Back to Journals » Clinical Ophthalmology » Volume 18
Prevalence and Distribution of Macular Fluid with Central Retinal Artery Occlusion and Anterior Ischemic Optic Neuropathy
Authors Fouad YA , Hamza MN, Wessam MM
Received 2 February 2024
Accepted for publication 18 March 2024
Published 21 March 2024 Volume 2024:18 Pages 887—893
DOI https://doi.org/10.2147/OPTH.S457503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yousef A Fouad, Mohamed Nabil Hamza, Moataz M Wessam
Department of Ophthalmology, Ain Shams University Hospitals, Cairo, Egypt
Correspondence: Yousef A Fouad, Department of Ophthalmology, Ain Shams University Hospitals, Ramses st., Abbassiya, Cairo, 11517, Egypt, Tel +201063781237, Email [email protected]
Purpose: To examine the prevalence and distribution of fluid within a cohort of eyes with acute central retinal artery occlusion (CRAO) and non-arteritic anterior ischemic optic neuropathy (AION) using optical coherence tomography (OCT).
Methods: A retrospective analysis of patient records and OCT imaging. Patients presenting with acute CRAO or AION who had available macular OCT imaging and no co-morbidities known to cause macular fluid were included in the analysis. Baseline characteristics, visual acuity (VA), and fluid presence and distribution among the retinal layers were recorded.
Results: In the 16 eyes with acute CRAO, fluid was noted in 5 eyes (31%), which was mainly subretinal (3 eyes) or intraretinal located within the outer retinal layers (3 eyes). Only one eye had inner retinal cysts. Of the 11 eyes with acute AION, fluid was present in 8 eyes (73%). Subretinal fluid was noted in 4 eyes and extended to the foveal area in 3 of them, and outer retinal versus inner retinal cysts were noted in 6 versus 3 eyes, respectively. None of the eyes showed hard exudate deposition. In the small subset of eyes with CRAO and macular fluid that were followed-up, VA improved, while in eyes with AION, VA remained stable.
Conclusion: Macular fluid on OCT is not an uncommon feature of acute CRAO and AION and is mainly distributed within the outer retinal layers or subretinal space. Fluid is an understudied feature of retinal and optic nerve head infarction and may have a role in predicting neuronal damage extent and visual outcome.
Keywords: anterior ischemic optic neuropathy, intraretinal fluid, optical coherence tomography, retinal artery occlusion, subretinal fluid
Background
The retina and optic nerve head (ONH) receives their blood supply from two circulations that share a common origin but very limited anastomosis.1 The inner retina down to the level of the inner nuclear layer (INL) is supplied by the central retinal artery (CRA), which also supplies the nerve fiber layer (NFL) at the surface of the optic nerve head.2 Outer retinal layers and deeper layers of the ONH are supplied by the posterior ciliary vessels.3 Acute insufficiency of either circulation can result in neural tissue infarction and catastrophic visual decline.4,5
Acute insufficiency of the CRA, known as CRA occlusion (CRAO), results in infarction of the inner retinal layers due to sudden deprivation of oxygen and nutrients. The ensuing damage becomes irreversible if the occluding thrombus or embolus persists for a few hours.6 In the acute phase, there is swelling of the retina, while in the chronic phase there is subsequent thinning of the inner retinal layers.7 Identifying imaging biomarkers that predict retinal viability during acute CRAO is an important area of research as it would prove crucial in predicting benefit from reperfusion therapy.8 Optical coherence tomography (OCT) is a non-invasive means of cross-sectionally examining the retinal layers. Studies utilizing OCT in eyes with acute CRAO demonstrate increased reflectivity of the inner retinal layers and progressive thickening of the inner and outer retinal layers, followed by thinning of the infarcted layers.7,9,10
Acute insufficiency of the posterior ciliary branches that supply the ONH is known as anterior ischemic optic neuropathy (AION) and is the second most common optic neuropathy following glaucoma.11 Non-arteritic AION, herein termed “AION” for simplicity, is much more common than the arteritic type which occurs due to inflammation of the inner lining of the blood vessels.12 The pathophysiology of AION is not completely understood11,13 and the condition has variable presentation and visual prognosis.14 Limited histopathological studies exist on eyes with AION, highlighting the importance of non-invasive means of investigating the condition.11 On OCT, eyes with acute AION exhibit peripapillary thickening and crowding of the NFL15,16 which later progresses into atrophy that is correlated with visual loss.14
Fluid detected on macular OCT is an important biomarker for multiple ocular pathologies.17–19 For example, intraretinal fluid (IRF) is the most common feature of macular edema in eyes with retinal vein occlusion and seems to be associated with poor visual prognosis, unlike subretinal fluid (SRF) which is less common and may have a more favorable influence on vision.20 Studying macular fluid with CRAO has been limited so far to case reports,21–23 with unclear prevalence rates or correlation to visual outcome. On the other hand, multiple groups have analyzed macular fluid in eyes with acute AION with variable prevalence and distribution across the different retinal layers.16,24–26
Thus, we set out to analyze the prevalence and exact distribution of macular fluid on OCT in a cohort of eyes with acute CRAO or AION.
Methods
This retrospective study was conducted in the setting of a single tertiary care center with adherence to the terms of the Declaration of Helsinki and after acquiring the approval of the local research ethics committee (Faculty of Medicine, Ain Shams University, approval identification: FMASU R329/2023). Informed consent was waived by the committee owing to the retrospective nature of the study and the utilization of anonymized data.
Records of patients referred to the ophthalmology department between the period of January 2021 and September 2023 who presented with acute CRAO or AION (within 30 days from the onset of symptoms) were queried for available OCT scans. Those with macular OCT studies of the affected eye that were of sufficient quality to detect fluid and discern the different retinal layers were included in the analysis. Patients with systemic or ocular co-morbidities that are known to cause macular fluid leakage (eg, diabetes mellitus, exudative age-related macular degeneration, central serous chorioretinopathy, uveitis, macular dystrophies, macular telangiectasia), recent ocular surgery or trauma, or those on medications reported in association with macular fluid were excluded from the analysis. Patients with arteritic AION (diagnosed based on symptomatology, elevated serum inflammatory markers and/or temporal artery biopsy) were also excluded.
Patient demographics (age, sex), time from symptoms onset, and presenting visual acuity (VA) were recorded. The VA was recorded as the best-corrected vision in Snellen format and converted to the logarithm of the minimum angle of resolution (logMAR). OCT macular B-scans acquired by a spectral domain OCT machine (Avanti, Optovue) through a raster scanning protocol centered over the fovea were analyzed for the presence or absence of IRF or SRF by two separate graders (YAF & MNH) with open adjudication. Upon detection, the location of the SRF was recorded as foveal or extrafoveal, and the distribution of the IRF across the different retinal layers was also noted. If follow-up of the case was available, follow-up duration and final VA were added to the analysis.
Descriptive statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS version 25).
Results
Of the 40 patients that presented with CRAO and 22 patients that presented with AION during the studied period, 16 (40%) and 11 (50%), respectively, met the inclusion criteria and had available OCT macular studies. The baseline characteristics of the included patients are summarized in Table 1. Patients in the CRAO group had a slightly higher mean age (60.5 years) compared to the AION group (58.1 years). The average time to presentation was shorter in patients with CRAO than in patients with AION (2.8 days vs 7 days), while VA was notably worse in the CRAO group than the AION group (mean LogMAR VA 2.2 vs 0.7).
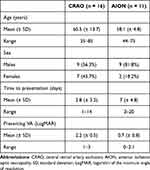 |
Table 1 Baseline Characteristics of the Analyzed Patients |
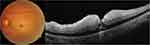
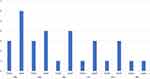
Of the 16 eyes with CRAO, signs of infarction were recorded on OCT in the outer plexiform layer (OPL), INL, and inner plexiform layer (IPL) of all eyes, and in the ganglion cell layer (GCL) and NFL of 12 (75%) and 9 (56.3%) eyes, respectively. Fluid was noted in 5 eyes (31.3%), SRF was noted in 3 eyes and IRF in 3 eyes (18.8%). One eye is presented with both SRF and IRF (Figure 1). The distribution of the IRF is shown in Figure 2. In all 3 eyes with IRF, the fluid was localized in the ONL, and in 1/3 of the eyes, the fluid extended to the inner retinal layers (INL, IPL, GCC and NFL). Follow-up was available for 5 patients with a mean duration of 2 months. Of the 3 eyes with fluid that had follow-up data, improvement in VA was noted (mean LogMAR VA improvement from 2.2 to 1.6). In the two eyes without fluid, one showed stable VA at counting fingers, and the other showed improvement in VA from light perception to hand motion.
In the studied eyes with AION (11 eyes), fluid was noted on OCT in 8 eyes (72.7%). Subretinal fluid was noted in 4 eyes (36.4%) which extended to the foveal area in 3 eyes (Figure 3) and was confined to the peripapillary area in 1 eye. Intraretinal fluid was noted in 6 eyes (54.5%) and its distribution was mainly within the outer retinal layers (Figure 2). Only half of the eyes with IRF had evidence of fluid within the GCL and only one eye had evidence of fluid within the NFL. Follow-up was available for 4 eyes (3 with fluid and 1 without fluid) with a mean duration of 10.8 months, with stable VA in all 4 eyes.
Discussion
The occurrence of macular fluid with acute infarction of the retina or ONH is an understudied topic. In our cohort, nearly a third of eyes with acute CRAO and three-quarters of eyes with acute AION had detectable fluid on macular OCT scans. Subretinal and outer retinal distribution of the fluid was the most common presentation. In the small subset of eyes with CRAO that were followed up, eyes that presented with macular fluid showed improvement in VA on final follow-up, unlike eyes with AION and fluid that showed stable VA.
Macular edema associated with acute CRAO has long been suggested to be distinct from the edema of other retinal vascular disorders (eg, diabetic retinopathy) in being intracellular swelling rather than extracellular fluid accumulation.27 The swelling is seen as increased thickness on OCT associated with increased reflectivity of the inner retinal layers, both features are thought to correlate well with visual outcome.28,29 The presence of extracellular fluid with acute CRAO is, however, less characterized in the literature. Ahn et al9 described retinal changes on OCT in a cohort of eyes with CRAO. The authors reported SRF occurrence in 14% of the eyes and inner retinal fluid in 5% of the eyes. Those numbers are close to our rates of SRF and inner retinal fluid of 19% and 6%, respectively. The authors also reported increased thickness of the outer retina in 73% of the eyes, with no report on cystic fluid accumulation.9 In our analysis, however, 19% of the eyes had cystic fluid accumulation within the ONL. Ng et al22 described a case of CRAO in an elderly female in which cystoid edema was noted in the ONL on OCT during the acute phase. More recently, Estawro et al23 reported on two cases with CRAO that developed IRF, one during the acute phase in which the fluid localized to the ONL, and the other during the chronic phase in which fluid localized mainly to the INL. The authors suggested that Müller cell alterations (swelling during the acute phase and gliotic changes during the chronic phase) may explain the cystoid edema seen on OCT.
Studies on macular fluid with acute AION have reported prevalence rates of 19–47%.24,26,30 In our cohort, macular fluid was noted in 73% of the eyes. However, SRF was only noted in 36% of the eyes and was reaching the subfoveal area in 75% of the eyes and limited to the peripapillary area in 25% of the eyes. These numbers are close to the SRF rates reported with AION in the literature. Donmez et al24 reported the presence of subretinal fluid in 35% of eyes with acute AION (27% of which was peripapillary and the rest had subfoveal extension). Chapelle et al26 reported the occurrence of SRF in 31% of eyes with acute AION, of which 90% had foveal localization. Yu et al31 also demonstrated that SRF was a consistent feature in a murine model of AION and that it resolved with time. Possible pathophysiological mechanisms include ischemic breakdown of the inner blood-retinal barrier with fluid leakage exceeding the capacity of removal by the retinal pigment epithelium, venous return impairment, and Muller cell dysfunction.31 This fluid is likely a transudate rather than an exudate since no hard exudates are deposited in association,26 a feature that was also found in our cohort.
Two distinct types of IRF with AION have been described. Chapelle et al25 reported microcystic macular edema in 56% of eyes with AION, which constitutes inner retinal cysts presumably associated with impaired axonal transport. In another report by the same group,26 outer retinal fluid (termed parafoveal fluid), presumably due to similar transudative mechanism that lead to SRF accumulation, was noted in 59% of eyes. In our analysis, outer retinal fluid was noted in 55% of the eyes while inner retinal microcysts were noted in 27% of the eyes. Variability in prevalence rates could be attributed to the cross-sectional design of our study, with analysis at a single point of time. Inner retinal microcysts are often a transient occurrence during the acute phase of AION and disappear as the ONH edema subsides and axonal transport recovers.25
Follow-up was available in a small subset of eyes with CRAO in which fluid was noted on OCT, and all showed improvement in VA. However, it is important to note that we did not classify the type of CRAO as complete or incomplete, or correlate VA to the extent of retinal layer involvement which limits our analysis of visual outcome. Studies have shown that eyes with incomplete or reversible CRAO, and those in which the infarction involves the middle retinal layers and spares the inner retina (termed paracentral acute middle maculopathy) fare visually better than eyes with complete CRAO.9,32 Chapelle et al26 also noted no significant difference in VA on follow-up of eyes with AION, whether or not SRF was noted on presentation, which is consistent with our findings.
Limitations to the current study include the retrospective design and the cross-sectional imaging analysis at a single point in time. The ischemic cascade is known to evolve with time and different presentations may be noted at different intervals.33 Further prospective work with close follow-up is needed to validate our findings. Another limitation is the small sample size owing to the low prevalence rates of both studied conditions. Studies on larger samples are needed to determine the exact prevalence rates and correlation of fluid to visual outcome. We must also emphasize that although both conditions (CRAO and AION) share features of NFL infarction and a commonly impaired ophthalmic circulation, the pathophysiology that underlies each condition may be different and findings may not be comparable between both groups.
Identifying biomarkers for CRAO is important in stratifying patients to determine who would benefit from possible interventions.34 The detection of fluid on OCT has become easier with newer technology allowing higher-resolution scans and rapid acquisition.35 Fluid leakage in eyes with acute CRAO and AION likely represents a feature of neuronal damage with blood-retinal barrier breakdown and understanding the implications of fluid accumulation and its distribution might help in understanding the extent of damage and in counseling the patients about their visual prognosis.
In conclusion, macular fluid is not an uncommon OCT feature of acute CRAO or AION. The fluid seems to accumulate within the outer retina and in the subretinal space and likely represents a transudate. This preliminary analysis could serve as a step for further investigation of fluid as a biomarker for ischemic neuronal damage within the retina and ONH, and as a possible biomarker for visual outcome.
Abbreviations
AION, anterior ischemic optic neuropathy; CRAO, central retinal artery occlusion; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; IRF, intraretinal fluid; LogMAR, logarithm of the minimal angle of resolution; NFL, nerve fiber layer; OCT, optical coherence tomography; ONH, optic nerve head; ONL, outer nuclear layer; OPL, outer plexiform layer; SD, standard deviation; SRF, subretinal fluid; VA, visual acuity.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wybar KC. Anastomoses between the retinal and ciliary arterial circulations. Br J Ophthalmol. 1956;40(2):65–81. doi:10.1136/bjo.40.2.65
2. Purnyn H. The mammalian retina: structure and blood supply. Neurophysiology. 2013;45(3):266–276. doi:10.1007/s11062-013-9365-6
3. Hayreh SS. The blood supply of the optic nerve head and the evaluation of it — myth and reality. Prog Retin Eye Res. 2001;20(5):563–593. doi:10.1016/S1350-9462(01)00004-0
4. Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140(3):376–391. doi:10.1016/j.ajo.2005.03.038
5. Berry S, Lin WV, Sadaka A, Lee AG. Nonarteritic anterior ischemic optic neuropathy: cause, effect, and management. Eye Brain. 2017;9:23–28. doi:10.2147/EB.S125311
6. Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye. 2013;27(6):688–697. doi:10.1038/eye.2013.25
7. Falkenberry SM, Ip MS, Blodi BA, Gunther JB. Optical coherence tomography findings in central retinal artery occlusion. Ophthalmic Surg Lasers Imaging Retina. 2006;37(6):502–505. doi:10.3928/15428877-20061101-12
8. Mac Grory B, Schrag M, Poli S, et al. Structural and functional imaging of the retina in central retinal artery occlusion – current approaches and future directions. J Stroke Cerebrovasc Dis. 2021;30(7):105828. doi:10.1016/j.jstrokecerebrovasdis.2021.105828
9. Ahn SJ, Woo SJ, Park KH, Jung C, Hong JH, Han MK. Retinal and choroidal changes and visual outcome in central retinal artery occlusion: an optical coherence tomography study. Am J Ophthalmol. 2015;159(4):667–676. doi:10.1016/j.ajo.2015.01.001
10. Ochakovski GA, Wenzel DA, Spitzer MS, et al. Retinal oedema in central retinal artery occlusion develops as a function of time. Acta Ophthalmol. 2020;98(6):680–684. doi:10.1111/aos.14375
11. Rizzo JFI. Unraveling the enigma of nonarteritic anterior ischemic optic neuropathy. J Neuro-Ophthalmol. 2019;39(4):529–544. doi:10.1097/WNO.0000000000000870
12. Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy: population-Based Study in the State of Missouri and Los Angeles County, California. J Neuro-Ophthalmol. 1994;14(1):38–44. doi:10.1097/00041327-199403000-00011
13. Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuro-Ophthalmol. 2003;23(2):157–163. doi:10.1097/00041327-200306000-00012
14. Salvetat ML, Pellegrini F, Spadea L, Salati C, Zeppieri M. Non-Arteritic Anterior Ischemic Optic Neuropathy (NA-AION): a comprehensive overview. Vision. 2023;7(4):72. doi:10.3390/vision7040072
15. Habib AM, Fouad YA, Mekkawy MO. Bilateral simultaneous anterior ischemic optic neuropathy following total knee arthroplasty with epidural block. Case Rep Ophthalmol Med. 2021;2021:1–5. doi:10.1155/2021/9952500
16. Han M, Zhao C, Han QH, Xie S, Li Y. Change of retinal nerve layer thickness in non-arteritic anterior ischemic optic neuropathy revealed by Fourier domain optical coherence tomography. Curr Eye Res. 2016;41(8):1076–1081. doi:10.3109/02713683.2015.1084640
17. van Dijk EHC, Boon CJF. Serous business: delineating the broad spectrum of diseases with subretinal fluid in the macula. Prog Retin Eye Res. 2021;84:100955. doi:10.1016/j.preteyeres.2021.100955
18. Fouad YA, Santina A, Bousquet E, Sadda SR, Sarraf D. Pathways of fluid leakage in age-related macular degeneration. Retina. 2023;43(6):873–881. doi:10.1097/IAE.0000000000003798
19. Arora S, Zur D, Iovino C, Chhablani J. Peripapillary fluid: obvious and not so obvious! Surv Ophthalmol. 2023. doi:10.1016/j.survophthal.2023.11.004
20. Michl M, Liu X, Kaider A, Sadeghipour A, Gerendas BS, Schmidt‐Erfurth U. The impact of structural optical coherence tomography changes on visual function in retinal vein occlusion. Acta Ophthalmol. 2021;99(4):418–426. doi:10.1111/aos.14621
21. Ishizaki N, Kida T, Fukumoto M, Sato T, Oku H, Ikeda T. Development of macular retinoschisis long after the onset of retinal arterial occlusion (RAO): a retrospective study. BMC Ophthalmol. 2018;18(1):59. doi:10.1186/s12886-018-0730-5
22. Ng WY, Wong DWK, Yeo IYS, Han DCY. Cystoid macular edema in acute presentation of central retinal artery occlusion. Case Rep Ophthalmol Med. 2012;2012:1–3. doi:10.1155/2012/530128
23. Estawro R, Abraham N, Fouad Y, Bousquet E, Sarraf D. Cystoid macular edema as a complication of central retinal artery occlusion. Am J Ophthalmol Case Rep. 2024;33:101998. doi:10.1016/j.ajoc.2024.101998
24. Donmez O, Kocaoglu G, Yaman A, Bajin MS, Saatci AO. Macular evaluation with spectral domain type optic coherence tomography in eyes with acute nonarteritic ischemic optic neuropathy at the presentation visit. Open Ophthalmol J. 2017;11:17–23. doi:10.2174/1874364101711010017
25. Chapelle AC, Rakic JM, Plant GT. Nonarteritic anterior ischemic optic neuropathy: cystic change in the inner nuclear layer caused by edema and retrograde maculopathy. Ophthalmol Sci. 2023;3(1):100230. doi:10.1016/j.xops.2022.100230
26. Chapelle AC, Rakic JM, Plant GT. The occurrence of intraretinal and subretinal fluid in anterior ischemic optic neuropathy. Ophthalmology. 2023;130(11):1191–1200. doi:10.1016/j.ophtha.2023.07.015
27. Chen SN, Hwang JF, Chen YT. Macular thickness measurements in central retinal artery occlusion by optical coherence tomography. Retina. 2011;31(4):730–737. doi:10.1097/IAE.0b013e3181f2a15c
28. Shinoda K, Yamada K, Matsumoto CS, Kimoto K, Nakatsuka K. Changes in retinal thickness are correlated with alterations of electroretinogram in eyes with central retinal artery occlusion. Graefes Arch Clin Exp Ophthalmol. 2008;246(7):949–954. doi:10.1007/s00417-008-0791-x
29. Chen H, Xia H, Qiu Z, Chen W, Chen X. Correlation of optical intensity on optical coherence tomography and visual outcome in central retinal artery occlusion. Retina. 2016;36(10):1964–1970. doi:10.1097/IAE.0000000000001017
30. Moon Y, Jung JH, Shin HJ, et al. Non-arteritic ischemic optic neuropathy following COVID-19 vaccination in Korea: a case series. J Korean Med Sci. 2023;38(12):95. doi:10.3346/jkms.2023.38.e95
31. Yu C, Ho JK, Liao YJ. Subretinal fluid is common in experimental non-arteritic anterior ischemic optic neuropathy. Eye. 2014;28(12):1494–1501. doi:10.1038/eye.2014.220
32. Liang S, Chen Q, Hu C, Chen M. Association of paracentral acute middle maculopathy with visual prognosis in retinal artery occlusion: a Retrospective Cohort Study. J Ophthalmol. 2022;2022:1–6. doi:10.1155/2022/9404973
33. Abtahi SH, Nourinia R, Mazloumi M, Nouri H, Arevalo JF, Ahmadieh H. Retinal ischemic cascade: new insights into the pathophysiology and imaging findings. Surv Ophthalmol. 2023;68(3):380–387. doi:10.1016/j.survophthal.2022.11.009
34. Lin JC, Song S, Ng SM, Scott IU, Greenberg PB. Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev. 2023;2023(1). doi:10.1002/14651858.CD001989.pub3
35. Trichonas G, Kaiser PK. Optical coherence tomography imaging of macular oedema. Br J Ophthalmol. 2014;98(Suppl 2):ii24–ii29. doi:10.1136/bjophthalmol-2014-305305
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.