Back to Journals » Clinical Epidemiology » Volume 10
Prevalence and risk factors of community-associated methicillin-resistant Staphylococcus aureus carriage in Asia-Pacific region from 2000 to 2016: a systematic review and meta-analysis
Authors Wong JWH , Ip M, Tang A , Wei VWI, Wong SYS, Riley S, Read JM, Kwok KO
Received 24 December 2017
Accepted for publication 8 March 2018
Published 12 October 2018 Volume 2018:10 Pages 1489—1501
DOI https://doi.org/10.2147/CLEP.S160595
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Irene Petersen
Jonathan WH Wong,1 Margaret Ip,2 Arthur Tang,3 Vivian WI Wei,1 Samuel YS Wong,1 Steven Riley,4 Jonathan M Read,5,6,* Kin On Kwok1,7,8,*
1Faculty of Medicine, The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong Special Administrative Region, China; 2Department of Microbiology, The Chinese University of Hong Kong, Hong Kong Special Administrative Region, China; 3Department of Software, Sungkyunkwan University, Seoul, South Korea; 4MRC Centre for Outbreak Analysis and Modelling, Department for Infectious Disease Epidemiology, Imperial College London, UK; 5Centre for Health Informatics Computing and Statistics, Lancaster Medical School, Faculty of Health and Medicine, Lancaster University, Lancaster, UK; 6Institute of Infection and Global Health, The Farr Institute@HeRC, University of Liverpool, Liverpool, UK; 7Faculty of Medicine, Stanley Ho Centre for Emerging Infectious Diseases, The Chinese University of Hong Kong, Shatin, Hong Kong, Hong Kong Special Administrative Region, China; 8Shenzhen Research Institute of the Chinese University of Hong Kong, Shenzhen, China
*These authors contributed equally to this work
Objective: Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is an emerging global public health threat. In response to a highlighted strategic priority of the World Health Organization Global Action Plan on Antimicrobial Resistance, to “strengthen the knowledge and evidence base through surveillance and research”, we synthesized published articles to estimate CA-MRSA carriage prevalence in the Asia-Pacific region.
Methods: A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PROSPERO CRD:42017067399). We searched MEDLINE, EMBASE, and PubMed for articles published from 1 January 2000 to 19 May 2017, which reported CA-MRSA carriage (defined as either colonization or infection) in Asia-Pacific region from 2000 to 2016. Studies were stratified according to settings (community or hospital where CA-MRSA was isolated) and study populations (general public or subpopulations with specified characteristics). Ranges of CA-MRSA carriage prevalence were reported for study groups.
Results: In total, 152 studies were identified. Large diversity was observed among studies in most study groups. In community-level studies, the CA-MRSA carriage prevalence among the general public ranged from 0% to 23.5%, whereas that ranged from 0.7% to 10.4% in hospital settings. From community-level studies, countries with the highest prevalence were India (16.5%–23.5%), followed by Vietnam (7.9%) and Taiwan (3.5%–3.8%). Children aged ≤6 (range: 0.5%–40.3%) and household members of CA-MRSA carriers (range: 13.0%–26.4%) are subgroups without specific health conditions but with much higher CA-MRSA carriage when compared to the general population.
Conclusion: Our CA-MRSA prevalence estimates serve as the baseline for future national and international surveillance. The ranges of prevalence and characteristics associated with CA-MRSA carriage can inform health authorities to formulate infection control policies for high-risk subgroups. Future studies should explore the heterogeneities in CA-MRSA carriage prevalence among subgroups and countries to clarify the predominant transmission mechanisms in Asia-Pacific and other regions.
Keywords: antimicrobial resistance, emerging global health threat, population studies, methicillin-resistant Staphylococcus aureus
Plain language summary
Methicillin-resistant Staphylococcus aureus (MRSA) can cause serious diseases in carriers and resist major antibiotics. MRSA was traditionally only found in healthcare settings. However, its variant, community-associated MRSA (CA-MRSA), is increasingly found in individuals without healthcare exposure. Despite the importance of CA-MRSA for health, generalizable knowledge about CA-MRSA carriage in the Asia-Pacific region is lacking. To address this gap, we reviewed published articles reporting CA-MRSA carriage among the general population and subpopulations in this region. From 2000 to 2016, CA-MRSA carriage among general population in the community ranged from 0% to 23.5%, with higher percentages in developing countries such as India (16.5%–23.5%) and Vietnam (7.9%). Children aged ≤6 (0.5%–40.3%) and household members of CA-MRSA carriers (13.0%–26.4%) are community subgroups with much higher CA-MRSA carriage than the general population. This study identifies the baseline CA-MRSA carriage prevalence and the corresponding high-risk subgroups, highlighting the circulation and spread of CA-MRSA in the Asia-Pacific region. We urge local and international agencies to formulate policies to reduce the spread of CA-MRSA.
Introduction
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is an emerging global public health threat, causing mild and life-threatening invasive infections related to skin, soft tissues, and respiratory system.1 Recent evidence suggested that CA-MRSA accounted for a significant proportion of overall MRSA infections. A prospective study in the USA revealed that the incidence rate of CA-MRSA infections was 243 cases/100,000 population in 2005, while that of health care–associated MRSA infections was 31 cases/100,000 population.2 CA-MRSA infections were also found among hospitalized patients,3 signifying the infiltration of CA-MRSA into hospitals. A previous study estimated that third-party payers’ cost for one CA-MRSA infection case was USD 2,277–3,200, and societal cost for one case of CA-MRSA was USD 7,070–20,489.4
Reported CA-MRSA outbreaks and infection cases in various Asia-Pacific countries suggest that the bacteria are circulating in this region.5–12 However, these reports have not been synthesized to provide an overview of CA-MRSA carriage prevalence among the general population and subgroups in this region. Effective infection control actions require thorough understanding of prevalence patterns and relative carriage prevalence for different individuals.
In addition, prevalence of CA-MRSA among various high-risk population subgroups, including but not limited to neonates, children, S. aureus carriers, and skin and soft tissue infection (SSTI) patients carrying CA-MRSA,1 is unknown in the Asia-Pacific region. Given the strong connectivity among countries within the region and with other countries, understanding the prevalence of CA-MRSA among population subgroups in the Asia-Pacific region might benefit the mitigation of the global transmission of CA-MRSA.
This study aimed to estimate the population-level prevalence and subgroup-specific prevalence of CA-MRSA carriage in the Asia-Pacific region. We also aimed to synthesize information about factors associated with CA-MRSA carriage. Our findings address the second strategic objective of the World Health Organization (WHO) Global Action Plan on Antimicrobial Resistance – “strengthen the knowledge and evidence base (of MRSA) through surveillance and research.”13 Our results help formulate evidence-based strategies for controlling CA-MRSA.
Methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.14 It was registered on PROSPERO (CRD: 42107067399).15 Two review authors (JWHW, KOK) were independently involved in the search strategy, study selection, data extraction, bias assessment, and statistical analysis. Disagreements between review authors were resolved through discussion. When consensus was not reached, opinions from the third review author (JMR) were sought to resolve disagreements.
Search strategy
The search was conducted in May 2017. We systematically searched MEDLINE, EMBASE, and PubMed databases for articles published from 1 January 2000 to 19 May 2017, with search terms relevant to CA-MRSA and names of countries of the Asia-Pacific region. Country names were retrieved from member states of WHO regional offices in South-East Asia and Western Pacific, with addition of Hong Kong Special Administrative Region and Taiwan. Appendix I describes the search strategy. To ensure literature saturation, the search was expanded by including relevant publications listed in the WHO Antimicrobial Resistance: Global Report on Surveillance 2014 (Appendix II).16
Selection criteria
Titles and abstracts were screened by reviewers for relevance of CA-MRSA in the Asia-Pacific region. When titles and abstracts appeared to be relevant, full-text articles were retrieved and considered for inclusion according to the eligibility criteria developed based on 1) study design, 2) population, 3) region, 4) definitions of CA-MRSA, 5) outcomes, 6) study period, 7) settings, and 8) language (Appendix III). Retrieved studies that reported information on either CA-MRSA carriage prevalence or associated factors among the general population or subgroups between 2000 and 2016 were included. Only Chinese or English articles were included. We excluded articles that reported only nonhuman isolates or failed to mention explicitly the location and setting in which the study was conducted. Review authors were not blinded to any journal information.
Data extraction
A standard data extraction form (Appendix IV) was adopted to extract information from each article, including but not limited to author, study year, country, study design, study population, study setting, study period, location(s), definition(s) of CA-MRSA, sample size, number of CA-MRSA-positive cases, factors for CA-MRSA carriage, and antibiotic resistance of CA-MRSA. For articles involving multiple studies, information was extracted separately for each study which therefore, led to the total number of studies greater than that of articles included. Each study was assigned a unique study number for identification purpose throughout this review.
Definitions of CA-MRSA carriage retrieved from included articles, when available, were grouped into three types: clinical, molecular, and epidemiologic. Clinical definition refers to identification of CA-MRSA through clinical symptoms or diseases relevant to CA-MRSA manifested in the population. Molecular definition refers to identification of CA-MRSA through the molecular structure and/or antimicrobial susceptibilities of CA-MRSA. Epidemiologic definition refers to MRSA diagnosed in the community or within a specific time period during hospital admission, with or without certain health care risk factors. These risk factors included but not limited to exposure to hemodialysis, surgery, residence in a long-term care facility or hospitalization during the previous year, and previous isolation of MRSA. If the article did not define CA-MRSA, review authors judged whether the MRSA described in the study conformed to the CA-MRSA definitions stated in the eligibility criteria (Appendix III).
Retrieved articles were stratified into two settings, according to the locations of participants from whom CA-MRSA was isolated: community and hospital. Community settings refer to urban and rural areas of communities, schools, day-care centers, outpatients, or emergency wards, whereas hospital settings refer to general and specific wards in hospitals. Articles were also stratified into three groups based on the study population: general population, subgroups without specific health conditions, and subgroups with specific health conditions. General population refers to participants enrolled in studies without specific inclusion criteria. Subgroups without specific health conditions refer to study participants from particular target populations without known health problems. Subgroups with specific health conditions refer to study participants with known health problems. Factors associated with CA-MRSA carriage reported in retrieved articles were summarized.
Bias assessment
The risk of bias of the articles, according to study types, was assessed using predefined assessment checklists for cross-sectional,17 cohort,18 and case–control18 design (Appendix V–VII). Sensitivity analyses were conducted by including only articles with low risk of bias in evaluating CA-MRSA carriage prevalence.
Statistical analysis
The primary outcome was the prevalence of CA-MRSA carriage. Both colonization and infection were regarded as the carriage. Prevalence was estimated as the number of people with CA-MRSA carriage divided by the number of people recruited in the study, and the 95% binomial confidence interval (bCI) of a single estimate is calculated by the Clopper–Pearson exact method.19 For subgroups with >1 study, ranges of estimates were reported. Pooled prevalence of CA-MRSA carriage was estimated with a DerSimonian–Laird random-effects model.20 The magnitude of between-study heterogeneity was assessed using I2 statistic.21,22
Meta-regression was used to explore the source of heterogeneity of CA-MRSA carriage prevalence. Potential sources of variance included gender, study settings, CA-MRSA definitions, isolation sites, countries’ status, and laboratory procedures. Study year (start-, mid-, and end-year of specimen) and publication year were also considered. Countries’ status were stratified based on the WHO mortality stratum into low-mortality developing, high-mortality developing, and developed nations.23 Laboratory procedures refer to whether the study adopted Clinical and Laboratory Standards Institute guidelines to isolate and cultivate samples.24 Before meta-analysis, variance of the prevalence was stabilized using the Freeman–Tukey transformed method because of the small proportions reported.25 A significance level of 0.05 was specified.
All analyses were performed using statistical software OpenMeta [Analyst] developed by the Center for Evidence Synthesis of Brown University in the USA.26
Results
Retrieved articles and studies
We identified 1,075 articles from the initial search: 573 articles were unique records and 293 were excluded based on their titles and abstracts (Figure 1). Hence, 280 full-text articles were assessed for eligibility. From these, 148 articles were excluded because of irrelevant outcomes (n=30), irrelevant or unknown study period (n=25), irrelevance to CA-MRSA (n=26), unknown or irrelevant study settings (n=21), irretrievable full text (n=15), unknown or irrelevant study participants (n=11), irrelevant study types (n=10), not being in Chinese or English (n=9), and not focusing on the Asia-Pacific region (n=1). Therefore, 132 articles met the inclusion criteria with full data extracted.
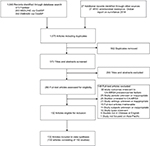  | Figure 1 Flow diagram of article selection. Abbreviations: CA-MRSA, community-associated methicillin-resistant Staphylococcus aureus; WHO, World Health Organization. |
The 132 identified articles reported 152 studies. Of these 152 studies, 119 provided CA-MRSA prevalence among general population or subgroups (Appendix XII), six provided information on associated factors of CA-MRSA carriage among general members (Appendix XIII–XIV), and eight provided the proportion of antibiotic resistance among general members (Appendix XVIII). Thirty three studies were excluded from meta-analysis because of various reasons (Appendix XXIV).
Study characteristics
The final dataset included 80 studies in community settings and 66 in hospital settings (Appendix X). Most studies were cross-sectional (community, 94%; hospital, 80%) and conducted before 2009 (76%). These studies were from 17 countries, mainly in Taiwan (n=41), China (n=18), India (n=20), Australia (n=19), and South Korea (n=18). The number of studies that collected isolates from single body sites (n=64, 43%) was comparable to that from multiple body sites (n=83, 55%). The nose was the most common site for single body site isolation (n=44).
Upon excluding 33 studies (Appendix XXIV), the remaining 119 studies were stratified into three groups based on the study population. Population-based studies of the general public accounted for 12%–20% of included studies (community, 9/73; hospital, 9/46), whereas the majority of studies were conducted among these subgroups: children aged ≤6 years (community, n=10, 14%); S. aureus carriers (community, n=63, 86%; hospital, n=41, 89%); and SSTIs (community, n=14, 19%).
Definition of CA-MRSA
Most studies defined CA-MRSA epidemiologically (community, n=20, 25%; hospital, n=40, 56%) (Appendix XI). A decreasing trend of adopting epidemiologic definitions was observed among studies in community settings, while an increasing definition diversity was observed among studies in hospital settings (Figure 2). Many studies did not explicitly define CA-MRSA, especially studies in community settings (n=54, 67%).
  | Figure 2 Distribution of CA-MRSA definitions across years, stratified by settings. Notes: Clinical definition refers to identification of CA-MRSA through different clinical symptoms or diseases manifested in the population. Molecular definition refers to identification of CA-MRSA through the molecular structure or/and antimicrobial susceptibilities of CA-MRSA. Epidemiological definition refers to MRSA diagnosed in the community or within a specific time period during hospital admission, with or without the presence of certain health care risk factors. Details of each included study are given in Appendix VIII. Abbreviation: CA-MRSA, community-associated methicillin-resistant Staphylococcus aureus. |
General community prevalence
In community settings, based on nine studies from seven countries, the prevalence of CA-MRSA ranged from 0% to 23.5% (pooled prevalence: 3.9%; 95% CI: 2.0, 6.3) (Table 1; Appendix XV). Large diversity was observed in these studies (I2=99.7). The three countries with the highest prevalence in community settings were India (study 32: 16.5%; 95% bCI: 13.9, 19.4; study 56: 23.5%; 95% bCI: 17.8, 30.0), Vietnam (study 131: 7.9%; 95% bCI: 6.3, 9.7), and Taiwan (study 88: 3.5%; 95% bCI: 2.7, 4.4; study 136: 3.8%; 95% bCI: 3.2, 4.6). In hospital settings, the prevalence of nine studies consisting of inpatients, health care workers, and janitors ranged from 0.7% to 10.4% (pooled prevalence: 2.5%; 95% CI: 1.7, 3.3; I2=81.2) (Table 1; Appendix XV). In addition, a change in CA-MRSA prevalence over time has been noted: Using study start year as the cutoff, prevalence reached a new peak from 2001–2005 (range: 0%–16.5%) to 2006–2010 (range: 0.9%–23.5%), then it dropped in 2011–2016 (range: 0.3%–7.9%).
  | Table 1 Country-specific CA-MRSA carriage prevalence Notes: aRefer to Appendix VIII for study details; bClopper–Pearson exact transformation; cThe precise value is 0.009%. Abbreviation: CA-MRSA, community associated methicillin-resistant Staphylococcus aureus. |
Prevalence among subgroups without specific health conditions
Except for children aged ≤6 years, studies involving other age groups were conducted in community settings. To maximize the number of studies contributing to age-specific prevalence, we defined the age groups as follows: 0–6, 7–18, and >18. In community settings, based on the overlapping age strata from 14 studies, respectively, CA-MRSA carriage prevalence among children aged ≤6 years, children aged 7–18, and adults >18 years ranged from 0.5% to 40.3% (pooled prevalence: 7.6%; 95% CI: 4.0, 12.2), 1.4%–6.5% (pooled prevalence: 3.2%; 95% CI: 0.7, 6.5), and 0.4%–4.2% (pooled prevalence: 1.6%; 95% CI: 0.7, 3.0) (Table 2; Appendix XII, XVI and XXV). Table 2 shows the prevalence of CA-MRSA carriage among other subgroups. The prevalence was the highest among household members of CA-MRSA carriers (range: 13.0%–26.4%), followed by mother of children aged two years (8.0%) and pediatricians (8.5%).
  | Table 2 CA-MRSA carriage prevalence among different population groups stratified based on settings Notes: aPrevalence within subgroups with >1 study included was presented as range, otherwise a single prevalence was presented. bTwo studies provided one combined data (Appendix XII, Appendix XXV). Abbreviations: BSI, blood stream infection; CA-MRSA, community-associated methicillin-resistant Staphylococcus aureus; DM, diabetes mellitus; ENT, ear, nose, and throat; HIV, human immunodeficiency virus; SSTIs, skin and soft tissue infections. |
Prevalence among subgroups with specific health conditions
Studies were conducted on individuals across a wide range of diseases. CA-MRSA prevalence among SSTI patients (I2=98.5) and S. aureus carriers (I2=97.9) varies substantially in both settings (Appendix XVI). The prevalence can be as high as 74.4% (range: 0%–74.4%) for S. aureus carriers and 49.1% (range: 1.0%–49.1%) for SSTI patients, respectively (Table 2). Other subgroups with high prevalence included S. aureus bacteremia (0.8%–38.6%) and ear, nose, and throat (ENT) conditions (community, 2.7%–23.8%). Regardless of settings, CA-MRSA was less common among subgroups with respiratory problems (range: 0%–2.4%) and cesarean section (hospital, 1.4%). Table 2 also shows the prevalence among subgroups with other specific health conditions.
Heterogeneity in studies among the general members
We explored the source of heterogeneity of CA-MRSA carriage prevalence among general population in community and hospital settings (Table 3, Appendix XIX). In community settings, univariate analysis showed a significant association between CA-MRSA prevalence and isolation site (p=0.019) and countries’ development and mortality pattern (p<0.0001), respectively. The highest prevalence estimates were observed when collected isolates were from multiple body sites (range: 1.1%–23.5%) and when studies were conducted in high-mortality developing countries (range: 16.5%–23.5%). Table 1 and Appendix XV detailed country-specific prevalence of CA-MRSA. In hospital settings, no factor explored appeared to explain the between-study heterogeneity.
Antibiotic resistance
Despite the reported resistance being highly heterogeneous (Appendix XVII), its pattern was twofold (Table 4, Appendix XVIII and XXVI): CA-MRSA resistance was high for erythromycin, tetracycline, cephalexin, cefoxitin, gentamicin, co-trimoxazole and clindamycin (up to 100%). On the other hand, the resistance was low for minocycline (community, 1.1%), moxifloxacin (community, 1.6%), and rifampin (community, 3.1%).
  | Table 4 Prevalence of CA-MRSA antibiotic resistance among general members stratified based on settings Notes: aPrevalence within subgroups with >1 study was presented as range, otherwise a single prevalence was presented. bTwo studies provided one combined data on antibiotic resistance (Appendix XVIII and Appendix XXVI). |
Factors associated with CA-MRSA carriage
Risk and protective factors were reported among general members (Appendix XIII and XIV). These factors varied in details across studies and could be classified into three types: demographic (including age, sex, ethnicity, education and wealthiness), medical (antimicrobial exposure, hospitalization, various forms of infections or diseases, and invasive devices), and others.
Risk of bias
A total of 24 studies (15.8%) were considered as presenting low risk of bias, and 128 studies (84.2%) as high risk (Appendix XX–XXII). Among 134 cross-sectional studies, only 69 studies (51.5%) provided CA-MRSA definition. Among 18 case–control and cohort studies, only ten studies (55.6%) specified a control group. After removing the high-risk studies, CA-MRSA carriage prevalence among the general population in both settings ranged from 0.3% to 23.5% (Appendix XXIII).
Discussion
This systematic review is the first study to synthesize published studies to provide recent point prevalence estimates of CA-MRSA carriage among the general population and population subgroups in the Asia-Pacific region. Responding to the WHO Global Action Plan on Antimicrobial Resistance,13 this review revealed that CA-MRSA is a regional public health concern.
More than half of the nine community-level studies in Asia-Pacific region had higher CA-MRSA carriage prevalence (range: 0%–23.5%) than that of the USA (2.0%).27 Given the median carriage prevalence (3.5%) from these nine studies and 4.5 billion28 individuals living in this region, ~157.5 million individuals are likely to be carrying CA-MRSA. Assuming that 19% of carriers develop infections (i.e., ~30 million) and the treatment of a single CA-MRSA infection case costs between USD 2,277 and USD 3,200 to third-party payers and between USD 7,070 and USD 20,489 to the society,4,29 the estimated potential treatment cost in this region could be as high as USD 68–USD 96 billion to third-party payers and USD 212–USD 615 billion to the society. The societal cost for CA-MRSA treatment could potentially be higher than the treatment cost of Methicillin-sensitive S. aureus in health care settings, which was USD 476.5 billion, ceteris paribus.30 To prevent further cost escalation for CA-MRSA treatment, we recommend increasing public health focus on hygienic standards in community settings,31 formulating guidelines for antibiotics use at local levels,32 and regulatory control of over-the-counter antibiotics sales.33
The detection of CA-MRSA carriage prevalence in hospital settings suggests that CA-MRSA has extended beyond community settings. This should be closely monitored, as health care environments can offer favorable conditions for the proliferation and amplification of CA-MRSA.34 Establishment of prevention and control infection measures in hospital and primary care settings is warranted to reduce transmission of CA-MRSA in health care environment.
Compared with children aged 7–18 and adults >18 (median: 1.8% and 1.0%), higher CA-MRSA carriage prevalence (median: 7.3%) was observed from the 11 studies on children ≤6 years old, which is consistent with previous studies focusing on newborns and children.35–37 We also collated information on identified associated factors. The high prevalence of CA-MRSA carriage among neonates and young children may be due to their naive immunity toward CA-MRSA and close contact with asymptomatic CA-MRSA carriers.38,39 Elevated hygienic standards are, therefore, recommended for people contacting infants and neonates. Infection control strategies may benefit schools, nurseries, and day-care centers, as these places facilitate contacts between asymptomatic CA-MRSA carriers and young children. Our findings also revealed the prevalence of CA-MRSA carriage among S. aureus carriers and SSTI patients are high in both settings, implying that relevant control strategies should be targeted to them irrespective of settings. Our study also found CA-MRSA prevalence in other population subgroups in community and hospital settings consistent with previous findings.40–41 However, the number of these studies representing each country was low, and a comparison between community and hospital settings is, therefore, not available. Notwithstanding, future CA-MRSA research might focus on these subgroups, especially for household members of CA-MRSA carriers and patients with ENT conditions.
The variability of CA-MRSA definitions among studies reported in this review was consistent with the conclusion of a previous study.34 We recommend that future studies consider both the epidemiologic and the molecular characteristics of CA-MRSA to define CA-MRSA,44 and standard laboratory procedures to identify CA-MRSA for improved comparison between studies.
Our study updates the baseline antibiotics to which CA-MRSA resists in Asia-Pacific region. Consistent with a previous study,45 the high erythromycin resistance of CA-MRSA observed in our review suggested that erythromycin may not be effective in treating CA-MRSA infections. Resistance toward clindamycin and tetracycline are also concerns, as they have been recommended for treating MRSA SSTIs.46 Clindamycin has even been recommended for treating MRSA pneumonia and MRSA joint and bone infections.46 To alleviate antibiotic resistance achieved by CA-MRSA, tailor-made antibiotic stewardship programs for both public and private physicians should be implemented to minimize irrational antibiotic prescription advocated by the WHO.47 Our findings further indicate that the public should be educated on hand hygiene and proper antibiotics usage, and that governments should play an active role in restricting over-the-counter antibiotic sales at local pharmacies to reduce misuse.
Isolation sites for CA-MRSA and country’s status were found to explain the heterogeneity of CA-MRSA prevalence among community-based studies. Higher CA-MRSA prevalence from multiple body sites was found comparing with nasal cavity alone in the general community, suggesting that only accounting isolation of CA-MRSA in the nasal cavity might underestimate CA-MRSA carriage, which led to reports of falsely low prevalence. Colonization of CA-MRSA in multiple body sites also suggests different transmission modes of CA-MRSA. Hand carriage is suggestive of skin-to-skin or contaminated-object transmission, while throat carriage indicates the possibility of aerosol transmission.48 Future research is suggested to investigate the potential of different transmission modes. The prevalence of CA-MRSA carriage in the general community is significantly higher in high-mortality developing countries. We advise high-mortality developing countries to mobilize resources for primary care and education to reduce the prevalence CA-MRSA. Policy makers should consider making MRSA a notifiable pathogen to identify the strains and trend of MRSA circulating in a country over time.48
There are limitations in this study. First, categorization of outpatients recruited in hospitals into community settings might be controversial as they might be colonized with CA-MRSA in hospital settings. Nevertheless, the reason for this classification was that contact mixing patterns were different between inpatients and outpatients, in the way that outpatients interact with other community members more frequently. Second, the included studies had an incomplete geographical representation of the Asia-Pacific region. Retrieved articles only covered 17 out of 48 countries and states searched (35%). The lack of publication in some countries poses difficulties in providing a complete picture of CA-MRSA carriage prevalence in the region. Our extrapolation is possibly an underestimation because of the considerable number of developing countries without reported CA-MRSA carriage prevalence. Third, classification of countries using WHO mortality stratum may overlook a country’s health care availability and sanitation access.24 These factors shape country-specific CA-MRSA epidemiology and are worth investigating. Fourth, a considerable proportion of studies included in the analyses were of high risk of bias (115/134). Absence of CA-MRSA definition in cross-sectional studies (65/134) and lack of control group in cohort and case–control studies (7/18) are reasons leading to these high risks of bias. Fifth, extrapolating prevalence of CA-MRSA infection rate as prevalence of carriage in our meta-analyses may underestimate the actual CA-MRSA carriage prevalence (Appendix VIII). Sixth, for studies with CA-MRSA isolated among newly admitted patients (within 48 or 72 hours) in hospital settings, we assume that the CA-MRSA pattern observed can be generalized to inpatients in general.49 Seventh, swabbing and cultivation methods for MRSA might have been optimized over the study years, which might alter the detection rate, hence prevalence of CA-MRSA. Eighth, consensus already exists across WHO and international communities on the uptrend of CA-MRSA prevalence;16 however, our review might not be “powered” to demonstrate this trend as only few community-level prevalence studies were published per 5-year period since 2000.
Conclusion
This review shows that CA-MRSA carriage is widespread in the Asia-Pacific region and poses a clear health threat with a potentially large health care cost. In addition to known high-risk groups, such as SSTI patients and S. aureus carriers, our study informs regional and local infection control strategies and suggests that targeting infants and children may be beneficial. Potential control strategies include hand hygiene promotion and proper antibiotics use among high-risk groups, together with infants and children. By responding to the WHO goals, the prevalence reported in this study facilitate future surveillance work. We urge health authorities, especially those from developing countries, to prioritize the control of CA-MRSA through community action plans and multilevel antibiotic stewardship. Future research should also consider the insights into transmission mechanisms inferred from the heterogeneities in prevalence among different population groups and countries.
Acknowledgments
SR acknowledges support from Wellcome Trust (UK, 200861/Z/16/Z); National Institute for General Medical Sciences (US, MIDAS U01 GM110721-01); National Institute for Health Research (UK, for Health Protection Research Unit funding). JMR acknowledges support from the ESRC (ES/K004255/1, RES-355-25-0019), EPSRC (EP/N014499/1) and MRC (MR/S004793/1). KOK acknowledges support from RFCID (number: CU-17-C18) and HMRF (Number: 17160302). MI acknowledges support from HMRF (number: 16150942) and RFCID (number: CU-15-B3). JWHW and KOK would like to express their gratitude to Professor Vincent Chi-ho Chung and Dr. Johnson Lau from JC School of Public Health and Primary Care of The Chinese University of Hong Kong, respectively, for their assistance with the systematic literature review, risk of bias assessment, and figure production. KOK also acknowledges Li Ka Shing Institute of Health Sciences for providing technical support in the research.
Author contributions
JWHW and KOK did the literature search, extracted and analyzed the data, designed and produced figures, and drafted the manuscript. KOK designed the study and oversaw analysis. JMR helped with the study design, interpreted the results, and edited the manuscript. MI, AT, VWWI, SYSW, and SR edited and contributed to the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–687. | ||
Liu C, Graber CJ, Karr M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clin Infect Dis. 2008;46(11):1637–1646. | ||
Hidron AI, Kourbatova EV, Halvosa JS, et al. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005;41(2):159–166. | ||
Lee BY, Singh A, David MZ, et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect. 2013; 19(6): 528–536. | ||
Schlebusch S, Price GR, Hinds S, et al. First outbreak of PVL-positive nonmultiresistant MRSA in a neonatal ICU in Australia: comparison of MALDI-TOF and SNP-plus-binary gene typing. Eur J Clin Microbiol Infect Dis. 2010;29(10):1311–1314. | ||
Chen J, Luo Y, Zhang S, et al. Community-acquired necrotizing pneumonia caused by methicillin-resistant Staphylococcus aureus producing Panton–Valentine leukocidin in a Chinese teenager: case report and literature review. Int J Infect Dis. 2014;26:17–21. | ||
Ho PL, Chuang SK, Choi YF, et al; Hong Kong CA-MRSA surveillance network. Community-associated methicillin-resistant and methicillin-sensitive Staphylococcus aureus: skin and soft tissue infections in Hong Kong. Diagn Microbiol Infect Dis. 2008;61(3):245–250. | ||
Manoharan A, Zhang L, Poojary A, et al. An outbreak of post-partum breast abscesses in Mumbai, India caused by ST22-MRSA-IV: genetic characteristics and epidemiological implications. Epidemiol Infect. 2012;140(10):1809–1812. | ||
Nagao M, Iinuma Y, Suzuki M, et al. First outbreak of methicillin-resistant Staphylococcus aureus USA300 harboring the Panton-Valentine leukocidin genes among Japanese health care workers and hospitalized patients. Am J Infect Control. 2010;38(9):e37–e39. | ||
Hsu LY, Koh YL, Chlebicka NL, et al. Establishment of ST30 as the predominant clonal type among community-associated methicillin-resistant Staphylococcus aureus isolates in Singapore. J Clin Microbiol. 2006;44(3):1090–1093. | ||
Park PD, Lee DG, Choi SM, et al. A case of perianal abscess due to Panton-Valentine leukocidin positive community-associated methicillin-resistant staphylococcus aureus: report in Korea and literature review from the far east. Infect Chemother. 2008;40(2):121–126. | ||
Tang CT, Nguyen DT, Ngo TH, et al. An outbreak of severe infections with community-acquired MRSA carrying the Panton-Valentine leukocidin following vaccination. PLoS One. 2007;2(9):e822. | ||
World Health Organization. Global Action Plan on Antimicrobial Resistance. Available from: http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf. Accessed August 7, 2017. | ||
Moher D, Shamseer L, Clarke M, et al; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. | ||
Wong JW, Ip M, Riley S, Read J, Kwok KO. Prevalence and risk factors of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) carriage in the Asia-Pacific region from 2000 to 2016: a systematic review. PROSPERO 2017 CRD42017067399. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017067399. Accessed May 7, 2018. | ||
World Health Organization. Antimicrobial resistance: global report on surveillance 2014. Available from: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1. Accessed August 7, 2017. | ||
Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. | ||
Wells GA, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed August 7, 2017. | ||
Clopper CJ, Pearson ES. The use of confidence or fuducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413. | ||
Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med. 1995;14(4):395–411. | ||
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
World Health Organization. The World Health report 2003 – shaping the future. Available from: http://www.who.int/whr/2003/en/whr03_en.pdf?ua=1. Accessed February 22, 2018. | ||
Brown DF, Edwards DI, Hawkey PM, et al; Joint Working Party of the British Society for Antimicrobial Chemotherapy; Hospital Infection Society; Infection Control Nurses Association. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J Antimicrob Chemother. 2005;56(6):1000–1018. | ||
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. | ||
Wallce BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as computational back-end. J Stat Softw. 2012;49(5):1–15. | ||
Centers for Disease Control and Prevention. General Information About MRSA in the Community. Available from: https://www.cdc.gov/mrsa/community/index.html. Accessed July 12, 2017. | ||
United Nations. 2016 ESCAP Population Data Sheet. Available from: http://www.unescap.org/sites/default/files/SPPS%20PS%20data%20sheet%202016%20v15-2.pdf. Accessed July 12, 2017. | ||
Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004;39(6):776–782. | ||
Flice GA, Nyman JA, Lexau C, et al. Excess costs and utilization associated with methicillin resistatnace for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2010;31(4): 365–373. | ||
World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care is Safer Care. Available from: http://apps.who.int/iris/bitstream/10665/44102/ 1/9789241597906_eng.pdf. Accessed November 25, 2017. | ||
Bhagwati A. Guidelines for antibiotic usage in common situations. J Assoc Physicians India. 2010;58(Supp):49–50. | ||
Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011;11(9):692–701. | ||
Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36(2):131–139. | ||
James L, Gorwitz RJ, Jones RC, et al. Methicillin-resistant Staphylococcus aureus infections among healthy full-term newborns. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F40–F44. | ||
Sattler CA, Mason EO, Kaplan SL. Prospective comparison of risk factors and demographic and clinical characteristics of community-acquired, methicillin-resistant versus methicillin-susceptible Staphylococcus aureus infection in children. Pediatr Infect Dis J. 2002;21(10): 910–917. | ||
Purcell K, Fergie JE. Exponential increase in community-acquired methicillin-resistant Staphylococcus aureus infections in South Texas children. Pediatr Infect Dis J. 2002;21(10):988–989. | ||
Al-Tawfiq JA. Father-to-infant transmission of community-acquired methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2006;27(6):636–637. | ||
Bertin ML, Vinski J, Schmitt S, et al. Outbreak of methicillin-resistant Staphylococcus aureus colonization and infection in a neonatal intensive care unit epidemiologically linked to a healthcare worker with chronic otitis. Infect Control Hosp Epidemiol. 2006;27(6):581–585. | ||
Hollis RJ, Barr JL, Doebbeling BN, Pfaller MA, Wenzel RP. Familial carriage of methicillin-resistant Staphylococcus aureus and subsequent infection in a premature neonate. Clin Infect Dis. 1995;21(2):328–332. | ||
Lee SY, Kim JY, Kim JH, et al. A case of primary infective endocarditis caused by community-associated methicillin-resistant Staphylococcus aureus in a healthy individual and colonization in the family. Yonsei Med J. 2009;50(1):152–155. | ||
Leonard FC, Markey BK. Meticillin-resistant Staphylococcus aureus in animals: a review. Vet J. 2008;175(1):27–36. | ||
Huang YC, Su LH, Lin TY. Nasal carriage of methicillin-resistant Staphylococcus aureus in contacts of an adolescent with community-acquired disseminated disease. Pediatr Infect Dis J. 2004;23(10):919–922. | ||
Millar BC, Loughrey A, Elborn JS, Moore JE. Proposed definitions of community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA). J Hosp Infect. 2007;67(2):109–113. | ||
Liu Y, Xu Z, Yang Z, Sun J, Ma L. Characterization of community-associated Staphylococcus aureus from skin and soft-tissue infections: a multicenter study in China. Emerg Microbes Infect. 2016; 5(12):e127. | ||
Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3): e18–e55. | ||
World Health Organization. Antibiotic resistance fact sheet. Available from: http://www.who.int/mediacentre/factsheets/antibiotic-resistance/en/. Accessed on November 25, 2017. | ||
Skov R, Christiansen K, Dancer SJ, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents. 2012;39(3):193–200. | ||
Centers for Disease Control and Prevention. 2003 National Hospital Discharge Survey. Available from: https://www.cdc.gov/nchs/data/ad/ad359.pdf. Accessed December 20, 2017. | ||
Munckhof WJ, Nimmo GR, Schooneveldt JM, et al. Nasal carriage of Staphylococcus aureus, including community-associated methicillin-resistant strains, in Queensland adults. Clin Microbiol Infect. 2009;15(2):149–155. | ||
Chen B, Dai X, He B, Pan K, Li H, Liu X, Bao Y, Lao W, Wu X, Yao Y, Huang S. Differences in Staphylococcus aureus nasal carriage and molecular characteristics among community residents and healthcare workers at Sun Yat-Sen University, Guangzhou, Southern China. BMC Infect Dis. 2015;15(1):303. | ||
Goud R, Gupta S, Neogi U, et al. Community prevalence of methicillin and vancomycin resistant Staphylococcus aureus in and around Bangalore, southern India. Rev Soc Bras Med Trop. 2011;44(3):309–312. | ||
Jain B, Agarwal J, Singh M. Observations on community associated methicillin resistant Staphylococcus aureus carriage. Clin Epidemiol Glob Health. 2014; 2(1):15–18. | ||
Lu PL, Chin LC, Peng CF, et al. Risk factors and molecular analysis of community methicillin-resistant Staphylococcus aureus carriage. J Clin Microbiol 2005;43(1):132–139. | ||
Wang JT, Liao CH, Fang CT, et al. Prevalence of and risk factors for colonization by methicillin-resistant Staphylococcus aureus among adults in community settings in Taiwan. J Clin Microbiol. 2009; 47(9):2957–2963. | ||
Ro YS, Do Shin S, Noh H, Cho SI. Prevalence of positive carriage of tuberculosis, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococci in patients transported by ambulance: a single center observational study. J Prev Med Public Health. 2012;45(3):174–180. | ||
Van Nguyen K, Zhang T, Thi Vu BN, et al. Staphylococcus aureus nasopharyngeal carriage in rural and urban northern Vietnam. Trans R Soc Trop Med Hyg. 2014;108(12):783–790. | ||
Verwer PE, Robinson JO, Coombs GW, et al. Prevalence of nasal methicillin-resistant Staphylococcus aureus colonization in healthcare workers in a Western Australian acute care hospital. Eur J Clin Microbiol Infect Dis. 2012;31(6):1067–1072. | ||
George K, Abdulkader JK, Sugumar M, Rajagopal GK. Prevalence of MRSA Nasal Carriage in Patients Admitted to a Tertiary Care Hospital in Southern India. J Clin Diagn Res. 2016;10(2):DC11. | ||
Chang CJ, Chen NC, Lao CK, Huang YC. Nasal Staphylococcus aureus and methicillin-resistant S. aureus carriage among janitors working in hospitals in northern Taiwan. PLoS One. 2015;10(9):e0138971. | ||
Chen CB, Chang HC, Huang YC. Nasal meticillin-resistant Staphylococcus aureus carriage among intensive care unit hospitalised adult patients in a Taiwanese medical centre: one time-point prevalence, molecular characteristics and risk factors for carriage. J Hosp Infect. 2010;74(3):238–244. | ||
Wang JT, Liao CH, Fang CT, et al. Incidence of and risk factors for community-associated methicillin-resistant Staphylococcus aureus acquired infection or colonization in intensive-care-unit patients. J Clin Microbiol. 2010;48(12):4439–4444. | ||
Park SY, Chung DR, Yoo JR, et al. Sequence type 72 community-associated meticillin-resistant Staphylococcus aureus emerged as a predominant clone of nasal colonization in newly admitted patients. J Hosp Infect. 2016;93(4):386–389. | ||
Joshi PR, Acharya M, Aryal R, et al. Emergence of staphylococcal cassette chromosome mec type I with high-level mupirocin resistance among methicillin-resistant Staphylococcus aureus. Asian Pac J Trop Biomed. 2017;7(3):193–197. | ||
Williamson DA, Roberts SA, Ritchie SR, et al. Clinical and molecular epidemiology of methicillin-resistant Staphylococcus aureus in New Zealand: rapid emergence of sequence type 5 (ST5)-SCC mec-IV as the dominant community-associated MRSA clone. PLoS One. 2013;8(4):e62020. | ||
Brennan L, Lilliebridge RA, Cheng AC, Giffard PM, Currie BJ, Tong SY. Community-associated meticillin-resistant Staphylococcus aureus carriage in hospitalized patients in tropical northern Australia. J Hosp Infect. 2013;83(3):205–211. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.