Back to Journals » Cancer Management and Research » Volume 12
Prevalence and Genotype Distribution of High-Risk Human Papillomavirus Infection in Women with Abnormal Cervical Cytology: A Population-Based Study in Shanxi Province, China
Authors Song L , Lyu Y, Ding L, Li X, Gao W, Wang M, Hao M, Wang Z , Wang J
Received 23 June 2020
Accepted for publication 21 October 2020
Published 8 December 2020 Volume 2020:12 Pages 12583—12591
DOI https://doi.org/10.2147/CMAR.S269050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ahmet Emre Eşkazan
Li Song,1,* Yuanjing Lyu,1,* Ling Ding,1 Xiaoxue Li,1 Wen Gao,1 Ming Wang,1 Min Hao,2 Zhilian Wang,2 Jintao Wang1
1Department of Epidemiology, School of Public Health, Shanxi Medical University, Taiyuan 030000, People’s Republic of China; 2Department of Obstetrics and Gynecology, Second Hospital of Shanxi Medical University, Taiyuan 030000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jintao Wang
Department of Epidemiology, School of Public Health, Shanxi Medical University, 56 Xinjian Nan Road, Taiyuan 030000, People’s Republic of China
Tel +86 0351-413-5245
Email [email protected]
Purpose: High-risk human papillomavirus (HR-HPV) infection is widely known as the major cause of cervical cancer and there are notable differences in HR-HPV prevalence and genotype distribution in different populations. Women with abnormal cervical cytology are at increased risk of cervical cancer; however, the genotype distribution of HR-HPV in women with abnormal cervical cytology remains unclear.
Methods: A total of 2,300 women with abnormal cervical cytology (from 39,988 women completing a baseline survey in a cohort established during June 2014 to December 2014) were enrolled in this study. All participants gave informed consent and completed a questionnaire about characteristics related to HPV infection. HPV genotypes were identified using flow-through hybridization, and cytology was assessed by the ThinPrep cytological test. Data were analyzed using SPSS 22.0 for Windows.
Results: The overall prevalence of HR-HPV in the 2,300 women with abnormal cervical cytology was 32%, with single and multiple HR-HPV infections making up 70.2% and 29.8%, respectively. The top-five HR-HPV genotypes were HPV16 (13.5%), HPV58 (5.7%), HPV52 (4.9%), HPV53 (2.5%), and HPV51 (2.3%). The prevalence of HR-HPV in atypical squamous cells of undetermined significance, low-grade squamous intraepithelial lesions, and high-grade squamous intraepithelial lesions or higher was 30.8%, 36.5%, and 54.9%, respectively, showing an increasing trend with severity of cervical cytology (χ2trend=13.952, p< 0.001). The prevalence of HPV16 and HPV33 increased significantly with the degree of cytological abnormality. HR-HPV infection risk was statistically higher in women aged 35– 45 years, with low education, infrequent bathing, multiple gravidity, multiple parity, history of gynecological diseases, and premenopause.
Conclusion: HR-HPV infection in women with abnormal cervical cytology was 32%, and the top-five HR-HPV genotypes were HPV16, HPV58, HPV52, HPV53, and HPV51 in Shanxi Province, China. These results shed light on demographic and behavioral characteristics related to HR-HPV infection in women with abnormal cervical cytology and provide an insight for the development of HPV vaccines.
Keywords: cervical cancer, high-risk human papillomavirus, genotype distribution, abnormal cervical cytology
Introduction
Human papillomavirus (HPV) infection has been identified as a definite human carcinogen,1 and accounts for >50% of infection-linked cancers in females.2 Cervical cancer ranks as the fourth-commonest cancer among women worldwide, with an estimated 570,000 new cases and 311,000 deaths in 2018.3 Annually, there are 102,000 new cases and 31,000 deaths in China, and the incidence of cervical cancer shows a substantially increasing trend.4 Shanxi Province is a high-incidence area of cervical cancer in China.5 High-risk HPV (HR-HPV) has been identified as an etiological factor in cervical cancer and precancerous lesions.6
To date, >100 HPV genotypes have been identified,7 of which approximately 40 are associated with genital tract infection.8 Among the numerous members of the HPV family, 15 HR-HPV genotypes (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) are etiologically associated with >98% of cervical cancers. Effective prophylactic vaccines against the most important carcinogenic HPV types are available, but they do not cover the main types of HR-HPV in different countries and regions.9 Moreover, prevalence and genotype distribution of HPV vary substantially by ethnicity, demographic and behavioral characteristics, and health status.10–12 However, few studies have been reported on women with abnormal cervical cytology.
Cervical cytology testing is used as a diagnostic aid for earlier detection of cervical cancer.13 Studies have found that incidence of cervical intraepithelial neoplasia (CIN) 2–3 and cervical cancer in women with smears revealing ASC-H (atypical squamous cells — cannot exclude high-grade squamous intraepithelial lesions) was 56.5% and 9.3%, respectively,14 and the incidence of CIN 2–3 and cervical cancer in women with high-grade squamous intraepithelial lesion (HSIL) smears was 62.9% and 25.8%.15 HPV-positive women with atypical squamous cells of undetermined significance (ASC-US)/low-grade squamous intraepithelial lesion (LSIL) cytology had a 5–year CIN3+ risk of 22.2%, while for those with normal cytology, this risk was 7.9%.16 These studies clearly demonstrated that women with abnormal cervical cytology had high rates of significant cervical lesions, particularly invasive cervical disease. Many studies have suggested that HR-HPV infection has a high probability of transferring persistent infection and subsequently leading to CIN and cervical cancer17 and that abnormal cervical cytology represents an early lesion of CIN and cervical cancer. It is obviously important to understand the major distribution characteristics and genotypes of HR-HPV in women with abnormal cervical cytology, in order to implement targeted measures to control the persistence of HR-HPV infection and reduce the risk of malignant progression in high-risk women.
The prevalence of HPV infection in women with abnormal cervical cytology is 47.0%–72.8%.18,19 HPV prevalence increases with increasing severity of cervical lesions: from 12% in normal cytology to 89% in invasive cervical cancer.20 The prevalence of HR-HPV in women with abnormal cervical cytology is 92.4%, significantly higher than those with normal cytology (71%).21 In Shanxi Province in China, the prevalence of HR-HPV in normal pathology, CIN1, and CIN2+ is 11.1%, 72.5%, and 91.4%, respectively.22 However, the distribution of HR-HPV infection varies in different countries and regions, and demographics as well as behavioral characteristics related to HR-HPV infection in women with cervical abnormalities are poorly understood. The primary aim of this study was to describe the prevalence and genotype distribution of HR-HPV infection in women with abnormal cervical cytology in Shanxi to provide a foundation for preventing and controlling cervical lesions.
Methods
Study Population
This study was based on data obtained from a baseline survey of a cohort established during June 2014 to December 2014 in Shanxi. The present survey was conducted in two counties (Jiexiu and Yangqv) of Shanxi. A woman was considered eligible to enter the study if she had resided in Shanxi for at least 1 year, had current or past sexual activity, was not pregnant, and had no history of cervical cancer, precancerous lesions, cervical treatments (eg, loop electrosurgical excision procedure, conization, and adnexectomy), or injected HPV vaccine and agreed to participate in the present study. A total of 39,988 women aged 19–65 years were included. All participants completed a demographic and behavioral characteristic–related questionnaire, medical examination, and ThinPrep cytological test (TCT). Of these, 37,219 were negative for intraepithelial lesion or malignancy and 2,769 showed abnormal cervical cytology: 2,305 with ASC-US, 82 with ASC-H, 316 with LSIL, 54 with HSIL, ten with squamous cervical carcinoma, and two with atypical glandular cells. Following that, 2,769 participants with abnormal cervical cytology were referred for HPV testing. Of those, 68 refused, 387 received inadequate investigation, eight had not fully completed medical examinations, and six specimens were not suitable for detection. Finally, 2,300 women were included in the present study (Figure 1). Informed consent was signed by all participants, and the study was approved by the Institutional Review Board of Shanxi Medical University (2013–003).
 |
Figure 1 Flowchart of participants in the study. ASC-US+, atypical squamous cells of undetermined significance and above. |
Data and Sample Collection
Details of demographic characteristics, history of gynecological diseases (including vaginitis, pelvic inflammation disease, uterine fibroids, hyperplasia endometria, and ovarian tumors), lifestyle habits, and sexual and reproductive history were collected by trained interviewers using a structured questionnaire. A sterile cotton swab was used to wipe a secretion specimen from the cervix, then a TCT-specimen brush was inserted into the cervical canal to a depth of approximately 1 cm to the junction of the squamous epithelium and columnar epithelium at the outer cervix and rotated clockwise for three to five circles. Next, the TCT-specimen brush was put into cell-preservation solution. The specimens were then subjected to TCT by an experienced cytologist. Afterward, cervical specimens were collected from the cervix using an HPV-testing brush and plugged into a vial containing transport medium. Eventually, samples were processed for HPV genotyping within 2 days.
ThinPrep Cytological Test
Exfoliated cervical cells were obtained from each participant using a TCT-specimen brush. All liquid-based cytology specimens were independently diagnosed by two experienced cytopathologists. If the diagnosis differed between the two cytopathologists, the sample was reviewed by a third cytopathologist and consensus obtained. Cytology results were diagnosed according to the 2001 Bethesda system and classified as negative for intraepithelial lesion or malignancy, ASC-US, ASC-H, LSIL, HSIL, squamous cervical carcinoma, and atypical glandular cells. A diagnosis of ASC-US or higher was considered cytological abnormality.
HR-HPV Detection
HPV detection and genotyping were tested using a HybriMax HPV GenoArray kit (Hybribio Biotechnology, Chaozhou, China) according to the manufacturer’s instructions. In sum, 21 types of HPV — 15 HR-HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66 and 68) and six low-risk HPV types (6, 11, 42, 43, 44, and CP8304) — were identified by flow-through hybridization using a TC96/G/H6 HPV DNA–amplification analyzer and an HMM2 fast nucleic acid molecule–hybridization instrument (HybriBio). Briefly, PCR was performed in a 25 μL reaction mixture containing 5 μL extracted DNA, 0.75 μL DNA Taq polymerase and 19.25 μL PCR-mix solution containing MY09/11 primer system. The PCR protocol was denaturion at 95°C for 9 minutes, followed by 40 cycles of 20 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 72°C, and finally an extension at 72°C for 5 minutes. Positive and negative controls were run in each PCR process. HPV-genotype results were determined by the position of the HPV-genotype probes on the microarray chip. With addition of NBT/BCIP solution to display the results, a positive result was indicated by a clearly visible indigo dot. Multiple dots indicated multiple infections. Participants with any of the HR-HPV–positive genotypes were regarded as HR-HPV infected. Single HR-HPV infection was defined as only one genotype of HR-HPV in a sample being detected, while multiple HR-HPV infections were two or more HR-HPV types being detected.
Statistical Analyses
Statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY, USA) for Windows. Numerical data were examined by χ2 tests, and ORs and 95% CIs were calculated using unconditional logistic regression. Graphics were constructed using GraphPad Prism 7.0 (GraphPad Software). All p-values were two-sided, and statistical significance was defined as p<0.05.
Results
Demographic Characteristics of Participants
The demographic characteristics of the study population are summarized in Table 1. Ultimately, a total of 2,300 females aged 19–65 (50±13) years were evaluated. Most patients (63.6%) had received education to junior high school and below, and only 838 (36.4%) to senior high school and above. Nearly 1,525 (66.3%) were housewives or farmers. Only 96 (4.2%) respondents were divorced, widowed, or separated. In this survey, 1877(81.6%) participants’ yearly income were <¥10,000.
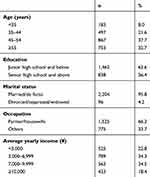 |
Table 1 Demographic Characteristics of the 2,300 Participants |
Distribution of HR-HPV Genotypes
A total of 736 of the 2,300 (32%) women had HR-HPV infection, with the top-five types being HPV16 (13.5%), HPV58 (5.7%), HPV52 (4.9%), HPV53 (2.5%), and HPV51 (2.3%). Prevalence of single and multiple-type HR-HPV infection was 22.5% (517 of 2,300) and 9.5% (219 of 2,300), with corresponding proportions of 70.2% (517 of 736) and 29.8% (219 of 736), respectively, in infected women. Among those with multiple infections, infection of two genotypes was most commonly observed — 76.7% (168 of 219). In addition, we found that HPV16, HPV58, and HPV39 occurred mainly as a single infection in all populations. The details are shown in Figure 2.
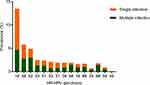 |
Figure 2 Genotype distribution of HR-HPV infection in women with abnormal cervical cytology. |
Demographic and Behavioral Characteristics Related to HR-HPV Infection
Age-specific prevalence of HR-HPV infection exhibited a peak of 46.3% (230 of 497) in the age-group 35–45 years and was lowest (23.9%, 180 of 753) in those aged 55–65 years. Age-group 35–45 years, low education, less frequent bathing, multiple gravidity (number of pregnancies), multiple parity (number of deliveries), history of gynecological diseases, and premenopause placed women at high risk of HR-HPV infection (Table 2), mainly for HPV16, HPV58, and HPV52. Of note, we found high infection rates for HPV16 and HPV51 in those <35 years old. No significant association was found between occupation, marital status, average annual income, or condom use and HR-HPV infection.
 |
Table 2 Associations Between Demographic/Behavioral Characteristics and HR-HPV Infection in Women with Abnormal Cervical Cytology |
Distribution of HR-HPV Genotypes in Various Abnormal Cervical Cytology Groups
Among the 2,300 women with abnormal cervical cytology, ASC-US, LSIL, and HSIL+ accounted for 86.5%, 11.3%, and 2.2%, respectively. The prevalence of HR-HPV in ASC-US, LSIL, and HSIL+ was 30.8% (613 of 1,989), 36.5% (95 of 260), and 54.9% (28 of 51), respectively, showing an increasing trend with severity of cervical cytology (χ2trend=13.952, p<0.001), particularly HPV16 and HPV33. The top-five HR-HPV types were HPV16 (13.1%), HPV58 (5.5%), HPV52 (4.9%), HPV53 (2.6%), and HPV51 (2.4%) in ASC-US, HPV16 (12.3%), HPV58 (8.1%), HPV52 (5.8%), HPV33 (3.1%), and HPV18 (2.7%) in LSIL, and HPV16 (33.3%), HPV33 (7.8%), HPV31 (3.9%), HPV56 (3.9%), and HPV58 (2.0%) in HSIL+ (Table 3).
 |
Table 3 HR-HPV Prevalence and Genotype Distribution Related to Cervical Cytology Status |
Discussion
In the present study, HR-HPV prevalence was 32% in women with abnormal cervical cytology, higher than population-based prevalence observed in Shanxi (15.2%).22 Castellsagué et al23 reported that HPV16, HPV18, HPV31, HPV58, and HPV52 were the top-five HR-HPV types in both normal and abnormal cervical cytology, according to estimates from 193 countries. Hernan et al24 found that HPV16, HPV53, HPV52, HPV58, and HPV59 were the top-five genotypes in women with abnormal cervical cytology from Bogotá, Colombia. Our findings indicated that the top-five HR-HPV genotypes were HPV16, HPV58, HPV52, HPV53, and HPV51 in women with abnormal cervical cytology in Shanxi, while HPV18 was in 12th position. The distribution of HR-HPV genotypes varies greatly worldwide, and these differences might be related to the complex geographical and biological interplay among different HPV genotypes and host immunogenetic factors.
Our results showed that the proportions of single and multiple HR-HPV infections were 70.2% and 29.8%, respectively. HPV16, HPV58, and HPV39 occur mainly as a single infection in all populations. A study conducted in Cyprus also showed that single HR-HPV infection accounted for 77.2% and multiple HR-HPV infections 22.8% in women with both abnormal cervical cytology and HR-HPV.19 There are currently three prophylactic vaccines targeting various HPV types: the bivalent vaccine (HPV16/18), the quadrivalent vaccine (HPV16/18/6/11) and the nine-valent vaccine (HPV16/18/6/11/31/33/45/52/58),25 but they do not cover the most frequent HR-HPV genotypes in Shanxi, such as HPV53 and HPV51, from our present study. Therefore, further work will be needed to develop next-generation vaccines that can target more HR-HPV types.
Li et al26 reported that HR-HPV prevalence presented two age peaks in the general Chinese population: one at age 15–24 years and the other in women aged 35–49 years. HR-HPV infection is a sexually transmitted disease, and the higher rate of infection in these women might point to more frequent sexual intercourse. As such, cervical cells might be more vulnerable to damage and HR-HPV infection. In our study, the prevalence of HR-HPV was much higher in those aged 35–45 years abnormal cervical cytology. These results were inconsistent with those for all age-groups, suggesting that women aged 35–45 years should be regarded as a high-risk group and a focus of prevention among women with abnormal cervical cytology. Additionally, we observed that HPV16, HPV58, and HPV52 presented the highest prevalence in women aged 35–45 years, suggesting that HPV16, HPV58, and HPV52 infection in this region should be given attention. Moreover, the nine-valent vaccine appears to be more valuable than the quadrivalent vaccine in women with abnormal cervical cytology.
Remarkably, it should be noted that the HR-HPV infection rate decreased with increasing education, the same as in a study conducted in Yunnan Province, China.27 Women with junior high school education and below had limited knowledge about cervical cancer and its prevention. It will be necessary to improve the education of women to prevent HR-HPV infection and cervical lesions. Our findings provide evidence that women bathing less frequently were more vulnerable to HR-HPV infection. Bathing infrequently results in increasing viral or bacterial reproduction, which can be curbed by frequently cleaning the lower genital tract.28
We found that the prevalence of HR-HPV significantly increased with increasing gravidity and parity, which might because of the hormonal changes of pregnancy. The female sex hormones — estrogen and progesterone — might influence susceptibility to HR-HPV infection. Changes in susceptibility to HR-HPV might also be the result of hormone-induced changes in host adaptive immunoresponse.29 Our previous studies have shown that menopause was a protective factor in HR-HPV infection, which could be ascribed to the decreased estrogen level of postmenopausal women.30–32 These results indicated that HPV DNA testing and effective HPV vaccines should be implemented in premenopausal women. Studies have reported that women with gynecological diseases were prone to HR-HPV infection, due to unstable sexual hormone levels, poor function of the immune system, lower resistance to HR-HPV, and weakviral clearance.33,34
Our findings indicated that HPV16 is the most frequently identified type in women with ASC-US, LSIL, and HSIL+ in Shanxi, confirming that HPV16 is the main HR-HPV type associated with cervical lesions. Furthermore, we found that infection rates of HPV16 and HPV33 increased significantly with degree of cytological abnormality. Our analyses suggest that HPV16 and HPV33 infection may be biomarkers to predict cervical lesion progression in women with abnormal cervical cytology.
Conclusion
HR-HPV prevalence was 32% and the top-five HR-HPV genotypes were HPV16, HPV58, HPV52, HPV53, and HPV51 in women with abnormal cervical cytology in Shanxi. Age 35–45 years, low education, less frequent bathing, multiple gravidity, multiple parity, history of gynecological diseases, and premenopause placed women at high risk of HR-HPV infection. Our results provide insight into HR-HPV infection–related demographic and behavioral characteristics in women with abnormal cervical cytology, and provide evidence for establishing strategies to control HR-HPV infection and reducing the risk of malignant progression in high-risk women. Genotype distribution in the region may point to a promising future for HPV-vaccine research and development. Based on these important leads, prospective cohort studies are needed to provide more powerful evidence.
Abbreviations
HR-HPV, high-risk human papillomavirus; CIN, cervical intraepithelial neoplasia; TCT, ThinPrep cytological test; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells — cannot exclude high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
Data-Sharing Statement
Anonymized data used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Ethical Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of Shanxi Medical University(2013-003). Written informed consent from every participant was obtained in this study.
Acknowledgments
We acknowledge the National Natural Science Foundation Commission of China and the National Health and Family Planning Commission of the People’s Republic of China for the great support.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was funded by the National Natural Science Foundation of China (81872705, 81473060, 81703313) and the National Health and Family Planning Commission of the People’s Republic of China (201402010).
Disclosure
The authors declare that they have no competing interests.
References
1. Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi:10.1016/j.vaccine.2012.07.055
2. Zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi:10.1016/j.virol.2008.11.046
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
5. Zhao FH, Lewkowitz AK, Hu SY, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int j Cancer. 2012;131(12):2929–2938. doi:10.1002/ijc.27571
6. Tota JE, Chevarie-Davis M, Richardson LA, Devries M, Franco EL. Epidemiology and burden of HPV infection and related diseases: implications for prevention strategies. Prev Med. 2011;53(Suppl 1):S12–21. doi:10.1016/j.ypmed.2011.08.017
7. Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen HZ, de Villiers E-M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401(1):70–79. doi:10.1016/j.virol.2010.02.002
8. Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24(Suppl 3):
9. Nicol AF, Andrade CV, Russomano FB, Rodrigues LL, Oliveira NS, Provance DW
10. de Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–459. doi:10.1016/S1473-3099(07)70158-5
11. Jalilvand S, Shoja Z, Nourijelyani K, Tohidi HR, Hamkar R. Meta-analysis of type-specific human papillomavirus prevalence in Iranian women with normal cytology, precancerous cervical lesions and invasive cervical cancer: implications for screening and vaccination. J Med Virol. 2015;87(2):287–295. doi:10.1002/jmv.24053
12. Chelimo C, Wouldes TA, Cameron LD, Elwood JM. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J Infect. 2013;66(3):207–217. doi:10.1016/j.jinf.2012.10.024
13. Lim AW, Landy R, Castanon A, et al. Cytology in the diagnosis of cervical cancer in symptomatic young women: a retrospective review. Br j General Pract. 2016;66(653):e871–e879. doi:10.3399/bjgp16X687937
14. Kietpeerakool C, Cheewakriangkrai C, Suprasert P, Srisomboon J. Feasibility of the ‘see and treat’ approach in management of women with ‘atypical squamous cell, cannot exclude high-grade squamous intraepithelial lesion’ smears. J Obstet Gynaecol Res. 2009;35(3):507–513. doi:10.1111/j.1447-0756.2008.00992.x
15. Aue-Aungkul APS, Natprathan A, Srisomboon J, Kietpeerakool C. “See and Treat” Approach is Appropriate in Women with Highgrade Lesions on either Cervical Cytology or Colposcopy. Asian Pacific J Cancer Prevent. 2011;12:1723–1726.
16. Uijterwaal MH, Polman NJ, Van Kemenade FJ, et al. Five-Year Cervical (Pre)Cancer Risk of Women Screened by HPV and Cytology Testing. Cancer Prevent Res. 2015;8(6):502–508. doi:10.1158/1940-6207.CAPR-14-0409
17. Wang HY, Park S, Lee D, et al. Prevalence of type-specific oncogenic human papillomavirus infection assessed by HPV E6/E7 mRNA among women with high-grade cervical lesions. Int j Infect Dis. 2015;37:135–142. doi:10.1016/j.ijid.2015.06.018
18. Tsedenbal B, Yoshida T, Enkhbat B, et al. Human papillomavirus genotyping among women with cervical abnormalities in Ulaanbaatar, Mongolia. Int j Infect Dis. 2018;77:8–13. doi:10.1016/j.ijid.2018.09.018
19. Krashias G, Koptides D, Christodoulou C. HPV prevalence and type distribution in Cypriot women with cervical cytological abnormalities. BMC Infect Dis. 2017;17(1):346. doi:10.1186/s12879-017-2439-0
20. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int j Cancer. 2012;131(10):2349–2359. doi:10.1002/ijc.27485
21. Wolday D, Derese M, Gebressellassie S, et al. HPV genotype distribution among women with normal and abnormal cervical cytology presenting in a tertiary gynecology referral Clinic in Ethiopia. Infect Agent Cancer. 2018;13:28. doi:10.1186/s13027-018-0201-x
22. Zhao XL, Hu SY, Zhang Q, et al. High-risk human papillomavirus genotype distribution and attribution to cervical cancer and precancerous lesions in a rural Chinese population. J Gynecol Oncol. 2017;28:e30. doi:10.3802/jgo.2017.28.e30
23. Castellsague SdS X. TA. HPV Cervical Cancer. 2007;25(Suppl 3):C1–C26.
24. Vargas H, Sánchez JP, Guerrero ML, et al. Type-Specific Identification of Genital Human Papillomavirus Infection in Women with Cytological Abnormality. Acta Cytol. 2016;60(3):211–216. doi:10.1159/000446389
25. Shi JF, Canfell K, Lew JB, Qiao YL. The burden of cervical cancer in China: synthesis of the evidence. Int J Cancer. 2012;130:641–652. doi:10.1002/ijc.26042
26. Li J, Huang R, Schmidt JE, Qiao Y-L. Epidemiological Features of Human Papillomavirus (HPV) Infection among Women Living in Mainland China. Asian Pacific J Cancer Prevent. 2013;14(7):4015–4023. doi:10.7314/APJCP.2013.14.7.4015
27. Baloch Z, Yasmeen N, Li Y, et al. Prevalence and risk factors for human papillomavirus infection among Chinese ethnic women in southern of Yunnan, China. Braz J Infect Dis. 2017;21:325–332. doi:10.1016/j.bjid.2017.01.009
28. Zou L. Life-style and genital human papillomavirus in a cross-sectional survey in Shanxi Province, China. Asian Pacific J Cancer Prevent. 2011;12:781–786.
29. Kaushic C, Roth KL, Anipindi V, Xiu F. Increased prevalence of sexually transmitted viral infections in women: the role of female sex hormones in regulating susceptibility and immune responses. J Reprod Immunol. 2011;88:204–209. doi:10.1016/j.jri.2010.12.004
30. Wang JT, Gao ES, Cheng YY, et al. Analysis on synergistic action between estrogen, progesterone and human papillomaviruses in cervical cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:370–373.
31. Wang JT, Gao ES, Ding L, et al. Association between endogenous hormones, hormone receptors and cervical cancer. Chin j Oncol. 2006;28:494–497.
32. Wang JT, Ding L, Jiang SW, et al. Folate Deficiency and Aberrant Expression of DNA Methyltransferase 1 were Associated with Cervical Cancerization. Curr Pharm Des. 2014;20:(11):1639–1646. doi:10.2174/13816128113199990543
33. Mongelos P, Mendoza LP, Rodriguez-Riveros I, et al. Distribution of human papillomavirus (HPV) genotypes and bacterial vaginosis presence in cervical samples from Paraguayan indigenous. Int J Infect Dis. 2015;39:44–49. doi:10.1016/j.ijid.2015.08.007
34. McInerney KA, Hatch EE, Wesselink AK, et al. The Effect of Vaccination Against Human Papillomavirus on Fecundability. Paediatr Perinat Epidemiol. 2017;31:531–536. doi:10.1111/ppe.12408
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
