables as needed. Descriptive statistics were used to analyze the data. The chi-square test was used to measure the association between the possibility of having a CV event and independent variables. One sub-analysis categorized patients into two groups: Group one included pantoprazole, H2RA, or no anti-acid (least likely to interact with clopidogrel), and group two included omeprazole, esomeprazole and lansoprazole (more likely to interact with clopidogrel), to calculate the association concerning cardiovascular events. Binary logistic regression was performed to identify which subgroup had a significant effect on the potential for developing CV events. Another sub-analysis among patients who developed CV events was conducted to assess the association between the time to CV events and PPIs administration using the Mann–Whitney test.
Ethical Consideration
This study was approved by the Institutional Review Board (IRB) committee of the Faculty of Pharmacy at Birzeit University. (Reference number: BZUPNH2101). This was a retrospective observational study; all patient data were anonymized and de-identified. Therefore, the requirement for written informed consent was waived. The study complies with the Declaration of Helsinki.
Results
Demographic Data
(Table 1) presents the characteristics of the study participants. A total of 443 patients who received 75 mg of clopidogrel and met the inclusion criteria were included in the study cohort. Among these, 359 (81%) were male, 156 (35.2%) were between 51–60 years old, and the most common comorbidities were hyperlipidemia (n=239), hypertension (n=211), and diabetes mellitus (n=187). Additionally, three-quarters of patients, 331 (74.7%), had used a PPI concurrently with clopidogrel (75 mg), and only 10 (2.3%) patients had received H2 blockers with clopidogrel. Fifty-nine (13.3%) patients experienced cardiovascular events.
 |
Table 1 The Characteristics of the Sample (N= 443) |
Cardiovascular Events Associations with Participant Characteristics of the Study
As shown in (Table 2), there was no significant association between PPI administration with clopidogrel and an increased risk of CV events (p= 0.768), as 13.6% of PPI participants developed CV events, while 12.5% of none PPI administrators developed CV events. Furthermore, there was no significant association between developing cardiac events after administration of clopidogrel and patient age, sex, or diagnosis of hypertension (P>0.05). However, a significant association was observed in patients who received statin therapy (p=0.028). Patients on statin therapy (10%) were significantly less likely to develop CV events than others (17.2%). Binary logistic regression revealed that patients with a CCI score >2 (21.8%) and a CCI score ≥ 3 (34.4%) were significantly more likely to develop CV events than those with a CCI score of 0 (p = 0.042 and p = 0.001, respectively).
 |
Table 2 Association Between CV Events and Other Variables. (N=443) |
PPIs and Incidence of CV Events
Approximately half of the patients used esomeprazole 198 (59.8%), followed by pantoprazole 113 (34.1%), omeprazole 11 (3.3%), and lansoprazole 9 (2.7%). Furthermore, no significant differences between the different PPIs and the occurrence of any cardiovascular events when taken concurrently with clopidogrel (p=0.408) (Table 3).
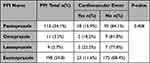 |
Table 3 Association Between PPI Molecules and CV Events. (N=331) |
As shown in (Table 4), among patients who developed CV events, 32 (14.2%) used clopidogrel alone or in combination with H2RA or pantoprazole as safe therapy. In comparison, 27 (12.4%) used clopidogrel combined with other PPIs (Esomeprazole, lansoprazole, and omeprazole) as an unsafe combination. Pantoprazole and H2RA are proposed to have a less significant interaction in decreasing the antiplatelet effects of clopidogrel and are not associated with an increased cardiovascular risk compared with other PPIs. There was no significant difference between the two groups, with a p-value of 0.579. Furthermore, Figure 2 shows that the median time to event for patients using the safe combination versus time to event for patients using the unsafe combination was 128 and 113 days, respectively. In addition, the Mann–Whitney test results revealed no significant difference in the potential to develop cardiovascular events with time between patients on a safe combination with clopidogrel and patients on an unsafe combination with clopidogrel (p= 0.637).
 |
Table 4 Association Between CV Events Comparing Interacting PPIs to Non-Interacting (N=443) |
 |
Figure 2 The median time to CV Events. |
Discussion
PPIs and Cardiovascular Events
This study is the first retrospective cohort study in Palestine that addresses the prevalence of prescribing clopidogrel in combination with PPIs and associated adverse cardiovascular events.
In this study, many participants were prescribed clopidogrel concomitantly with an interacting PPI, an alarming indicator of prescribers’ none compliance with FDA recommendations to avoid specific PPIs with clopidogrel. A similar finding was in a regional study in Qatar, where the rate of PPI co-prescribing with clopidogrel was relatively high.23 In addition, healthcare providers in Palestine have reported a lack of awareness and knowledge of drug-drug interaction as a barrier to identifying inappropriate prescribing and preventing medication errors.24 Increasing awareness can be accomplished by offering continual education on significant drug-drug interactions and utilizing clinical pharmacists in medication therapy management.
Although there was a high prevalence of prescribing the combination of Clopidogrel with PPIs, no significant association was found between clopidogrel use alone or in combination with any of the PPIs studied (pantoprazole, omeprazole, lansoprazole, and esomeprazole) (p> 0.05). This finding was supported by a systematic review and meta-analysis evaluating this interaction and increased cardiovascular risk in patients receiving clopidogrel. Data from 156,823 patients from 27 trials conducted between 2009 and 2016 were analyzed. Subgroup analysis revealed no significance in randomized controlled trials (RCTs) (RR = 0.99, 95% CI = 0.76–1.28, p = 0.93) in the major adverse cardiac event (MACE) outcome group. There was no association between PPI and clopidogrel therapy on CV death outcome (RR = 1.21, 95% CI = 0.97–1.50, p = 0.09).25
A regional retrospective longitudinal cohort study was performed in Jeddah, Saudi Arabia, to assess the effect of this interaction on readmission and mortality risk within 30 days and one year, respectively. A total of 464 participants were included, with 107 (24.4%) receiving PPI and clopidogrel concurrently. Participants taking clopidogrel and PPI did not have a higher one-year mortality risk than those in the non-PPI cohort (p = 0.7). The risk of unplanned 30-day readmission was lower in patients who received clopidogrel and PPI (p = 0.03). The use of clopidogrel and PPI decreased readmission rates and was not associated with increased mortality compared with non-PPI participants.26 In contrast to this finding a recent cohort population-based study of 99,836 patients who received clopidogrel after primary PCI, the concomitant use of PPI appeared to increase the risk of major cardiovascular events; the primary outcome was myocardial infarction (HR= 1.23, 95% CI 1.15–1.32), the secondary outcome was coronary heart disease (HR=1.28, 95% CI 1.24–1.33), stroke (HR=1.21, 95% CI 1.05–1.40) and death due to coronary heart disease (HR=1.52, 95% CI 1.37–1.69).7
Moreover, in a Chinese study, the concomitant use of dual antiplatelet therapy (DAT) and a PPI may be associated with a high risk of a major cardiovascular event.5 Most participants were on DAT, which could have affected the results. The concomitant use of medications such as aspirin would increase antiplatelet activity in patients undergoing dual therapy when used with omeprazole, which may bias the results towards a lower risk of CV events in patients taking PPIs.27
Pantoprazole vs Other PPIs in Increased Cardiovascular Risk
Pantoprazole is proposed to be the least among the PPIs to inhibit CYP2C19 enzymes leading to a decreased ability to affect clopidogrel’s antiplatelet response, according to studies comparing and evaluating PPI’s pharmacokinetic properties through markers such as the inhibition constant (Ki), which turned out to be 14 to 69 μM for pantoprazole and 17 to 21 μM for rabeprazole. The higher the Ki value, the higher the drug required to inhibit CYP2C19 enzyme activity.28 In another study, pantoprazole had the highest half-maximal inhibitory concentration (IC50) value of 93 μM among other PPIs. This measure reveals the amount of drug needed to inhibit a biological process by half; thus, the higher the value, the higher the amount required to inhibit CYP2C19 activity.29
No significant differences were found when comparing PPIs with known CYP-450 enzyme inhibitors (esomeprazole, lansoprazole, and omeprazole) with pantoprazole. This finding was supported by a propensity score-adjusted cohort study that was carried out for adults (n=325,559) who were concomitantly on clopidogrel and a PPI (esomeprazole, lansoprazole, omeprazole, rabeprazole, and pantoprazole), where hospitalization for acute ischemic stroke was considered to be the endpoint.17 The identification of 1667 ischemic strokes occurred with an annual incidence of 2.4% (95% confidence interval, 2.3–2.5). The adjusted hazard ratios (HRs) for ischemic stroke versus pantoprazole were 0.98 (0.82–1.17) for esomeprazole; 1.06 (0.92–1.21) for lansoprazole; 0.98 (0.85–1.15) for omeprazole; and 0.85 (0.63–1.13) for rabeprazole. Pantoprazole was chosen as the reference PPI for comparison in the study as it does not possess CYP2C19 inhibitory potency, lacks the potential for drug-drug interactions with clopidogrel, and has been recommended by Food and Drug Administration as a PPI of choice in clopidogrel-treated patients.30,31
Clopidogrel-PPIs Interactions and Patients Characteristics
In addition to assessing the PPI-clopidogrel interactions and prevalence, the present study categorized patients according to their Charlson comorbidity index (CCI) to minimize confounding variables. In this study, patients with a CCI score of more than two had a significant risk of cardiovascular events. Risk factors and comorbidities may increase or confound the effect of PPI on clopidogrel use. Some studies suggest that patients with cardiovascular diseases and comorbidities and being treated with chronic medications have a greater possibility that these medications may compete with or inhibit the CYP isoenzymes responsible for the bioactivation of clopidogrel.32,33 Therefore, prescribing clopidogrel to these patients might explain why the active metabolite of clopidogrel is reduced below the needed concentration to inhibit platelet aggregation. A recent randomized crossover study conducted on 28 healthy patients also failed to show a consistent increase in cardiovascular events in patients treated with clopidogrel and PPI concurrently (pantoprazole p-value= 0.786 (SD= 0.557), omeprazole P-value= 0.653 (SD= 0.615), rabeprazole p-value= 0.726 (SD= 0.592), esomeprazole p-value= 0.807 (SD= 0.574), lansoprazole p-value= 0.887 (SD =0.592), dexlansoprazole p-value=0.998 (SD= 0.592)).6
An increase in cardiovascular events might be present in certain clinical conditions, such as severe cardiovascular disease or co-administration of other medications that may affect the activity of CYP isoenzymes.6 In this study, statin use was significantly associated with a decreased risk of cardiovascular events (Table 2). Drug-Drug Interaction between statins and PPIs or clopidogrel has been reported in the literature. Since clopidogrel is activated mainly by CYP2C19 and CYP3A4 enzymes, its use with statins, such as atorvastatin, would lower its antiplatelet efficacy due to competitive binding to the enzyme receptors inactivating clopidogrel.34 As for PPIs, some studies revealed no interaction between PPIs and statins and that PPIs may moderately augment statin-mediated lipid reduction.35
Other factors, such as genetics and acquired alteration of CYP2C19, may influence this interaction, including polymorphism and drug-drug interactions. For example, a 2019 study investigated the influence of CYP2C19 G681A polymorphism on clopidogrel platelet activity in patients with ACS and concluded that clopidogrel might be hyporesponsive in patients with the CYP2C19 G681A polymorphism.36 Thus, patients with this allele are at higher risk of major cardiovascular events. Understanding the prevalence rate of this polymorphism with a specific population or race will aid in providing personalized medicine.37
Clopidogrel-PPI Interactions Management
In clinical situations where antacid secretory agents are indicated in patients on clopidogrel for gastrointestinal bleeding prophylaxis (GIBP), such as the indication in the current study, most of the PPIs used GIBP prophylaxis in patients on dual antiplatelet therapy requires the patient’s assessment of risk factors and comorbidities. A safer alternative to a PPI is H2RA due to the lack of interactions or effects on clopidogrel antiplatelet activity. For example, famotidine showed no more than 20% inhibition of clopidogrel activation by CYP2C19 and CYP3A4 at up to 100 µmol/L of clopidogrel, and long-term use of famotidine was not associated with CYP2C19 and CYP3A4 inhibition.38 Another prospective observational study was carried out on patients receiving DAT due to prior PCI; 25 received omeprazole or rabeprazole, and 30 did not take PPI or H2 blockers, but famotidine was added. The study found that omeprazole significantly reduced the antiplatelet effect of clopidogrel (p=0.0051), whereas concomitant use of famotidine did not affect the antiplatelet effect of clopidogrel.39
Doubling the dose of clopidogrel could restore the loss of the antiplatelet effect induced by esomeprazole, a phenomenon suggested by some studies to overcome this interaction.40 This phenomenon is supported by a study that found that esomeprazole decreased the effect of clopidogrel (75 mg/day) in patients with stable coronary artery disease. In contrast, ranitidine did not alter the antiplatelet effect of clopidogrel. Furthermore, the esomeprazole-clopidogrel interaction was narrowed by increasing the dose of clopidogrel (75 mg) to (150 mg) or using ranitidine or famotidine as alternatives.41 As for clopidogrel alternatives; prasugrel may be a good choice as a P2Y12 inhibitor that can avoid the adverse interactions produced by PPIs.40
In this retrospective cohort observational study, none of the PPIs tested significantly increased cardiovascular events with concomitant use of clopidogrel. Therefore, the interaction may only be significant in specific clinical circumstances, such as severe cardiovascular disease or co-administration of other medications that affect the activity of CYP isoenzymes. There are conflicting studies on the relationship between different PPIs and clopidogrel combinations. Given the inconsistent results of different studies, making correct decisions regarding this interaction is complex and challenging.
Limitation
This study has some limitations that may help explain the results. The main limitation of the present study was its small sample size. Further prospective clinical studies are required to confirm the biological interactions.
Another limitation of this study was the absence of genotype data, as these play a major role in clopidogrel metabolism. This study had no access to bio-samples that assessed genetic polymorphisms in CYP enzymes, p-glycoprotein transporters, or P2Y12 receptors. A larger study involving genotype data from Palestine is necessary to determine whether this is the case. In addition, the study could not capture other patient factors, such as lifestyle, social habits, family history, obesity, and medication adherence. However, this was the first study to address this drug-drug interaction among patients in Palestine and assess prescribers’ adherence to FDA recommendations.
Conclusion
This study found a high prevalence of co-prescribing PPIs with clopidogrel. Furthermore, there is no association between PPI use and an increased risk of cardiovascular events in patients taking clopidogrel; until more data are available, the concomitant use of specific PPIs with clopidogrel may not be the optimal choice of therapy when acid-suppression therapy is indicated. Further research and testing should be considered because multiple confounders, such as genetics and comorbidities, can affect this interaction. Prescribing clopidogrel with PPIs is a gray area that requires healthcare providers to complete patient assessments of medications and comorbidities to minimize the risk of cardiovascular events associated with this interaction and select the appropriate medication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Patti G, Micieli G, Cimminiello C, Bolognese L. The Role of Clopidogrel in 2020: a Reappraisal. Cardiovasc Ther. 2020;2020:8703627. doi:10.1155/2020/8703627
2. Beavers C, Naqvi I. Clopidogrel. Antiplatelet Ther Cardiovasc Dis; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470539/.
3. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e18–e114. doi:10.1161/CIR.0000000000001038
4. Cuisset T, Frere C, Quilici J, et al. Comparison of omeprazole and pantoprazole influence on a high 150-mg clopidogrel maintenance dose the PACA (Proton Pump Inhibitors And Clopidogrel Association) prospective randomized study. J Am Coll Cardiol. 2009;54(13):1149–1153. doi:10.1016/j.jacc.2009.05.050
5. Zou JJ, Chen SL, Tan J, et al. Increased risk for developing major adverse cardiovascular events in stented Chinese patients treated with dual antiplatelet therapy after concomitant use of the proton pump inhibitor. PLoS One. 2014;9(1):e84985. doi:10.1371/journal.pone.0084985
6. Przespolewski ER, Westphal ES, Rainka M, Smith NM, Bates V, Gengo FM. Evaluating the effect of six proton pump inhibitors on the antiplatelet effects of clopidogrel. J Stroke Cerebrovasc Dis. 2018;27(6):1582–1589. doi:10.1016/j.jstrokecerebrovasdis.2018.01.011
7. Maret-Ouda J, Santoni G, Xie S, Rosengren A, Lagergren J. Proton pump inhibitor and clopidogrel use after percutaneous coronary intervention and risk of major cardiovascular events. Cardiovasc Drugs Ther. 2022;36(6):1121–1128. doi:10.1007/s10557-021-07219-6
8. Ali Khan M, Howden CW. The Role of Proton Pump Inhibitors in the Management of Upper Gastrointestinal Disorders. Gastroenterol Hepatol. 2018;14(3):169–175.
9. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122(24):2619–2633. doi:10.1161/CIR.0b013e318202f701
10. Ma TK, Lam YY, Tan VP, Yan BP. Variability in response to clopidogrel: how important are pharmacogenetics and drug interactions? Br J Clin Pharmacol. 2011;72(4):697–706. doi:10.1111/j.1365-2125.2011.03949.x
11. Dean L, Kane M. Omeprazole therapy and CYP2C19 genotype. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries. Bethesda (MD): National Center for Biotechnology Information (US); 2012.
12. Drepper MD, Spahr L, Frossard JL. Clopidogrel and proton pump inhibitors--where do we stand in 2012? World J Gastroenterol. 2012;18(18):2161–2171. doi:10.3748/wjg.v18.i18.2161
13. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
14. Jeridi D, Pellat A, Ginestet C, et al. The safety of long-term proton pump inhibitor use on cardiovascular health: a meta-analysis. J Clin Med. 2022;11(14):4096. doi:10.3390/jcm11144096
15. Pang J, Wu Q, Zhang Z, et al. Efficacy and safety of clopidogrel only vs. clopidogrel added proton pump inhibitors in the treatment of patients with coronary heart disease after percutaneous coronary intervention: a systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2019;23:100317. doi:10.1016/j.ijcha.2018.12.016
16. Norgard NB, Mathews KD, Wall GC. Drug-drug interaction between clopidogrel and the proton pump inhibitors. Ann Pharmacother. 2009;43(7):1266–1274. doi:10.1345/aph.1M051
17. Leonard CE, Bilker WB, Brensinger CM, et al. Comparative risk of ischemic stroke among users of clopidogrel together with individual proton pump inhibitors. Stroke. 2015;46(3):722–731. doi:10.1161/STROKEAHA.114.006866
18. Yasu T, Sato N, Kurokawa Y, Saito S, Shoji M. Efficacy of H2 receptor antagonists for prevention of upper gastrointestinal bleeding during dual-antiplatelet therapy. Int J Clin Pharmacol Ther. 2013;51(11):854–860. doi:10.5414/CP201917
19. Abrahami D, McDonald EG, Schnitzer M, et al. Prescribing PPIs. Drug Ther Bull. 2017;55(10):117–120. doi:10.1136/dtb.2017.10.0541
20. Sarnaik MK, Modi S, Pisipati Y, et al. Proton pump inhibitors: exploring cardiovascular complications and prescription protocol. Cureus. 2021;13(7):e16744. doi:10.7759/cureus.16744
21. Westergaard N, Tarnow L, Vermehren C. Use of clopidogrel and proton pump inhibitors alone or in combinations in persons with diabetes in Denmark; potential for CYP2C19 genotype-guided drug therapy. Metabolites. 2021;11(2):96. doi:10.3390/metabo11020096
22. Abukhalil AD, Ali O, Saad A, et al. Evaluation of proton pump inhibitors prescribing among hospitalized patients: a cross-sectional study. Int J General Med. 2023;Volume 16:141–150. doi:10.2147/IJGM.S396202
23. Awaisu A, Hamou F, Mekideche L, et al. Proton pump inhibitor co-prescription with dual antiplatelet therapy among patients with acute coronary syndrome in Qatar. International Journal of Clinical Pharmacy. 2016;38(2):353–361. doi:10.1007/s11096-016-0250-4
24. Abukhalil AD, Shaloudi AY, Shamasneh NM, Aljamal AM. Awareness of Beers criteria and potentially inappropriate medications among physicians and pharmacists in Palestine. J Pharm Pract Res. 2021;51(5):381–389. doi:10.1002/jppr.1728
25. Demcsák A, Lantos T, Bálint ER, et al. PPIs are not responsible for elevating cardiovascular risk in patients on clopidogrel-A systematic review and meta-analysis. Front Physiol. 2018;9:1550. doi:10.3389/fphys.2018.01550
26. Alghamdi RA, Marzoughi S, Alghamdi MS, Alghamdi A, Almekhlafib M. Outcome of stroke patients on clopidogrel plus proton-pump inhibitors: a single-center cohort study. Ann Saudi Med. 2019;39(2):82–86. doi:10.5144/0256-4947.2019.82
27. Stockl KM, Le LB, Zakharyan A, et al. Risk of rehospitalization for patients using clopidogrel with a proton pump inhibitor. Archiv Int Med. 2010;170(8):704–710. doi:10.1001/archinternmed.2010.34
28. Li XQ, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32(8):821–827. doi:10.1124/dmd.32.8.821
29. Ogilvie BW, Yerino P, Kazmi F, et al. The proton pump inhibitor, omeprazole, but not lansoprazole or pantoprazole, is a metabolism-dependent inhibitor of CYP2C19: implications for coadministration with clopidogrel. Drug Metab Dispos. 2011;39(11):2020–2033. doi:10.1124/dmd.111.041293
30. Mathews S, Reid A, Tian C, Cai Q. An update on the use of pantoprazole as a treatment for gastroesophageal reflux disease. Clin Exp Gastroenterol. 2010;3:11–16. doi:10.2147/ceg.s6355
31. Johnson DA, Chilton R, Liker HR. Proton-pump inhibitors in patients requiring antiplatelet therapy: new FDA labeling. Postgrad Med. 2014;126(3):239–245. doi:10.3810/pgm.2014.05.2772
32. Raza JA, Babb JD, Movahed A. Optimal management of hyperlipidemia in primary prevention of cardiovascular disease. Int J Cardiol. 2004;97(3):355–366. doi:10.1016/j.ijcard.2003.07.039
33. Hsieh C-F, Huang W-F, Chiang Y-T, Chen C-Y. Effects of clopidogrel and proton pump inhibitors on cardiovascular events in patients with type 2 diabetes mellitus after drug-eluting stent implantation: a nationwide cohort study. PLoS One. 2015;10(8):e0135915. doi:10.1371/journal.pone.0135915
34. Lau WC, Waskell LA, Watkins PB, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation. 2003;107(1):32–37. doi:10.1161/01.CIR.0000047060.60595.CC
35. Barkas F, Elisaf M, Rizos CV, Klouras E, Kostapanos MS, Liberopoulos E. Proton pump inhibitors and statins: a possible interaction that favors low-density lipoprotein cholesterol reduction? Hippokratia. 2015;19(4):332–337.
36. Jirungda S, Pussadhamma B, Komanasin N, Senthong V, Leuangwatthananon W. Interaction of CYP2C19 G681A polymorphism and omeprazole on clopidogrel responsiveness and impact in patients with acute coronary syndrome. Coronary Artery Dis. 2020;31(3):266–272. doi:10.1097/MCA.0000000000000808
37. Akkaif MA, Daud NAA, Sha’aban A, et al. The role of genetic polymorphism and other factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD). Molecules. 2021;26(7):1987. doi:10.3390/molecules26071987
38. Ohbuchi M, Noguchi K, Kawamura A, Usui T. Different effects of proton pump inhibitors and famotidine on the clopidogrel metabolic activation by recombinant CYP2B6, CYP2C19 and CYP3A4. Xenobiotica. 2012;42(7):633–640. doi:10.3109/00498254.2011.653655
39. Yamane K, Kato Y, Tazaki J, et al. Effects of PPIs and an H2 blocker on the antiplatelet function of clopidogrel in Japanese patients under dual antiplatelet therapy. J Atheroscler Thromb. 2012;19(6):559–569. doi:10.5551/jat.11601
40. Wang ZY, Chen M, Zhu LL, et al. Pharmacokinetic drug interactions with clopidogrel: updated review and risk management in combination therapy. Ther Clin Risk Manag. 2015;11:449–467. doi:10.2147/TCRM.S80437
41. Moceri P, Doyen D, Cerboni P, Ferrari E. Doubling the dose of clopidogrel restores the loss of antiplatelet effect induced by esomeprazole. Thromb Res. 2011;128(5):458–462. doi:10.1016/j.thromres.2011.06.029
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
