Back to Journals » Journal of Asthma and Allergy » Volume 13
Prevalence and Predictors of Uncontrolled Asthma in Children Referred for Asthma and Other Atopic Diseases
Authors Kansen HM , Le TM, Uiterwaal CSPM, van Ewijk BE, Balemans WAF, Gorissen DMW, de Vries E , van Velzen MF , Slabbers GHPR, Meijer Y, Knulst AC, van der Ent CK, van Erp FC
Received 20 September 2019
Accepted for publication 13 December 2019
Published 30 January 2020 Volume 2020:13 Pages 67—75
DOI https://doi.org/10.2147/JAA.S231907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
HM Kansen,1,2 TM Le,2 CSPM Uiterwaal,3 BE van Ewijk,4 WAF Balemans,5 DMW Gorissen,6 E de Vries,7 MF van Velzen,8 GHPR Slabbers,9 Y Meijer,1 AC Knulst,2 CK van der Ent,1 FC van Erp2
1Department of Pediatric Pulmonology and Allergology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, The Netherlands; 2Department of Dermatology/Allergology, University Medical Center Utrecht, Utrecht, The Netherlands; 3Julius Center for Health Science and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands; 4Department of Pediatrics, Tergooi Hospital, Blaricum, The Netherlands; 5Department of Pediatrics, St. Antonius Hospital, Nieuwegein, The Netherlands; 6Department of Pediatrics, Deventer Hospital, Deventer, The Netherlands; 7Department of Pediatrics, Jeroen Bosch Academie (Research), Jeroen Bosch Hospital, ‘S Hertogenbosch, The Netherlands; 8Department of Pediatrics, Meander Medical Center, Amersfoort, The Netherlands; 9Department of Pediatrics, Bernhoven Hospital, Uden, The Netherlands
Correspondence: HM Kansen
Department of Pediatric Pulmonology and Allergology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Lundlaan 6, Utrecht 3508 AB, The Netherlands
Tel +31 30 88 75 75 2 84
Email [email protected]
Background: Uncontrolled asthma in children is still highly prevalent despite the availability of effective asthma treatment. We investigated 1) the prevalence of uncontrolled asthma among children referred for asthma and referred for atopic diseases other than asthma (ie food allergy, allergic rhinitis or atopic dermatitis) to secondary care; and 2) the predictors associated with uncontrolled asthma.
Methods: All children (4 to 18 years) referred for asthma or atopic diseases other than asthma to 8 secondary care centers in The Netherlands were invited to an electronic portal (EP). The EP is a web-based application with several validated questionnaires including the ISAAC questionnaires and the Asthma Control Test (ACT). Children were eligible for inclusion in this study when their parents reported in the EP that their child had asthma diagnosed by a physician. The ACT was used to assess asthma control. Multiple predictors of asthma control (patient, asthma and atopic characteristics) were evaluated by univariable and multivariable logistic regression analyses.
Results: We included 408 children: 259 children (63%) with asthma referred for asthma and 149 children (37%) with asthma referred for atopic diseases other than asthma. Thirty-nine percent of all children had uncontrolled asthma: 47% of the children referred for asthma and 26% of the children referred for atopic diseases other than asthma. Predictors associated with uncontrolled asthma were a family history of asthma (odds ratio [OR] 2.08; 95% confidence interval [95% CI] 1.34 to 3.24), and recurrent upper and lower respiratory tract infections in the past year (OR 2.40; 95% CI 1.52 to 3.81 and OR 2.00; 95% CI 1.25 to 3.23, respectively).
Conclusion: Uncontrolled asthma is highly prevalent in children with asthma referred to secondary care, even if children are primarily referred for atopic diseases other than asthma. Thus, attention should be paid to asthma control in this population.
Keywords: asthma, asthma control, atopic diseases, respiratory tract infections, prognosis
Background
Asthma is one of the most common chronic inflammatory diseases in the Western world, and a notable cause of morbidity in children.1 Good asthma control is the main management goal according to current asthma management guidelines, as this decreases the risk of asthma exacerbations and improves the quality of life.2 However, uncontrolled asthma is still highly prevalent despite the availability of effective asthma treatment.3 In order to achieve adequate asthma control in children with asthma, it is necessary to gain insight into the current prevalence of uncontrolled asthma among children and the associated predictors.
Several predictors of uncontrolled asthma in children have been previously described such as age, male gender, maternal education level, exposure to indoor smoking, pet ownership, high use of short-acting β2-agonists, incorrect inhaler technique, poor adherence to asthma medication and the presence of co-existing diseases.2,4,5 Many children with asthma have co-existing atopic diseases – including food allergy, allergic rhinitis and atopic dermatitis – or recurrent respiratory tract infections. Food allergy has been reported in around 25% of children with asthma, allergic rhinitis in 60%, atopic dermatitis in 45% and recurrent respiratory tract infections in 60%.6–10 Atopic comorbidities and recurrent respiratory tract infections are associated with increased asthma morbidity. Children with asthma and food allergy have increased asthma symptoms and a significantly lower lung function compared to asthmatic children without food allergy.6,11 Children with asthma and allergic rhinitis have a lower level of asthma control compared to asthmatic children without allergic rhinitis.12,13 In addition, the presence of allergic rhinitis increases the risk of asthma exacerbations, emergency visits and hospitalizations. Furthermore, recurrent respiratory tract infections are associated with asthma exacerbations in children and a lower level of asthma control.14
Although predictors of uncontrolled asthma have been previously investigated in various studies, there are still some gaps in knowledge. The majority of research investigating asthma control is conducted among children referred for asthma4,15 or among children in the general population5,6,8,12 but not among children that are referred with atopic diseases other than asthma to secondary care. The aim of this cross-sectional study was 1) to investigate the prevalence of uncontrolled asthma among children referred for asthma and among children referred for atopic diseases other than asthma, and 2) to investigate the predictors, including atopic comorbidities, associated with uncontrolled asthma among these children.
Methods
Domain and Data Collection
We conducted a cross-sectional study among children aged 4 to 18 years who were referred to a participating secondary care center (n=8) in the Netherlands between June 2011 and December 2017. All children who were referred for asthma or for atopic diseases other than asthma (ie food allergy, allergic rhinitis or atopic dermatitis) were invited to fill in an electronic portal (EP) as part of their outpatient visit. There were no exclusion criteria for participation in the EP. Children were eligible for inclusion in this study when their parents reported in the EP that their child had a physician-diagnosed asthma.
The structure of the EP has been published previously.16 In brief, the EP is a web-based application for children and their parents which has been developed and used by a nationwide collaborative network of Dutch caregivers in primary-, secondary- and tertiary health care. The aim of the EP is to collect information on asthma, atopic comorbidities and recurrent respiratory tract infections using validated questionnaires including the ISAAC core questionnaires for wheezing, allergic rhinitis, and atopic dermatitis, the Asthma Control Test, the Pediatric Asthma Quality of Life Questionnaire and the RAND general health questionnaire.17–22 The study has been approved by the Medical Ethics Committee of the University Medical Center Utrecht (No. 10/348) and all parents and/or children gave informed consent in the EP.
Outcome
Asthma control during the past month was assessed using the standardized and validated Child-Asthma Control Test (C-ACT) in children 4 to 12 years of age and the Asthma Control Test (ACT) in children ≥12 years of age.18,19 The final asthma control score is the summed score ranging from 5 through 27 (C-ACT) or 5 through 25 (ACT) with higher scores indicating better asthma control. A score of <20 indicates uncontrolled asthma.18,19
Potential Predictors of Asthma Control
Potential predictors of asthma control were selected based on clinical knowledge and prior research: age (4 to 12 years or 12 to 18 years), gender, maternal education level (categories "low", "middle" or "high"), a family history of asthma in one or both parent(s), current smoke exposure, current pet ownership, use of daily inhaled corticosteroids (categories “no”, “yes, adherent” or “yes, non-adherent”), food allergy, allergic rhinitis, atopic dermatitis, recurrent upper respiratory tract infections in the past year, recurrent lower respiratory tract infections in the past year and the referral reason (categories “referral for asthma” or “referral for atopic diseases other than asthma”). Food allergy was defined as self-reported relevant allergic complaints (urticaria, oral allergy symptoms, angioedema, upper or lower respiratory complaints, vomiting, a loss of consciousness or anaphylaxis) within 2 hrs after ingestion of a food (yes or no). Allergic rhinitis was defined as having problems with sneezing, or a runny, or a blocked nose when the child did not have a cold or the flu, accompanied by itchy-watery eyes in the past 12 months (yes or no). Atopic dermatitis was defined as the presence of an itchy rash for 6 months or longer ever and complaints of an itchy rash in the past 12 months (yes or no). Recurrent upper respiratory tract infections included middle ear infections, croup, a cold or laryngitis and was defined as ≥8 episodes/year for children aged 2 to 5 years, ≥6 for ages 5–10 years and ≥4 for ages 10 years or older. Recurrent lower respiratory tract infections included severe bronchitis or pneumonia and were defined as ≥2 episodes/year for children of all ages. Definitions of all predictors are displayed in Supplemental Table 1.
Adherence to asthma medication was assessed among all parents of children using daily inhaled corticosteroids by the Medication Adherence Rating Scale (MARS).23 The MARS is a 5-item questionnaire on medication use behavior. Children with a total MARS score ≥21 were considered adherent.24
Statistical Analysis
Differences between children with complete and incomplete data and differences between children referred for asthma and referred for atopic diseases other than asthma were statistically evaluated by the chi-square test for categorical variables, the unpaired t-test for normally distributed continuous variables and the Mann–Whitney U-tests for non-normally distributed continuous variables with Bonferroni correction for multiple testing. Univariable and multivariable logistic regression analyses of all potential predictors as independent variables and asthma control (well-controlled versus uncontrolled) as the dependent variable were performed. We manually removed predictors one by one based on the p-value (p > 0.20 based on the likelihood ratio test) and change in model fit (Akaike information criterion).25 The odds ratios (ORs) with 95% confidence intervals (CIs) were estimated. A p-value < 0.05 was considered statistically significant. In addition to backward selection, forward selection was conducted to evaluate whether the identified predictors were robust based on the sample. Both methods identified the same predictors. All analyses were performed in SPSS Statistics for Windows (Version 25.0. Armonk, NY: IMB Corp).
Results
Patients
The study population flowchart is displayed in Figure 1. A total of 1676 children aged 4 to 18 years were invited for the electronic portal and 1556 (93%) completed the screening questionnaire. Parents of 579 children reported their child to have asthma diagnosed by a physician. Asthma diagnosis was established by a pediatrician in 284 (49.1%) children, by a general practitioner in 235 (40.6%) children and by a pulmonologist in 60 (10.4%) children. Complete data on the ACT and all predictors were available in 408 of 579 children (70%). These children did not differ in the level of asthma control, the frequencies of atopic comorbidities and the frequencies of other predictors (data not shown). Only children with complete data were included in the analysis of asthma control.
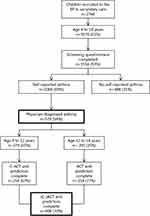 |
Figure 1 Study population flowchart. Abbreviations: (C-) ACT, (Child) Asthma Control Test; EP, electronic portal; n, number. |
Asthma Control in Children Referred for Asthma or Atopic Diseases Other Than Asthma
Clinical characteristics of 408 children with asthma are displayed in Table 1, stratified by the referral reason: 259 children (63%) with asthma were referred for asthma and 149 children (37%) with asthma were referred for atopic diseases other than asthma including food allergy, allergic rhinitis, atopic dermatitis or other. Children had a mean (SD) age of 10.6 (3.5) years and were predominantly Dutch (83%). Uncontrolled asthma (ACT score < 20) was found in 160 children (39%): in 121 of 259 children (47%) referred for asthma and in 39 of 149 children (26%) referred for atopic diseases other than asthma. Children referred for asthma were more likely to use daily inhaled corticosteroids compared to children referred for atopic diseases other than asthma (80% versus 56%, respectively), and were less likely to report at least on other atopic comorbidity (61% versus 89%, respectively). The proportion of atopic comorbidities in children in both referral groups is displayed in Figure 2. Almost 40% of children in both referral groups reported recurrent upper respiratory tract infections in the past year.
 |
Table 1 Clinical Characteristics of Children with Asthma |
 |
Figure 2 Venn diagrams displaying the proportion of atopic comorbidities among children with asthma stratified by referral reason (A and B). Data are n (%). |
Predictors of Asthma Control
Univariable logistic regression analyses showed that a family history of asthma, the use of daily inhaled corticosteroids in children who were considered non-adherent, recurrent upper and lower respiratory tract infections in the past year and a referral for asthma were associated with uncontrolled asthma (Table 2). In a multivariable logistic regression analysis, a family history of asthma (odds ratio [OR] 2.08; 95% CI 1.34 to 3.24), recurrent upper respiratory tract infections in the past year (OR 2.40; 95% CI 1.52 to 3.81), recurrent lower respiratory tract infections in the past year (OR 2.00; 95% CI 1.25 to 3.23) and a referral for asthma (OR 2.60; 95% CI 1.61 to 4.26) were independently and significantly associated with uncontrolled asthma (Table 2). Uncontrolled asthma was observed in 47% of children with a family history of asthma versus 32% of children without a family history of asthma, in 51% of children with recurrent upper RTIs versus 32% of children without recurrent upper RTIs and in 53% of children with recurrent lower RTIs versus 34% of children without recurrent lower RTIs. The full multivariable logistic regression analysis is presented in Supplemental Table 2. Atopic comorbidities, including food allergy, allergic rhinitis and atopic dermatitis, were not associated with uncontrolled asthma.
 |
Table 2 Univariable and Fitted Multivariable Logistic Regression Analysis with Uncontrolled Asthma (ACT Score <20) as the Outcome |
Discussion
Our study indicates that a high percentage of uncontrolled asthma is not only observed in children with asthma who were referred for asthma to secondary care (47%) but uncontrolled asthma is also highly prevalent in children with asthma who were referred for atopic diseases other than asthma (26%). The prevalence of uncontrolled asthma in our population of children with asthma referred for atopic diseases other than asthma is notably higher than the prevalence observed in children with asthma among the general population (15%).5 In addition, only 56% of the children with asthma referred for atopic diseases other than asthma were prescribed daily inhaled corticosteroids suggesting these children were inappropriately treated.26 Our results suggest that assessment of asthma control and optimization of asthma treatment are not only important for children referred for asthma but also for children primarily referred for allergic complaints other than asthma.
We found a family history of asthma, recurrent upper and lower respiratory tract infections in the past year to be independently associated with uncontrolled asthma. Children with a family history of asthma might have more frequent uncontrolled asthma due to increased asthma severity, as suggested in previous research.27 Furthermore, children with a family history of asthma might have more frequent uncontrolled asthma as parents with asthma may be less concerned about the asthma symptoms of their child as they are familiar with asthma symptoms themselves. Finally, recurrent upper and lower respiratory tract infections were associated with uncontrolled asthma. This observation is concordant with previous studies that showed that respiratory tract infections can have a profound effect on loss of control and asthma exacerbations in both children and adults.28 Furthermore, asthma attacks are often triggered by respiratory tract infections. Another explanation for the observed association between respiratory tract infections and uncontrolled asthma might be that asthma has been misdiagnosed.29 Previous studies indicate that parent-reported physician-diagnosed asthma in children is often not confirmed upon lung function testing and respiratory symptoms were due to, eg viral illnesses.29,30
Atopic comorbidities, ie food allergy, allergic rhinitis and atopic dermatitis, were not independently associated with asthma control in our population of children referred with respiratory or allergic complaints. Previous research in children with asthma reported an increased risk of uncontrolled asthma and increased asthma morbidity (ie hospitalization, use of controller medication) in children with asthma and food allergy6 or allergic rhinitis.4,5 We might not have detected an association between atopic comorbidities and asthma control because the atopic comorbidities were self-reported. Furthermore, most studies focused on an individual comorbidity and included additional relevant predictors associated with the comorbidity in the analysis, eg the season of the questionnaire, the severity of the atopic comorbidity or medication use. The sample size of our study was too small to consider all these additional relevant predictors per atopic comorbidity in the regression model. This may explain why the association was not found.
The major limitation of the present study is that all data were self-reported, lacking objective data such as lung function testing or specific IgE measurement. The diagnosis of asthma was based on a parent-reported physician-diagnosis and the diagnoses of atopic comorbidities were mainly based on the ISAAC questionnaire. Therefore, the prevalence of these diseases may have been overestimated31,32 or underestimated.33 In addition, we only included the children with complete data which may have caused selection bias. However, the children with complete data were comparable to the children with incomplete data in the frequencies of all predictors and the level of asthma control providing confidence that the children included in the study are a fair representation of the overall study population. Finally, we did not assess long-term asthma control and the frequency of asthma exacerbations, emergency department visits and hospitalizations. However, our study is strengthened by the inclusion of a large population of children with parent-reported physician-diagnosed asthma in eight secondary care centers in the Netherlands, rigorous data collection using several validated questionnaires in children and parents, as well as the assessment of asthma, atopic comorbidities and respiratory tract infections simultaneously.
Conclusions
In conclusion, this study demonstrates that assessment of asthma control is not only important for all children referred for asthma to secondary care but also for children with asthma who are referred for atopic diseases other than asthma. Special attention should be paid to children with asthma who have a family history of asthma, or have a history of recurrent upper and/or lower respiratory tract infections in the past year, as these predictors are associated with uncontrolled asthma.
Abbreviations
ACT, Asthma Control Test; C-ACT, Child Asthma Control Test; CI, Confidence interval; EP, electronic portal; ISAAC, International Study of Asthma and Allergies in Childhood; IQR, Interquartile range; MARS, Medication Adherence Rating Scale; OR, Odds ratio; PAQLQ, Pediatric Asthma Quality of Life Questionnaire; RAND, RAND (no abbreviation) general health-rating index; SD, Standard deviation.
Ethics Approval and Consent to Participate
The study has been approved by the Medical Ethics Committee of the University Medical Center Utrecht (No. 10/348). All parents and/or children gave informed consent.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank the members of the Expert Network P.F. Eskes (Department of Pediatrics, Meander Medical Center, Amersfoort, the Netherlands), R. van Gent (Department of Pediatrics, Máxima Medical Center, Veldhoven, the Netherlands; R. van Gent passed away on May 28, 2014) and A.G. Ketel (Department of Pediatrics, Spaarne Hospital, Hoofddorp, the Netherlands). Furthermore, the authors would like to thank P.M.J. Welsing (Department of Dermatology, University Medical Center Utrecht, Utrecht, the Netherlands) for his statistical advice and L.M. Verhagen (Department of Pediatrics, University Medical Center Utrecht, Wilhelmina Children’s Hospital, Utrecht, the Netherlands) for her help to define recurrent respiratory tract infections in children. An abstract of this paper was presented at the European Academy of Allergy and Clinical Immunology Congress, 2019, as a poster presentation with interim findings. The poster’s abstract was published in “Abstracts LB PDS” in Allergy (https://onlinelibrary.wiley.com/toc/13989995/2019/74/S106).
Author Contributions
HK substantially contributed to design, concept, acquisition of data, analysis and interpretation of data, and drafting the article. TL, FE and CE substantially contributed to design, interpretation of data, and drafting the article. BE, WB, DG, EV, MV and GS substantially contributed to acquisition of data and revising the article critically for important intellectual content. CU, YM and AK substantially contributed to interpretation of data and revising the article critically for important intellectual content. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Stichting Astma Bestrijding Nederland. The electronic portal was supported by an unrestricted grant of GlaxoSmithKline and ALK-Abelló.
Disclosure
Esther de Vries reports grants from Takeda, outside the submitted work. HM Kansen reports grants from Stichting Astma Bestrijding Nederland, GlaxoSmithKline, and ALK-Abéllo, during the conduct of the study. Cornelis van der Ent reports grants from GlaxoSmithKline during the conduct of the study. The authors have declared that they have no competing interests in relation to this study.
References
1. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–2235. doi:10.1056/NEJMra054308
2. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; 2018. Available from: https://ginasthma.org.
3. Rabe KF, Adachi M, Lai CKW, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114(1):40–47. doi:10.1016/j.jaci.2004.04.042
4. Oka A, Hirano T, Yamaji Y, et al. Determinants of incomplete asthma control in patients with allergic rhinitis and asthma. J Allergy Clin Immunol Pract. 2017;5(1):160–164. doi:10.1016/j.jaip.2016.08.002
5. Sasaki M, Yoshida K, Adachi Y, et al. Factors associated with asthma control in children: findings from a national web-based survey. Pediatr Allergy Immunol. 2014;25(8):804–809. doi:10.1111/pai.12316
6. Friedlander JL, Sheehan WJ, Baxi SN, Kopel LS. Food allergy and increased asthma morbidity in a school-based inner-city asthma study. J Allergy Clin Immunol Pract. 2013;1(5):479–484. doi:10.1016/j.jaip.2013.06.007
7. Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000;106(5):S201–S205. doi:10.1067/mai.2000.110151
8. Ballardini N, Kull I, Lind T, et al. Development and comorbidity of eczema, asthma and rhinitis to age 12 - data from the BAMSE birth cohort. Allergy. 2012;67(4):537–544. doi:10.1111/all.2012.67.issue-4
9. de Oliveira TB, Klering EA, da Veiga ABG. Is recurrent respiratory infection associated with allergic respiratory disease? J Asthma. 2019;56(2):160–166. doi:10.1080/02770903.2018.1445266
10. Tay TR, Hew M. Comorbid “treatable traits” in difficult asthma: current evidence and clinical evaluation. Allergy. 2018;73:1369–1382. doi:10.1111/all.13370
11. Simpson AB, Glutting J, Yousef E. Food allergy and asthma morbidity in children. Pediatr Pulmonol. 2007;42(6):489–495. doi:10.1002/(ISSN)1099-0496
12. Deliu M, Belgrave D, Simpson A, Murray CS, Kerry G, Custovic A. Impact of rhinitis on asthma severity in school-age children. Allergy Eur J Allergy Clin Immunol. 2014;69(11):1515–1521. doi:10.1111/all.2014.69.issue-11
13. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen). Allergy Eur J Allergy Clin Immunol. 2008;63(Suppl. 86):8–160.
14. Edwards MR, Strong K, Cameron A, Walton RP, Jackson DJ, Johnston SL. Viral infections in allergy and immunology: how allergic inflammation influences viral infections and illness. J Allergy Clin Immunol. 2017;140(4):909–920. doi:10.1016/j.jaci.2017.07.025
15. Franklin JM, Grunwell JR, Bruce AC, Smith RC, Fitzpatrick AM. Predictors of emergency department use in children with persistent asthma in metropolitan Atlanta, Georgia. Ann Allergy Asthma Immunol. 2017;119(2):129–136. doi:10.1016/j.anai.2017.04.008
16. Zomer-Kooijker K, van Erp FC, Balemans WAF, van Ewijk BE, van der Ent CK. The expert network and electronic portal for children with respiratory and allergic symptoms: rationale and design. BMC Pediatr. 2013;13(1):9. doi:10.1186/1471-2431-13-9
17. Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491. doi:10.1183/09031936.95.08030483
18. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi:10.1016/j.jaci.2003.09.008
19. Liu AH, Zeiger R, Sorkness C, et al. Development and cross-sectional validation of the childhood asthma control test. J Allergy Clin Immunol. 2007;119:817–825. doi:10.1016/j.jaci.2006.12.662
20. Raat H, Bueving HJ, De Jongste JC, Grol MH, Juniper EF, Van Der Wouden JC. Responsiveness, longitudinal- and cross-sectional construct validity of the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) in Dutch children with asthma. Qual Life Res. 2005;14(1):265–272. doi:10.1007/s11136-004-6551-4
21. Lewis CC, Pantell RH, Kiekhefer GM. Assessment of children’s health status: field test of new approaches. Med Care. 1989;27(3 Suppl):S54–S65. doi:10.1097/00005650-198903001-00005
22. Post M, Kuyenhoven M, Verheij M, de Melker R, The Dutch HA. “Rand general health rating index for children”: a questionnaire measuring the general health status of children. Ned Tijdschr Geneeskd. 1998;142(49):2680–2683.
23. Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: the medication adherence report scale for asthma. Ann Allergy Asthma Immunol. 2009;103(4):325–331. doi:10.1016/S1081-1206(10)60532-7
24. Koster ES, Raaijmakers JAM, Vijverberg SJH, Maitland van der Zee AH. Inhaled corticosteroid adherence in paediatric patients: the PACMAN cohort study. Pharmacoepidemiol Drug Saf. 2011;20:1064–1072. doi:10.1002/pds.2228
25. Hilbe JM. Logistic Regression. Berlin, Heidelberg: Springer; 2011.
26. Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. European Respiratory Journal. 2008;31:143–178. doi:10.1183/09031936.00138707
27. Zhao J, He Q, Zhang G, et al. Status of asthma control in children and the effect of parents’ knowledge, attitude, and practice (KAP) in China: a multicenter study. Ann Allergy Asthma Immunol. 2012;109(3):190–194. doi:10.1016/j.anai.2012.07.005
28. Busse WW, Lemanske RF
29. Yang CL, Simons E, Foty RG, Subbarao P, To T, Dell SD. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52(3):293–302. doi:10.1002/ppul.v52.3
30. Looijmans-van den Akker I, van Luijn K, Verheij T. Overdiagnosis of asthma in children in primary care: a retrospective analysis. Br J Gen Pract. 2016;66(644):e152–e157. doi:10.3399/bjgp16X683965
31. Hederos CA, Hasselgren M, Hedlin G, Bornehag CG. Comparison of clinically diagnosed asthma with parental assessment of children’s asthma in a questionnaire. Pediatr Allergy Immunol. 2007;18(2):135–141. doi:10.1111/j.1399-3038.2006.00474.x
32. Nwaru BI, Hickstein L, Panesar SS, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69(1):62–75. doi:10.1111/all.2014.69.issue-1
33. Van Gent R, Van Essen-zandvliet LEM, Rovers MM, Kimpen JLL, De Meere G, Van Der Ent CK. Poor perception of dyspnoea in children with undiagnosed asthma. Eur Respir J. 2007;30(5):887–891. doi:10.1183/09031936.00031407
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
