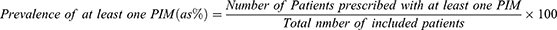Back to Journals » Clinical Interventions in Aging » Volume 17
Prevalence and Predictors of Potentially Inappropriate Medications Among Patients Aged ≥65 Years on Hospital Admissions in Kuwait
Authors Alshammari H, Al-Saeed E, Ahmed Z, Aslanpour Z
Received 2 March 2022
Accepted for publication 11 May 2022
Published 6 July 2022 Volume 2022:17 Pages 1025—1036
DOI https://doi.org/10.2147/CIA.S328693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Hesah Alshammari, Eman Al-Saeed, Zamzam Ahmed, Zoe Aslanpour
Department of Clinical and Pharmaceutical Sciences, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, UK
Correspondence: Hesah Alshammari, Department of Clinical and Pharmaceutical sciences, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, UK, Email [email protected]
Background: Potentially inappropriate medications are major health concerns for patients aged ≥ 65 years. To investigate the prevalence of potentially inappropriate medications, Beer’s criteria can be used. We estimated the prevalence of potentially inappropriate medications prescription among patients aged ≥ 65 years admitted to Kuwait’s largest hospital and identified the predictors of prescribing a potentially inappropriate medication.
Methods: A cross-sectional study was conducted retrospectively using inpatient records from the medical department at the Hospital in Kuwait from 1 January 2019 to 31 December 2019. The latest version of Beer’s criteria was used to identify potentially inappropriate medications in patients’ medical records. Data were analyzed descriptively to estimate the prevalence of potentially inappropriate medications and to describe participant characteristics. The predictors of potentially inappropriate medications prescribing were determined using binary logistic regression.
Results: A total of 423 medical records of patients were collected. The mean age of the patients admitted was 76 ± 7 years, and 222 of them (52.5%) were women. Upon hospital admission, potentially inappropriate medication was prevalent in 58.4% of patients. The most prevalent potentially inappropriate medications identified were proton pump inhibitors (27.3%), diuretics (21.5%), antipsychotic agents (9%), selective serotonin reuptake inhibitors (5%), and methyldopa (4%). Polypharmacy, Alzheimer’s disease, depression, irritable bowel syndrome, hypothyroidism, chronic kidney disease were predictors of potentially inappropriate medications prescription.
Conclusion: A high prevalence of potentially inappropriate medication prescription was observed among patients aged ≥ 65 years admitted to a hospital in Kuwait. The most likely predictor of potentially inappropriate medication prescription was polypharmacy.
Keywords: older people, potentially inappropriate medications, Kuwait, hospital, beers criteria
Introduction
With ageing, the burden of multimorbidity (ie the presence of at least two chronic conditions) increases.1 Multimorbid patients aged ≥65 years can present a challenge in terms of treatment because alterations in pharmacodynamic and pharmacokinetic properties due to aging may affect the fate of medicines in the body and, in turn, the clinical outcome. Several studies have documented the direct relationship between multimorbidity and polypharmacy, which is often defined as using ≥5 prescription medications on a regular basis.2,3 Polypharmacy can increase the likelihood of adverse drug events (ADEs) due to drug interactions, non-adherence to treatment, decline in cognitive function, falls, and increases in healthcare-related costs. in people aged ≥65 years.4,5 Furthermore, polypharmacy can be associated with prescription of potentially inappropriate medications (PIMs).
A PIM is defined as a medication that should be avoided in those aged ≥65 years because it is associated with a higher risk of adverse drug events (ADEs), and/or poor evidence of its efficacy with the availability of a safer or more efficacious alternative.6,7 It has been documented that a PIM is associated with ADE, functional decline, morbidity and mortality,8,9 and hospitalization.10
The prevalence of a PIM among patients aged 65 years and older is alarming, despite the availability of various screening tools, such as Beers Criteria (BC) and Screening Tool of Older Persons’ Prescriptions/Screening Tool to Alert to Right Treatment (STOPP/START). This has been documented in a large volume of research centered around investigating the prevalence of PIM prescribing.11–13 The settings, methodologies, and tools used to detect and investigate PIM prescription differ among studies. The reported prevalence of PIMs in hospitals in USA ranged from 40% to 87.4% using BC,12–14 whereas a lower prevalence has been reported in European hospitals using BC (22.7% to 43.3%).15,16
Fewer studies focusing on the prevalence of PIM prescription in the Middle East and North Africa (MENA) region have been published compared with those published in the USA and Europe. Review of the literature on the prevalence of PIM prescription among healthcare settings in the MENA region reveals the study sites in primary-care settings to be in Qatar,17,18 Oman,19 Jordan,20 Lebanon21 and Kuwait.22 The prevalence of PIM prescription in primary care in the MENA region ranged from 12.7% to 60.7%. This value may not reflect the prevalence of PIM prescription in hospitals because the complexity of medical conditions and variety of medications can influence the availability of specialist healthcare professionals (HCPs).
Four studies on the prevalence of PIM prescription were conducted in hospitals in the Gulf Cooperation Council (GCC), which are geographically near countries to Kuwait. Three of the studies were undertaken in different locations in Saudi Arabia.23–25 One study was carried out in a military hospital in Riyadh,23 one in a tertiary care hospital in Jeddah,24 and one in a tertiary hospital in Riyadh.25 The prevalence of PIM prescription reported in Saudi Arabia ranged from 43.6% to 80%. The fourth study was a nationwide study carried out in a geriatric hospital in Qatar, and the estimated prevalence of PIM prescription was 62.6%.26 Even though the GCC region has a health cooperation council, the practices in hospitals are different. Importantly, none of the studies were conducted in a general hospital.
The high prevalence of PIM that was reported in the GCC region necessitates investigating the prevalence of PIMs in hospitals in Kuwait. We aimed to estimate the prevalence of PIM prescription among patients aged ≥65 years admitted to the largest hospital in Kuwait and to identify the predictors of PIM prescription. Such a study has not been conducted previously in this setting.
Methods
Study Design
A cross-sectional retrospective study was conducted to estimate the prevalence of PIM prescription among people aged ≥65 years admitted to largest governmental hospital in Kuwait in terms of inpatient beds (945). Hospital admissions were selected because they better reflect the practice of prescribing to patients aged ≥65 years than that upon hospital discharge.
The MRs of patients were reviewed using the hospital information system (HIS). A researcher was trained on use of the HIS during 15–22 December 2019. A username and password to access the HIS was created for the researcher with a start date and end date. Only the patient profile, case view, laboratory tests, imaging, prescriptions, and clinic appointments were accessed. Access for medication orders, medication supplies, patient report requests, or medication stocks was not granted.
The literature was reviewed to establish which tool to use. The recent update of BC was chosen because it is updated regularly and is less reliant on data that may or may not be available in electronic and paper-based MRs.27
“Polypharmacy” was defined as a prescription with ≥5 medications. “Multimorbidity” was defined as the presence of ≥2 medical conditions. “Medication upon hospital admission” was defined as medication that was reconciled upon hospital admission by an HCP. “A single pill-combined medication” was defined as a tablet that consisted of ≥2 medications.
Sample Size
A statistician was consulted to calculate the appropriate sample size to estimate the prevalence of PIM prescription for patients aged ≥65 years, and the Cochran equation was used:30
where n = desired sample size, t = level of confidence, p = estimated prevalence of the characteristic of interest in the project area, and m = error margin. To calculate the maximum sample size needed, the prevalence was assumed to be 50%,29 with a 95% confidence interval (CI) and 5% margin of error. The sample size was calculated to be 385 MRs. To account for missing data or errors, an extra 10% was added to the sample size, thereby resulting in 428 MRs required to calculate the prevalence of PIM prescription upon hospital admission in Kuwait.
Study Population
A stratified sampling strategy was designed to ensure maximum variety of included MRs. We considered: (i) different days of the week (including weekdays and weekends); (ii) different months of the year (busy seasons versus quiet seasons). A matrix of the months of the year was developed and color-coded for busy seasons and quiet seasons.
Review of the MRs of patients aged ≥65 years focused on admission to a medical department through the emergency department or outpatient clinics from 1 January 2019 to 31 December 2019. Cancer patients and deceased patients after hospital admission were not included. Transfers from private hospitals were not included. Other departments in the hospital (eg, surgical department) were not included.
Data Collection
To ensure the extraction of homogenous, comparable, and complete data, a data-collection form (DCF) (Appendix 1) was developed. The researcher developed the DCF after reviewing the literature15,25,30,31 and reading BC 2019 thoroughly to meet the study objectives and to identify the information required to detect PIMs. The DCF had two sections: (i) extracting data from the MR of the patient; (ii) applying BC and identifying the number of PIMs and name and type of PIMs. Before data collection, the DCF was checked by the research team for content validity, and then piloted on 22 December 2019 using the MRs of 10 patients. Changes were not introduced to the DCF.
A computer-generated list of hospital admissions in a medical department from 1 January 2019 to 31 December 2019 was created by the Information Technology department. The list included the hospital-visit number and file number to facilitate the retrieval of MRs from the HIS. The list did not provide patient-identifiable information (eg, name, date of birth, civil identification number).
The list in the HIS included all the data required for the patient: file number, age, sex, nationality, allergies, medications on hospital admission (name, dose and frequency of the medication), creatinine clearance, date and reason for hospital admission, diagnosis, and medical history. The information was extracted from the HIS into the paper-based forms and number-coded for data confidentiality.
Management and Analyses of Data
Throughout the data-collection period (31 December 2019 to 23 January 2020), the data extraction from the MRs in the HIS was double-checked by the main researcher for completeness. Bias and the reliability of the extracted data were controlled through double-data extraction of 10% of DCFs by a clinical pharmacist working in the hospital. Paper-based DCFs were stored in a locked cabinet at the University of Hertfordshire (Hatfield, UK).
We detected PIMs by applying all categories of BC to the DCFs. Subsequently, the number and type of the identified PIM were transferred to the second part of the DCF. A member of the research team applied BC to a random 10% sample of cases. Disagreement with PIM detection based on BC was discussed until consensus was reached.
Then, the data were entered by the main researcher using SPSS 26 (IBM, Armonk, NY, USA). The SPSS file was saved in an encrypted drive to protect the data. A member of research team validated the data entry of a random 10% of cases.
Descriptive statistics were used to detail the characteristics of the study. Prevalence was calculated using the following equation:
Binary forward (conditional) logistic regression was employed to test the association between the prevalence of a PIM and independent variables (sex, nationality, medical conditions, polypharmacy, and number of comorbidities) to identify PIM predictors.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was followed in reporting of our findings.32
Ethical Approval of the Study Protocol
The study protocol was approved (LMS/PGR/UH/03951) by the University of Hertfordshire and Ministry of Health in Kuwait (2019/1154).
Results
The MRs of 496 patients were accessed. Then, 73 MRs were discarded (44 MRs were from patients transferred from private hospitals and 29 from deceased patients). Hence, 423 MRs of patients aged ≥65 years admitted to a medical department were included. The age (mean ± SD) of the included patients was 76 ± 7 (range, 65–98) years, and 222 (52.5%) were women. Most admissions to a medical department were of patients of Kuwaiti nationality (72.1%, n = 305) Table 1 summarizes patients characteristics.
 |
Table 1 Patient Characteristics and Relevant Clinical Information |
The mean number of multimorbidities was 4.77 ± 1.5. Hypertension was the most frequent presenting condition in included patients (99.1%), followed by type-2 diabetes mellitus (71.9%). Table 2. Describes the comorbidities of aged ≥65 years upon hospital admission.
 |
Table 2 Comorbidities of Study Participants |
The total number of prescribed medications upon hospital admission was 3626. Polypharmacy33 was present in 96.9% (n = 410) of cases. The maximum number of prescribed medications was 21 and the minimum was 3 (mean = 8.6). A single pill-combined medication was prescribed for 64 patients (15.1%) and 3 out of 64 patients were prescribed two single pill-combined medications.
Of the 3626 medications prescribed upon hospital admission, 462 (12.7%) were found to be a PIM. The prevalence of PIMs upon hospital admission was 58.4%. Table 3 summarizes the proportion of PIMs upon hospital admission.
 |
Table 3 Proportion of PIMs for Patients Aged ≥65 Years Upon Hospital Admission |
Based on BC, proton pump inhibitors were the most prescribed PIMs upon hospital admission, and should be avoided if no indication of use or is used for more than 8 weeks. The most prescribed PIM that should be used with caution were diuretics. The most prescribed PIMs in the category of “creatinine clearance” were antiepileptics and direct oral anticoagulants. Table 4 demonstrates the prevalence of PIMs using BC 2019.
 |
Table 4 Prevalence of PIMs Using BC 2019 |
Predictors of PIM Prescription
Seven variables were found to be associated significantly (P < 0.05) with prescribing a PIM (Table 5). Six variables were predictors, and one variable was a protective factor. Patients aged ≥65 years with Alzheimer’s disease (P = 0.001, odds ratio (OR) = 3.940, 95% CI = 1.771–8.763), depression (P = 0.030, OR = 10.742, 95% CI = 1.260–91.550), inflammatory bowel syndrome (P = 0.049, OR = 8.892, CI = 1.012–78.100), hypothyroidism (P = 0.028, OR = 1.982, 95% CI = 1.076–3.653), chronic kidney disease (CKD; P = 0.034, OR = 1.662, 95% CI = 1.038–2.661), or receiving polypharmacy (P < 0.001, OR = 1.624, 95% CI = 1.441–1.831) were more likely to have a PIM prescribed to them. However, patients aged ≥65 years diagnosed with type-1 diabetes mellitus (P = 0.033, OR = 0.349, 95% CI = 0.133–0.917) were less likely to be prescribed a PIM. Two variables were nearly significant variables: heart failure (P = 0.57) and deep-vein thrombosis (P = 0.51).
 |
Table 5 Predictors of PIM Prescription. Logistic Regression |
Discussion
To our knowledge, this is the first study to report PIM prescription among patients aged ≥65 years admitted to a Kuwaiti hospital. Using BC, the prevalence of PIM prescription was 58.4% in patients aged ≥65 years. The high prevalence of PIM prescriptions could be attributed to two main factors. First, the morbidity and complexity of caring for patients in hospitals and polypharmacy are linked to an increased risk of being prescribed a PIM. Second, guidelines in Kuwait have not been defined, so PIMs could be prescribed across different sectors and healthcare teams where patients are being treated or have access to healthcare.
Our findings are in accordance with those published in USA that utilised BC to report PIM.6,7 However, our findings are not consistent with studies conducted in European countries who reported a lower prevalence of PIM using BC.16,34–36 One study conducted in Ireland examined the association between the detected PIM and ADEs in patients aged ≥65 years in hospitals.16 That study revealed the prevalence of PIM prescriptions to be 28.8% by applying BC. This finding could be due to the limited suitability of BC in European practice as relevant tools are available specific to their context.
A comparable prevalence of PIM prescription has been reported in the GCC region.23–26 Alhawassi et al conducted a study in a hospital in Riyadh to estimate the prevalence of PIM prescription in patients aged ≥65 years. They retrieved data retrospectively using the electronic MRs of 4073 patients, and the prevalence was 57.6% using BC 2015.25 One limitation of that study was that only two categories of BC 2015 were examined to detect PIMs, which may have caused the prevalence of PIM prescription to be underestimated. In a study conducted in Qatar using two criteria from BC 2019, 62.6% of participants had been prescribed PIMs.26 The higher prevalence of PIM prescription found in Kuwait and geographically nearby areas should be explored to identify the causes and possible interventions to optimize patient care.
A literature review intended to identify the predictors of PIM prescription focused on 12 studies.37 These predictors were the number of medications, multiple prescribers, and multimorbidity, including anemia, heart failure, hypothyroidism, chronic lung disease, and depression. That review had limited generalizability because it focused only on studies conducted in the USA.37 According to our study, several predictors were associated with prescribing a PIM, with polypharmacy being the most likely predictor. In accordance with our results, studies have demonstrated that polypharmacy is strongly associated with prescribing a PIM regardless of the healthcare structure.22,25,38–40 Multimorbidity is strongly associated with polypharmacy, even though multimorbidity was not significant for PIM prescribing in our study. Comparison of our findings with those of other studies revealed a significant association between PIM prescription and polypharmacy, but not multimorbidity.22,40 Our findings also suggest hypothyroidism, chronic kidney disease, depression, irritable bowel syndrome, and Alzheimer’s disease to be significant variables and linked to prescribing a PIM.
Alhawassi et al utilized two categorizes of BC. They concluded that polypharmacy, hypertension, diabetes mellitus, dyslipidemia, heart failure, ischemic heart disease, chronic kidney disease, cancer, chronic obstructive pulmonary disease, osteoarthritis, osteoporosis, and anxiety were predictors of being prescribed a PIM.24 Dementia and depression were not considered to be risk factors to PIM use. Those observations further support the notion of context-specific predictors because they are sensitive to the location and setting of the study, as well as the type of tool used to detect PIMs. Therefore, predictors of PIM prescription reported in our study may aid the development of a screening tool targeting patients aged ≥65 years to minimize PIM prescriptions.
A high prevalence of PIMs in hospitalized patients upon hospital admission is alarming, and multistage clinical interventions are required to combat this problem. A possible intervention in Kuwaiti hospitals could be addition of geriatric wards and specialized geriatric services.41 Piau et al evaluated medication optimization in a real-life geriatric ward for 6 months, and concluded that 83% of patients in a geriatric ward had medication adjustment.42 That was an observational study in a geriatric ward reflecting the daily practice of optimizing medications, but it may have been influenced by the Hawthorne effect, and the latter can be reduced slightly by the study duration. One study in the UK demonstrated the benefits of a geriatric ward because the change of prevalence of PIM prescription was significant.43 In that study, 195 patients were assessed, and the prevalence of PIMs upon hospital admission was 26.7% (95% CI = 20.5–32.9; 52 patients; 74 PIMs) and upon hospital discharge was 22.6% (95% CI 16.7–28.5; 44 patients; 51 PIMs).43 Limited generalizability could be attributed to the small study cohort and the period for which data were collected.
Johansen et al measured the impact of hospitalization in geriatric wards on PIM prescription and medication use.44 They analyzed 715 records of prescribed medications before and after hospitalization from a prescription database and applied the European Union (EU)(7)-PIM list and Norwegian General Practice–Nursing Home (NORGEP–NH) list parts A and B. They revealed that the number of prescribed medications increased after hospitalization and found no improvement in PIM prescription.45 Those results could have been due to noncompliance with the EU(7)-PIM list and NORGEP–NH. One limitation that could have influenced measurement of PIM prescriptions before and after hospitalization was assessment of the appropriateness of PIMs because data were retrieved from a prescriptions database. In addition, a patient and his/her carer may have a role in abating the reduction of PIMs. Application of geriatric wards in Kuwait could yield different results if several aspects were considered, such as specialization in geriatric medicine, collaborative HCPs, and a PIM-detection tool.
One systematic review summarized interventions designed to reduce PIM prescriptions in patients aged ≥65 years: medication reviews, pharmaceutical interventions, computer-assisted interventions, educational interventions, and other interventions.46 One of these strategies is “deprescribing”, which is defined as a method for identifying, discontinuing, or reducing the doses of a medicine that may impose more harm than good (potential or actual).47 Deprescribing in hospital has been suggested to be feasible and efficacious in several systematic reviews. However, the limited number of studies published on deprescribing has reduced its importance in improving quality of life and mortality.48–50
Deprescribing PIMs in hospital has advantages and disadvantages. One advantage is that a patient is under the supervision of a team of HCPs. Therefore, withdrawal effects or changes in his/her homeostasis can be detected, which allows for timely interventions. Another advantage is the availability of a multidisciplinary team of physicians, clinical pharmacists, and nurses, which can contribute to better patient care. One disadvantage is the deteriorating physical status of patients aged ≥65 years. Despite evidence supporting the relationship between PIM prescription and hospital admission for patients aged ≥65 years, this may not be considered a priority for in-hospital deprescribing.51,52 Another disadvantage is the hospital culture and unfamiliarity with the idea of deprescribing rather than prescribing medications to manage healthcare conditions. An important disadvantage is a lack of willingness by the patient or caregiver to deprescribe53 because some caregivers believe that the positive effects of medications are more important than their side-effects.53 A direct approach based on highly prescribed PIMs and predictors of prescribing a PIM can be used to identify PIM prescriptions that might be susceptible to being deprescribed. This approach must be determined by the context because prescription predictors and prevalent PIMs between primary-care centers and hospitals are different.
Our study had several strengths. This is the first study estimating the prevalence of PIM prescription on hospital admissions in Kuwait. In addition, this is the first study in GCC to explore PIMs in a general hospital with no specific targeted population as in, for example, a military hospital, and in tertiary care. We utilized all categories of the recent update of BC, which is based on strong evidence. We applied a matrix to ensure that patients in quiet seasons and busy seasons of hospital admission were captured equally, thereby reducing the risk of a selection bias. Our study was conducted in the largest hospital in Kuwait, which provides a good insight in the prevalence of PIM prescription in Kuwait.
Our findings are subject to three main limitations. First, our study was conducted in a single center (though the hospital was the largest in terms of bed capacity and population served) in Kuwait. Second, PIMs were assessed at one point in the healthcare system: hospital admission. Comparing the prevalence of PIM prescription with medications prescribed upon hospital discharge might reflect the current practice of detecting and reducing PIMs while in hospital. Third, the prevalence of PIM prescription must be interpreted with caution due to the absence of information about previously used medications by patients.
Conclusions
We estimated the prevalence of PIM prescription upon hospital admission among patients aged ≥65 years to be 58.4%. The high prevalence of PIM highlights the need to put effective interventions in place. Polypharmacy and several chronic conditions were revealed to be predictors for prescribing PIMs, and they can be utilized to develop a screening tool in directed deprescribing.
Acknowledgment
We express our gratitude to Dr. Keith Sullivan for his guidance and support on statistical analyses.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work..
References
1. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. doi:10.1016/j.arr.2011.03.003
2. Almirall J, Fortin M. The coexistence of terms to describe the presence of multiple concurrent diseases. J Comorb. 2013;3:4–9. doi:10.15256/joc.2013.3.22
3. Sinnott C, Bradley CP. Multimorbidity or polypharmacy: two sides of the same coin? J Comorb. 2015;5(1):29–31. doi:10.15256/joc.2015.5.51
4. Maher RL, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. doi:10.1517/14740338.2013.827660
5. Wastesson JW, Morin L, Tan ECK, Johnell K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17(12):1185–1196. doi:10.1080/14740338.2018.1546841
6. Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–2724. doi:10.1001/archinte.163.22.2716
7. Zhang X, Zhou S, Pan K, et al. Potentially inappropriate medications in hospitalized older patients: a cross-sectional study using the Beers 2015 criteria versus the 2012 criteria. Clin Interv Aging. 2017;53:1697–1703. doi:10.2147/CIA.S146009
8. Bradley MC, Fahey T, Cahir C, et al. Potentially inappropriate prescribing and cost outcomes for older people: a cross-sectional study using the Northern Ireland enhanced prescribing database. Eur J Clin Pharmacol. 2012;68(10):1425–1433. doi:10.1007/s00228-012-1249-y
9. Passarelli MCG, Jacob-Filho W, Figueras A. Adverse drug reactions in an elderly hospitalised population. Drugs Aging. 2005;22(9):767–777. doi:10.2165/00002512-200522090-00005
10. Xing XX, Zhu C, Liang HY, et al. Associations between potentially inappropriate medications and adverse health outcomes in the elderly: a systematic review and meta-analysis. Ann Pharmacother. 2019;53(10):1005–1019. doi:10.1177/1060028019853069
11. Bhagavathula AS, Gebreyohannes EA, Fialova D. Prevalence of polypharmacy and risks of potentially inappropriate medication use in the older population in a developing country: a systematic review and meta-analysis. Gerontology. 2021;68(2):136–145. doi:10.1159/000516075
12. Alosaimy S, Vaidya A, Day K, Stern G. Effect of a pharmacist-driven medication management intervention among older adults in an inpatient setting. Drugs Aging. 2019;36(4):371–378. doi:10.1007/s40266-018-00634-9
13. Sheikh-Taha M, Dimassi H. Potentially inappropriate home medications among older patients with cardiovascular disease admitted to a cardiology service in USA. BMC Cardiovasc Disord. 2017;17(1):189. doi:10.1186/s12872-017-0623-1
14. Fick DM, Mion LC, Beers MH, Waller JL. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health. 2008;31(1):42–51. doi:10.1002/nur.20232
15. Gallagher P, Lang PO, Cherubini A, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol. 2011;67(11):1175. doi:10.1007/s00228-011-1061-0
16. Hamilton H, Gallagher P, Ryan C, Byrne S, O’Mahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Int Med. 2011;171(11):1013–1019.
17. Alhmoud E, Khalifa S, Bahi AA. Prevalence and predictors of potentially inappropriate medications among home care elderly patients in Qatar. Int J Clin Pharm. 2015;37(5):815–821. doi:10.1007/s11096-015-0125-0
18. Al-Dahshan A, Kehyayan V. Prevalence and predictors of potentially inappropriate medication prescription among older adults: a cross-sectional study in the state of Qatar. Drugs Real World Outcomes. 2020;8(1):95–103. doi:10.1007/s40801-020-00220-9
19. Al-Busaidi S, Al-Kharusi A, Al-Hinai M, et al. potentially inappropriate prescribing among elderly patients at a primary care clinic in Oman. J Cross Cult Gerontol. 2019;35(2):209–216. doi:10.1007/s10823-019-09393-5
20. Bulatova N, Elayeh E, Abdullah S, et al. Assessment of inappropriate medication use in Jordanian elderly hospitalized patients using 2015 beers criteria. Turk J Geriatr. 2019;22(3):258–268.
21. Saab YB, Hachem A, Sinno S, El-Moalem H. Inappropriate medication use in elderly Lebanese outpatients. Drugs Aging. 2006;23(9):743–752. doi:10.2165/00002512-200623090-00004
22. Awad A, Hanna O. Potentially inappropriate medication use among geriatric patients in primary care setting: a cross-sectional study using the Beers, STOPP, FORTA and MAI criteria. PLoS One. 2019;14(6):e0218174. doi:10.1371/journal.pone.0218174
23. Al‐Omar HA, Al‐Sultan MS, Abu‐Auda HS. Prescribing of potentially inappropriate medications among the elderly population in an ambulatory care setting in a Saudi military hospital: trend and cost. Geriatr Gerontol Int. 2013;13(3):616–621. doi:10.1111/j.1447-0594.2012.00951.x
24. Jastaniah NA, Almaqati AS, Alsuraihi AK, Abughanim SA, Aseeri M. Inappropriate prescribing in elderly inpatients at a university hospital in Saudi Arabia. Drugs Real World Outcomes. 2018;5(4):211–216. doi:10.1007/s40801-018-0142-0
25. Alhawassi TM, Alatawi W, Alwhaibi M. Prevalence of potentially inappropriate medications use among older adults and risk factors using the 2015 American Geriatrics Society Beers criteria. BMC Geriatr. 2019;19(1):154. doi:10.1186/s12877-019-1168-1
26. Alyazeedi A, Fouad Algendy A, Sharabash M, Karawia A. Prevalence, determinants and associated risk of potentially inappropriate prescribing for older adults in Qatar: a national retrospective study. Clin Interv Aging. 2019;14:1889–1899. doi:10.2147/CIA.S222532
27. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. doi:10.1111/jgs.15767
28. Cochran W. Sampling Techniques.
29. Niang L, Winn T, Rusli BN. Practical issues in calculating the sample size for prevalence studies. Med Stat. 2006;1:9–14.
30. Arellano C, Saldivia G, Córdova P, et al. Using two tools to identify potentially inappropriate medications (PIM) in elderly patients in Southern Chile. Arch Gerontol Geriatr. 2016;67:139–144. doi:10.1016/j.archger.2016.08.001
31. Kersten H, Hvidsten LT, Gløersen G, Wyller TB, Wang-Hansen MS. Clinical impact of potentially inappropriate medications during hospitalization of acutely ill older patients with multimorbidity. Scand J Prim Health Care. 2015;33(4):243–251. doi:10.3109/02813432.2015.1084766
32. Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi:10.1016/j.jclinepi.2007.11.008
33. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi:10.1186/s12877-017-0621-2
34. Damoiseaux-Volman BA, Raven K, Sent D, et al. Potentially inappropriate medications and their effect on falls during hospital admission. Age Ageing. 2021;51:afab205.
35. Tosato M, Landi F, Martone AM, et al. Potentially inappropriate drug use among hospitalised older adults: results from the CRIME study. Age Ageing. 2014;43(6):767–773. doi:10.1093/ageing/afu029
36. Dalleur O, Spinewine A, Henrard S, Losseau C, Speybroeck N, Boland B. Inappropriate prescribing and related hospital admissions in frail older persons according to the STOPP and START criteria. Drugs Aging. 2012;29(10):829–837. doi:10.1007/s40266-012-0016-1
37. Nothelle SK, Sharma R, Oakes AH, Jackson M, Segal JB. Determinants of potentially inappropriate medication use in long term and acute care settings: a systematic review. J Am Med Dir Assoc. 2017;18(9):
38. Abdulah R, Insani WN, Destiani D, Rohmaniasari N, Mohenathas N, Barliana MI. Polypharmacy leads to increased prevalence of potentially inappropriate medication in the Indonesian geriatric population visiting primary care facilities. Ther Clin Risk Manag. 2018;14:1591–1597. doi:10.2147/TCRM.S170475
39. Harugeri A, Joseph J, Parthasarathi G, Ramesh M, Guido S. Potentially inappropriate medication use in elderly patients: a study of prevalence and predictors in two teaching hospitals. J Postgrad Med. 2010;56(3):186–191. doi:10.4103/0022-3859.68642
40. Kondo N, Nakamura F, Yamazaki S, et al. Prescription of potentially inappropriate medications to elderly hemodialysis patients: prevalence and predictors. Nephrol Dial Transplant. 2015;30(3):498–505. doi:10.1093/ndt/gfu070
41. Moquaddam F, Al-Jeheidli A, Salmin N. General practitioner’s view of geriatric care in Kuwait. Kuwait Med J. 2005;37(4):251–256.
42. Piau A, Huet Y, Gallini A, Andre L, Vellas B, Nourhashemi F. Optimization of drug therapy in elderly individuals admitted to a geriatric unit. Clin Interv Aging. 2017;12:1691–1696. doi:10.2147/CIA.S132309
43. Onatade R, Auyeung V, Scutt G, Fernando J. Potentially Inappropriate prescribing in patients on admission and discharge from an older peoples’ unit of an acute UK hospital. Drugs Aging. 2013;30(9):729–737. doi:10.1007/s40266-013-0097-5
44. Johansen JS, Halvorsen KH, Svendsen K, Havnes K, Garcia BH. The impact of hospitalisation to geriatric wards on the use of medications and potentially inappropriate medications - a health register study. BMC Geriatr. 2020;20(1):190. doi:10.1186/s12877-020-01585-w
45. Santos NS, Marengo LL, Moraes FDS, Barberato-Filho S. Interventions to reduce the prescription of inappropriate medicines in older patients. Rev Saúde Pública. 2019;53:7. doi:10.11606/S1518-8787.2019053000781
46. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Int Med. 2015;175(5):827–834. doi:10.1001/jamainternmed.2015.0324
47. Thillainadesan J, Gnjidic D, Green S, Hilmer SN. Impact of deprescribing interventions in older hospitalised patients on prescribing and clinical outcomes: a systematic review of randomised trials. Drugs Aging. 2018;35(4):303–319. doi:10.1007/s40266-018-0536-4
48. Pruskowski JA, Springer S, Thorpe CT, Klein-Fedyshin M, Handler SM. Does deprescribing improve quality of life? A systematic review of the literature. Drugs Aging. 2019;36(12):1097–1110. doi:10.1007/s40266-019-00717-1
49. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Brit J Clin Pharmacol. 2016;82(3):583–623. doi:10.1111/bcp.12975
50. Wang P, Wang Q, Li F, Bian M, Yang K. Relationship Between potentially inappropriate medications and the risk of hospital readmission and death in hospitalized older patients. Clin Interv Aging. 2019;14:1871–1878. doi:10.2147/CIA.S218849
51. Varga S, Alcusky M, Keith SW, et al. Hospitalization rates during potentially inappropriate medication use in a large population‐based cohort of older adults. Br J Clin Pharmacol. 2017;83(11):2572–2580. doi:10.1111/bcp.13365
52. Scott S, Clark A, Farrow C, et al. Attitudinal predictors of older peoples’ and caregivers’ desire to deprescribe in hospital. BMC Geriatr. 2019;19(1):108. doi:10.1186/s12877-019-1127-x
53. Pohontsch NJ, Löffler A, Luck T, et al. Informal caregivers’ perspectives on health of and (potentially inappropriate) medication for (relatively) independent oldest-old people – a qualitative interview study. BMC Geriatr. 2018;18(1):169. doi:10.1186/s12877-018-0849-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.