Back to Journals » Research and Reports in Tropical Medicine » Volume 12
Prevalence and Intensity of Schistosoma mansoni Infection and Its Associated Risk Factors Among Patients with and without HIV at Chuahit Health Center, Dembia District, Northwest Ethiopia
Authors Kahisay M, Birhanie M, Derso A
Received 29 November 2020
Accepted for publication 3 February 2021
Published 16 February 2021 Volume 2021:12 Pages 25—32
DOI https://doi.org/10.2147/RRTM.S292899
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Mulubrhan Kahisay,1 Meseret Birhanie,2 Adane Derso2
1University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia; 2Department of Medical Parasitology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Adane Derso
University of Gondar, College of Medicine and Health Science, Gondar, 196, Ethiopia
Tel + 251-930716000
Email [email protected]
Background: Human Immunodeficiency Virus-1/AIDS and Schistosoma mansoni are widely spread in sub-Saharan Africa including Ethiopia and the co-infection is also prevalent, occurs commonly. Schistosoma mansoni infection has been suggested to be a risk factor for HIV transmission and progression. This study aims to assess the prevalence and intensity of Schistosoma mansoni infection and associated risk factors among individuals with and without human immunodeficiency virus (HIV) at Chuahit Health Center, West Dembia, Northwest Ethiopia.
Methods: Institutional based cross-sectional study was conducted from March to April 2019. Two hundred sixty-six study subjects were included in the study by using a systemic and convenient sampling technique. Pretested structured questionnaire was employed to collect data. Single stool samples were collected and examined for S. mansoni eggs. Finger prick and venous blood samples were collected for HIV-1 screening and viral load count. Data were analyzed using SPSS version 20. Independent t-test and one-way ANOVA were used to compare the mean of egg counts with HIV status and viral load counts, respectively. A P-value of less than 0.05 was taken as statistically significant.
Results: The overall prevalence and intensity of S. mansoni infection was 41 (15.4%) and 162.24 egg per gram of faeces (EPG), respectively. Prevalence of S. mansoni was higher in seronegative study participants though the difference is statistically insignificant. Higher intensity of infection was observed among seropositive study participants with high viral load counts (> 1000 copies/mL).
Conclusion: Relatively higher prevalence and intensity of S. mansoni infection were found. Study participants’ occupation was identified as potential risk factor to S. mansoni infection. Further studies are needed to know the impact of HIV on the prevalence and intensity of S. mansoni infection in the study area.
Keywords: Schistosoma mansoni, prevalence, intensity, viral load, Ethiopia
Background
Schistosomiasis is an acute and chronic parasitic disease caused by blood flukes of the genus Schistosoma. The species Schistosoma mansoni and Schistosoma haematobium are the etiological agents for the main human infections in sub-Saharan Africa1 and are endemic in 78 countries,2 whereas S. mansoni is endemic in 54 countries.3
According to the World Health Organization report, schistosomiasis is an infectious disease caused by parasitic worms that affects more than 230 million people worldwide, 90% of whom are in Africa.4 Moreover, in Sub Sahara Africa, it has been estimated that about 54 million are infected and 393 million individuals are at risk of infection due to S. mansoni.5 In Ethiopia, 37.3 million people are living in schistosomiasis endemic areas, comprising 3.4 million pre-school children, 12.3 million school-aged children, and 21.6 million adults.6
Various factors are responsible for the continuous and persistent transmission of schistosomiasis in sub-Saharan Africa. These include climatic changes and global warming, proximity to water bodies, irrigation and dam construction as well as socio-economic factors such as occupational activities and poverty.7
Globally, HIV-1 infections remain a major public health problem. In 2019, an estimated 38 million people were living with the disease and new cases of the disease were estimated to be 1.7 million.8 In Ethiopia, the first evidence of HIV epidemic was detected in 1984. Since then, Acquired Immunodeficiency Syndrome (AIDS) has claimed the lives of millions and has left hundreds of thousands orphans.9 According to Ethiopian Public Health Institute (EPHI), in 2018 HIV related estimations and projections indicated the national HIV prevalence was 0.96%.10
Intestinal parasites are endemic in many regions of the world where HIV/AIDS is also prevalent. Sub-Saharan Africa is among the regions where intestinal parasitic infections are entrenched11 and the largest burden of AIDS cases exist.12
The overlap of multiple risk factors associated with the two diseases in the same geographical setting or the biological interaction between HIV-1 and S. mansoni has been proposed to increase the risk of being coinfected with both HIV-1 and S. mansoni.13
Schistosomiasis also can alter the immune responses to HIV. The immunoregulatory responses associated with helminth infection downregulate the T-helper-1-type immune response associated with control of viral infections. HIV replicates more readily in the T-helper-2-type cells associated with helminth infections.14,15
Although Ethiopia is among the Sub-Saharan countries with a high rate of HIV and Schistosomiasis coinfection, most previous studies conducted on schistosomiasis were focusing on school-age children and other study groups but only a few studies reported prevalence and intensity of S. mansoni infection in individuals with and without HIV. Thus, the present study intended to assess the prevalence and intensity of S. mansoni infection and the possible associated risk factors among study participants who live with and without HIV.
Materials and Methods
Study Design, Period and Area
An Institutional based cross-sectional study was conducted among patients attending Chuahit Health Center, Dembia District, Northwest Ethiopia, from March to April 2019.
The study was conducted among patients attending Chuahit Health Center at west Dembia district in central Gondar zone, Northwest Ethiopia. Chuahit is located 62km from Gondar town and 789 km Northwest of Addis Ababa (Capital City of Ethiopia). Based on the 2007 census result, the district has a total population of 21,479. The area had an elevation of 1850 to 2000 meters above sea level. It is one of the four small rural towns found in Dembia District. Under its current administrative area, Chuahit comprises nine kebeles (the smallest administrative units of Ethiopia). The average temperature and humidity are 28°C and 22%, respectively.16
The topography of the districts shows mountains and plain land with lakes, springs, streams, and the four rivers namely Tanti Kura, Ambizina, Ambagenin, and Chigero, which are often used as source of water for drinking and cooking activities. Moreover, the communities use the water sources for swimming, bathing, washing clothes, and for recreational purpose around the river side. In prevention and control of the infection, health extension workers and other health professionals engaged in providing health education at schools, in the community through house to house, and at the health centers. Besides, both governmental and non-governmental organizations are working in providing potable water to the communities. Furthermore, mass treatment also administered since the area was one of most the endemic for STH and intestinal schistosomiasis in the region. In relation to ART program at the health center, tuberculosis positive patient’s treatment follow-up, antenatal and postnatal care, provider initiated counseling and testing (PICT), prevention mothers to child transmission (PMTC), and family planning have been provided.
Study Population and Sampling Technique
During the study period, patients who were clinically suspected to had intestinal schistosomiasis at ART Clinic and Outpatient department of Chuahit Health Center were considered as study population.
The sample size was determined by using double population proportion formula. By considering the previous study conducted in Malawi on the prevalence of schistosomiasis among individuals with and without HIV which accounted for 4.1% and 10.6%, respectively.17 95% confidence interval (Z=1.96), the value of β is 0.8, p is expected prevalence, So P1 is 10.6% and P2 is 4.1%, the minimum required sample size was 242. By considering a 10% non-response rate total sample size was 266, from this 133 were HIV seropositive individuals.
A systematic random sampling technique was used to select study participants from the Out Patient Department (OPD). By considering Chuahit Health Center’s annual plan of outpatient department, 6480 patients were anticipated to visit the health center. Based on the information 540 outpatients were expected to visit the health center monthly (K = N/n) K=540/133=4.1. So, every 4th patient who came to the OPD from March to April was included in the study until the required sample size was achieved. The other wing of study participants were HIV-positive individuals. A total of 956 ART clients were registered in the ART clinic. Based on this information and considering the inclusion criteria, a convenient sampling technique was used to select 133 study participants from 361 seropositive patients who visited ART clinic during the study period at Chauhit Health Center.
Inclusion and Exclusion Criteria
The study participants who were volunteer to provide stool and blood samples and who had no history of having been treated with Praziquantel (PZQ) and anti-helminthic drugs in the last four weeks prior to screening were included in this study and those who were not willing to give stool and blood samples were excluded from the study.
Data Collection and Processing
A pre-tested structured questionnaire was used to collect socio-demographic, behavioral, and environmental characteristics of study participants. After consent was signed, HIV screening was done for all study participants to confirm positivity. Clinical data were collected from seropositive individuals at the ART clinic. Finger prick and venous blood samples were collected for HIV-1 screening and viral load count, respectively. Single stool samples were provided by all study participants for examination.
HIV testing was carried out using rapid HIV-1 diagnostic test kits following manufacturer’s instructions. Results were then interpreted following the national algorithm for screening of HIV-1 infection that was adopted from WHO. In brief, the samples were first tested with HIV-1/2 STAT-PAK. If the result was found to be negative it will be taken as negative. If not, it was further being tested with SD BIOLINE HIV-1/2. If the result of SD BIOLINE HIV-1/2 was found positive, then the whole blood was considered as positive for HIV-1 antibodies, if not it will be tested for a third time with a tiebreaker, HIV 1/2/O Tri-line Human immunodeficiency virus rapid test, and it was reported as negative or positive depending on the result.
Stool Sample Collection and Processing
A labeled, clean, leak-proof empty container with unique identification (ID) numbers was distributed to the study participants and 5 grams of stool samples were collected. Each stool specimen was processed and it was examined by direct wet mount method using normal saline (0.85% NaCl solution) and formol ether concentration (FEC). The remaining portion of stool samples was processed for Kato Katz techniques.18 Slides were independently examined for the presence of S. mansoni eggs. The intensity of infection of S. mansoni was estimated by multiplying the total number of eggs counted by 24, which gives the EPG of stool. The intensity of infection was categorized based on WHO’s criteria as, light infection (1–99 EPG), moderate (100–399 EPG), and heavy (greater than 400 EPG).11
Viral Load Count
HIV RNA is most useful in measuring the effectiveness of ART after initiation. Study participants were under follow-up and as currently WHO recommends, viral load is better option for the treatment monitoring tool. In the present study, 4mL of blood sample was collected from each participant in a labeled plasma separator tubes for viral load analyses, viral load was quantified using the COBASR AmpliPrep/COBASR TaqManR HIV-1 Test (Roche Molecular Systems Inc., Pleasanton, California, USA). The test can quantitate HIV-1 RNA over the range of 20–10,000,000 copies/mL in EDTA plasma.19 Viral load counts which were below lower detection limit (for viral load counts less than 20 copies/mL) in this study were represented as NTD (Not Detected), low (≤1000 copies/mL), and high (>1000 copies/mL).20
Quality Control
The quality of the test result was maintained strictly by following laboratory standard operating procedures (SOP) and manufacturer’s instructions throughout the procedures. Specimen collection and laboratory tests were performed independently with two experienced senior laboratory technologists and 10% of the total slides were randomly selected and examined by a third experienced laboratory technologist working at the University of Gondar.
Data Management and Analysis
After the data were cleaned and checked for completeness, data were entered and analyzed by statistical package for social sciences (SPSS) statistical software version 20. Descriptive statistics like frequency, mean, and percentage was calculated to describe the study population characteristics. Study findings were explained in words, tables, and other statistical summary techniques. A binary logistic regression model was used to identify factors associated with infection of S. mansoni. An independent t-test was used to compare the intensity of S. mansoni infection and viral load count between study subjects with or without HIV. Multiple logistic regression was employed to control the possible effect of confounders, and finally, the variables having an independent association with dependent variables were identified based on the odds ratio (OR) with a 95% confidence interval (CI) and P-value less than 0.05.
Result
Socio-Demographic Characteristics of the Study Participants
A total of two hundred sixty-six (n= 266), one hundred thirty-three HIV seronegative, and 133 HIV seropositive, study participants were enrolled in the study. Female participants account for 158 (59.4%). The mean age of the study participants were 35.1 ± 11.5 years. The mean age was 38.7 (18–66 years) for HIV seropositive and 31.5 (18–87 years) for the HIV seronegative study participants. Majority of them 116 (43.6%) were between 18 and 30 years. As far as the educational level is concerned, 126 (47.4%) were illiterate, 65 (24.4%) had primary school education, and 75 (28.2%) attended secondary school/college. One hundred fifty-eight participants (59.4%) were married, and 77 (28.9%) of the participants were farmers (Tables 1 and 2).
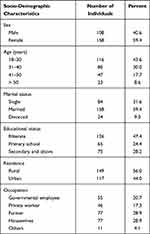 |
Table 1 Socio-Demographic Characteristics of Study Participants in Chuahit Health Center, West Dembia, Northwest Ethiopia, March to April 2019 |
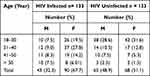 |
Table 2 Age and Sex Distribution of HIV Seropositive and HIV Seronegative Study Participants Attending Chuahit Health Center, West Dembia, March to April 2019 |
Prevalence and Intensity of S. mansoni Infection Among Study Participants
The prevalence of S. mansoni using the wet mount, FEC, and Kato-Katz was 32 (12.0%), 40 (15.0%), and 41 (15.4%), respectively. The overall prevalence of S. mansoni was 41 (15.4%). Eighteen (13.5%) were from HIV seropositive and 23 (17.3%) were from HIV seronegative study subjects. Statistically, there was no association between S. mansoni infections with individuals’ HIV status (P> 0.05). The peak prevalence of S. mansoni infection was found in 18–30 years of age (17.2%) followed by those whose age lies between 31 and 40 years (13.7%). The overall prevalence of infection was (23.1%) for male and (10.1%) for female participants (P < 0.05). Schistosoma mansoni infections were predominantly light (68.3%), (21.9%) moderate, and (9.8%) were a heavy. Similarly, from 18 HIV seropositive study participants 13 (72.2%), 3 (16.6%), and 2 (11.1%) had light, moderate and heavy intensity of infection, respectively.
The Intensity of S. mansoni Infection with Viral Load of Study Participants
Overall mean intensity of infection was 162.24 EPG (95% CI: 102.47–240.61) with parasitic load ranging from 24 to 1104 eggs per gram of faeces. The mean intensity of infection was also higher in males (196 EPG) than females (110 EPG) (P= 0.15). The mean intensity of infection was also numerically higher in the age group 18–30 years (387 EPG), though there is no significant difference between age groups (P =0.79).
HIV seropositive individuals had a slightly higher intensity of infection (172.22epg, 95% CI; 135.35–250.86) than HIV seronegative (154.43epg, 95% CI; 68.90–276.52) (P = 0.8) (Table 3). In the present study, study participants who had a viral load count below the lower detection limit and low viral load level had the lowest intensity of infection compared to those who have a high viral load count (Figure 1). Moreover, statistically, no difference was observed in the mean intensity of infection across viral load categories (P=0.2).
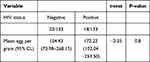 |
Table 3 HIV Status in Relation to the Mean Intensity of S. mansoni Infection Among Study Participants Attending Chuahit Health Center, West Dembia, Northwest Ethiopia, March to April 2019 |
 |
Figure 1 Intensity of S. mansoni infection mean plot in relation with viral load count, one way ANOVA. |
Risk Factor Analysis for S. mansoni Infection
In bivariate analysis, the prevalence of S. mansoni infection was associated with gender, occupational status, residence, and swimming habit (P< 0.05). After adjustment for significantly associated variables, farmers (odds ratio (OR) = 5.6; 95% CI 1.1 to 29.1), governmental employees (OR = 4.8; 95% CI 1.1 to 20.4), private work (OR = 11.6; 95% CI 2.2 to 61.6) and housewife (OR = 24.1; 95% CI 3.9 to 146.9) had a higher risk of parasitic infection than those study participants who had other jobs. But, the association of gender, residence, swimming habit and parasitic infection was insignificant (Table 4).
Discussion
This study determined the prevalence and intensity of S. mansoni infection and associated risk factors among HIV positive and negative individuals. The study also addressed whether the intensity of S. mansoni infection was associated with viral load.
The overall prevalence of S. mansoni was 15.4% among the study subjects. The present finding agreed with the study in Zimbabwe (18.1%).21 In contrast, a lower prevalence was found at Hawassa Teaching and Referral Hospital, Ethiopia,22 and at the 1° De Maio Health Centre in Maputo, Mozambique (1.3%).23 Compared to the current study, a higher rate of parasitic infection (31.4%) was found among individuals with and without HIV/AIDS in Wonji Shoa sugar estate residents24 in Ethiopia. The variation in study findings might be due to the difference in study subjects, study design, geographical location, and diagnostic methods used.
The prevalence of S. mansoni was found 13.5% and 17.3% in HIV seropositive and seronegative participants, respectively. Though the difference was statistically insignificant. These findings are similar to the study conducted in Ethiopia24 and other countries studies like; Mozambique, Zimbabwe, and Uganda.21,23,25 However, a cross-sectional study focusing on women on the Tanzanian shores of Lake Victoria26 and studies done by Brown and his colleagues found a positive association between S. mansoni infection and HIV infection.27 The lower prevalence of S. mansoni in seropositive individuals might be related to ART drugs, which affect the parasite clearance possibly due to the mitochondrial toxicity of the ART on the worms.28,29 Besides, other studies indicated that the colonization of the intestinal tract by parasites might have been influenced by HIV enteropathy, thereby causing both structural and functional impairment of the gut and thus making the luminal environment unfavorable for these parasites to thrive.30
In contrast, the overall parasitic intensity in HIV seropositive study participants was slightly higher (EPG= 172.2) than that of HIV seronegative (EPG=154.4), although there was not a statistically significant difference (P= 0.8) (Table 3). Similarly, a previous cohort study conducted in Tanzania showed that patients who had low CD4 counts (high viral counts) had high S. mansoni intensity of infection.31 It might be explained based on the impairment of the immune system in those patients, which is not allowing the clearance of the infection. HIV infection induces cellular depletion and early abnormalities of CD4+ T cells decreases CD8+ T-cell and function causes deterioration of specific antigen responses and leads to alteration of innate immunity through impairment of cytolytic activity and cytokine production by natural killer cells, all-important pathways to necessary resolve infection.29
In agreement with the present study, the study done by Per Kallestrup et al, in Zimbabwe showed that intensities of Schistosoma infections did not differ between HIV negative and HIV positive participants.21 However, this study was inconsistent with reports from previous similar studies in Ethiopia and Western Kenya,24,32 where individuals co-infected with HIV-1 and S. mansoni infections excreted fewer egg counts per gram of faeces than HIV negative individuals infected with S. mansoni. This study indicated that the efficiency of fecal egg excretion rate decreased with a decrease in immune system, to mean the immune system facilitated the excretion of schistosome eggs from patients co-infected with HIV. Complementary to these observations in human hosts, the CD4+ T-helper cells lymphocyte responses are thought to play a central role in excretion efficiencies of S. mansoni eggs, with decreasing CD4+ cell counts correlating with reduced excretion efficiencies of S. mansoni eggs in murine models.33–35
In our study, participants whose viral counts below the lower detection limit (LDL) (<20 copy/ul) were associated with higher parasitic prevalence (7.5%). Individuals with high viral load (>1000 copy/ul) had higher mean parasitic intensity (264.67 EPG). This difference may also reflect the impairment of both cell-mediated and humoral immunity by HIV with the consequent inability of the host to completely clear up the infection.29
Limitation of the Study
The present study used a single stool sample only. This might lead to under-estimate the prevalence and number of egg counts due to the intermittent release of eggs by the adult worms.
Conclusion
The overall prevalence of S. mansoni infection was relatively high. Statistically, no difference was observed in the prevalence of S. mansoni infection between HIV seronegative and positive individuals. Higher intensity of infection was found in seropositive individuals who had high viral load count (P<0.05) though statistically, the difference was insignificant between the two groups. Except for the occupation of study participants, none of the variables included in the present study showed statistically significant associations with S. mansoni infections.
Abbreviations
WHO, World Health Organization; S. mansoni, Schistosoma mansoni; EPG, egg per gram; HIV, human immunodeficiency virus; ART, antiretroviral therapy.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from the Research and Ethical Review Committee of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar (Ref.No.SBMLS/2177/11). Besides, a letter of support was also taken from the Clinical Director of Chuahit Health Center and the Head of ART Clinic. Informed consent was obtained from all study participants before testing and starting the study. A code identification number was given to each study subject. The diagnosis results remained confidential and necessary treatments were given by linking them with Chuahit Health Center.
Acknowledgments
We would like to thank the University of Gondar. We are grateful to all study participants, the Head of Chuahit Health center, and all staff members. Last but not least, our appreciation go to the data collectors.
Author Contributions
All authors made a significant contribution to this work in the conception, study design, execution, acquisition of data, analysis and interpretation; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the University of Gondar.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Organization WH. Preventive Chemotherapy in Human Helminthiasis. Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Program Managers. World Health Organization; 2006.
2. World Health Organization. Schistosomiasis: progress report 2001–2011, strategic plan 2012–2020. 2013.
3. Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77(1):41–51. doi:10.1016/S0001-706X(00)00122-4
4. Organization WH. Integrating Neglected Tropical Diseases into Global Health and Development: Fourth WHO Report on Neglected Tropical Diseases. World Health Organization; 2017.
5. Van der Werf MJ, de Vlas SJ, Brooker S, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86(2–3):125–139. doi:10.1016/S0001-706X(03)00029-9
6. Health FDRoEMo. Of Ethiopia National Master Plan for Neglected Tropical Diseases. Ethiopia: Federal Ministry of Health Ethiopia Addis Ababa; 2016.
7. Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Brazilian J Infectious Dis. 2015;19(2):196–205. doi:10.1016/j.bjid.2014.11.004
8. HIV/AIDS/factsheet/2020.
9. Ethiopia Ministry of Health. National Guidelines for Comprehensive HIV Prevention, Care, and Treatment. ETHIOPIA: FEDERAL MINISTRY OF HEALTH; 2017.
10. Ethiopia Ministry of Health. National Comprehensive HIV Prevention, Care, and Treatment Training for Pharmacy Professionals. Participant Manual. Ethiopia: Ministry of Health; May 2018
11. Schistosomiasis WECotCo, Organization WH. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis: Report of a WHO Expert Committee. Who; 2002.
12. UNAIDS/WHO. AIDS epidemic update. 2006.
13. Mazigo HD, Nuwaha F, Wilson S, et al. Epidemiology and interactions of human immunodeficiency Virus–1 and Schistosoma mansoni in sub-Saharan Africa. Infectious Diseases Poverty. 2013;2(1):2. doi:10.1186/2049-9957-2-2
14. Fairfax K, Nascimento M, Huang S-C-C, Everts B, Pearce EJ Th2 responses in schistosomiasis. Paper presented at Seminars in immunopathology 2012.
15. Turner JD, Jenkins GR, Hogg KG, et al. CD4+ CD25+ regulatory cells contribute to the regulation of colonic Th2 granulomatous pathology caused by schistosome infection. PLoS Negl Trop Dis. 2011;5(8):e1269. doi:10.1371/journal.pntd.0001269
16. Commission PC. Summary and statistical report of the 2007 population and housing census. Population Size by Age Sex. 2008.
17. Hosseinipour MC, Napravnik S, Joaki G, et al. HIV, and parasitic infection and the effect of treatment among adult outpatients in Malawi. J Infect Dis. 2007;195(9):1278–1282. doi:10.1086/513274
18. Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2. Cambridge university press; 2006.
19. Organization WH. Prequalification of Diagnostics Programme PUBLIC REPORT, COBAS® Ampliprep/COBAS® TaqMan® HIV-1 Test, Version 2.0 (Taqman 96); 2018.
20. Doherty M WHO guidelines on the use of CD4, viral load, and EID tests for initiation and monitoring of ART. World Health Organization; 2014. http://www.who.int/HIV/aids/102_WHO_Guidelines_on_CD4_and_VL_for_ART_Doherty.pdf.
21. Kallestrup P, Zinyama R, Gomo E, et al. Schistosomiasis and HIV-1 infection in rural Zimbabwe: implications of coinfection for excretion of eggs. J Infect Dis. 2005;191(8):1311–1320. doi:10.1086/428907
22. Assefa S, Erko B, Medhin G, Assefa Z, Shimelis T. Intestinal parasitic infections in relation to HIV/AIDS status, diarrhea, and CD4 T-cell count. BMC Infect Dis. 2009;9(1):155. doi:10.1186/1471-2334-9-155
23. Noormahomed EV, Tucuzo RM, Madureira AC. Prevalence of intestinal parasites among HIV infected and HIV uninfected patients treated at the 1º De Maio Health Centre in Maputo, Mozambique. EC Microbiol. 2017;9:231–240.
24. Fontanet A, Woldemichael T, Sahlu T, et al. Epidemiology of HIV and Schistosoma mansoni infections among sugar-estate residents in Ethiopia. Ann Trop Med Parasitol. 2000;94(2):145–155. doi:10.1080/00034983.2000.11813523
25. Sanya RE, Muhangi L, Nampijja M, et al. Schistosoma mansoni, and HIV infection in a Ugandan population with high HIV and helminth prevalence. Tropical Med Int Health. 2015;20(9):1201–1208. doi:10.1111/tmi.12545
26. Downs JA, Van Dam GJ, Changalucha JM, et al. Association of Schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg. 2012;87(5):868–873. doi:10.4269/ajtmh.2012.12-0395
27. Brown M, Mawa P, Kaleebu P, Elliott A. Helminths, and HIV infection: epidemiological observations on immunological hypotheses. Parasite Immunol. 2006;28(11):613–623. doi:10.1111/j.1365-3024.2006.00904.x
28. Kiros H, Nibret E, Munshea A, Kerisew B, Adal M. Prevalence of intestinal protozoan infections among individuals living with HIV/AIDS at felegehiwot referral hospital, Bahir Dar, Ethiopia. Int j Infectious Dis. 2015;35:80–86. doi:10.1016/j.ijid.2015.04.012
29. Janssen S, Hermans S, Knap M, et al. Impact of anti-retroviral treatment and cotrimoxazole prophylaxis on helminth infections in HIV-Infected patients in Lambaréné, Gabon. PLoS Negl Trop Dis. 2015;9(5):e0003769. doi:10.1371/journal.pntd.0003769
30. Conlon CP, Pinching AJ, Perera CU, Moody A, Luo NP, Lucas SB. HIV-related enteropathy in Zambia: a clinical, microbiological, and histological study. Am J Trop Med Hyg. 1990;42(1):83–88. doi:10.4269/ajtmh.1990.42.83
31. Mazigo HD, Dunne DW, Wilson S, et al. Co-infection with Schistosoma mansoni and Human Immunodeficiency Virus-1 (HIV-1) among residents of fishing villages of north-western Tanzania. Parasit Vectors. 2014;7(1):587. doi:10.1186/s13071-014-0587-2
32. Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya: i. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg. 1997;56(5):515–521. doi:10.4269/ajtmh.1997.56.515
33. Doenhoff M, Bain J. The immune-dependence of schistosomicidal chemotherapy: relative lack of efficacy of an antimonial in Schistosoma mansoni-infected mice deprived of their T-cells and the demonstration of drug-antiserum synergy. Clin Exp Immunol. 1978;33(2):232.
34. Dunne D, Hassounah O, Musallam R, et al. Mechanisms of Schistosoma mansoni egg excretion: parasitological observations in immunosuppressed mice reconstituted with immune serum. Parasite Immunol. 1983;5(1):47–60. doi:10.1111/j.1365-3024.1983.tb00722.x
35. Harrison R, Doenhoff M. Retarded development of Schistosoma mansoni in immunosuppressed mice. Parasitology. 1983;86(3):429–438. doi:10.1017/S0031182000050629
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

