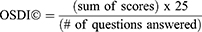Back to Journals » Clinical Ophthalmology » Volume 17
Prevalence and Determinants of Symptomatic Dry Eye Disease Among Adult Urban Residents of High-Altitude Areas of Southwest Saudi Arabia – A Survey
Authors Aldawsari SA, Alzaidi N , Abdalla Elsayed ME, Alhammadi AA, Alharthi HK, Alosaimi A, Al-Najmi Y
Received 22 June 2023
Accepted for publication 30 August 2023
Published 11 September 2023 Volume 2023:17 Pages 2687—2695
DOI https://doi.org/10.2147/OPTH.S427101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Saad Abbas Aldawsari,1 Nazih Alzaidi,2 Maram EA Abdalla Elsayed,3 Abdullah Ahmed Alhammadi,4 Hadeel Khaled Alharthi,1 Abdulrahman Alosaimi,5 Yahya Al-Najmi6
1Ophthalmology Department, King Faisal Medical Complex, Taif, Saudi Arabia; 2Ophthalmology Department, Alhada Military Hospital, Taif, Saudi Arabia; 3Ophthalmology Department, Jeddah Eye Hospital, Jeddah, Saudi Arabia; 4Ophthalmology Department, Asir Central hospital, Abha, Saudi Arabia; 5Ophthalmology Department, College of Medicine, Majmaah University, Majmaah, Saudi Arabia; 6Ophthalmology Department, Saggaf Eye Center, Jeddah, Saudi Arabia
Correspondence: Yahya Al-Najmi, Ophthalmology Department, Saggaf Eye Center, Abdullah Salman St., Al Faiha’a Dist., P.O. Box: 31903, Jeddah, 21418, Saudi Arabia, Tel +966 564844281, Email [email protected]
Purpose: To estimate the prevalence and determinants of Ocular Surface Disease Index (OSDI) score based dry eye disease (DED) among the adult urban population of four cities located at high altitudes in Southwest Saudi Arabia.
Methods: This cross-sectional survey was held in 2023. OSDI questionnaire was used to collect the responses of the adult participants. The score was further graded into none, mild, moderate, and severe DED to estimate age-sex-adjusted DED prevalence. The OSDI score was correlated to demographic (age group, gender, education, occupation, city) and risk factors like smoking and co-morbidities.
Results: Of the 401 adults, 388 (response rate of 97.8%) participated. The age-sex-adjusted prevalence of mild, moderate, and severe DED was 21.7%, 13.1%, and 32%, respectively. The median ODSI score was 22.9 [Interquartile range (IQR) 10.4; 47.9)]. The score was significantly higher in females (Mann–Whitney U-test P = 0.038), residents of Taif city (KW P = 0.05), those with primary/middle school education (Kruskal–Wallis P = 0.004), comorbidities like hypertension, asthma (KW P < 0.001) and risk factors like past refractive surgeries, arthritis (KW P = 0.013). Education status (P < 0.001) [B = − 9.0 95%] and presence of comorbidity (P = 0.022), [B = − 0.823] were significant predictors of DED.
Conclusion: The prevalence of DED and severe grade was high. The level of education and presence of comorbidities significantly influenced DED in the adult urban Saudi population of cities at high altitudes.
Keywords: dry eye disease, Ocular Surface Disease Index
Introduction
Dry eye disease (DED) is a rapidly evolving health issue globally. Its prevalence ranges from 5% to 50%.1 People suffering from DED complain of irritation, stinging, dryness, ocular fatigue, and fluctuating visual disturbances.2 DED negatively affects person’s health-related quality of life and causes an economic burden on the health care system and society.3 Both environmental and personal factors cause DED. They include advancing age, female sex, low humidity environments, systemic medications, and autoimmune disorders.4
During Covid 19 pandemic, restriction of outdoor activities, use of face masks and exposure to excessive screen time by using visual display terminals, and non-availability of eye care in need further increased the problem of DED both in adults and children.5,6
For effective public health intervention and better preventive and therapeutic care to the identified persons with DED, information on the magnitude and determinants are crucial. The health services including medications are provided free of cost by the government to the Saudi citizen. As a part of VISION 2030, the Kingdom periodically reviews the need and implements revision of services.7 Being a large country with nearly 30 million population in 215,000 KM2, DED is likely to vary in different parts. Most of the KSA has a dry and hot climate for nearly 10 months of the year. But in the southwest part of the Kingdom, provinces are hilly, and the climate is relatively humid with different vegetation compared to the rest of northern and central regions of the kingdom.8 The eastern and western provinces although have coastal areas, the hot climate, and humidity affect a limited part adjoining the sea. Several studies on the prevalence and risk factors of DED are conducted in these provinces.9–13
To the best of our knowledge, the magnitude and determinants of DED in the adult population residing in cities at high altitudes in Saudi Arabia are not studied.
We conducted a survey targeting the adult population of Abha, Taif, Al-Baha, and Khamis Mushait cities in southwest Saudi Arabia to determine the prevalence of DED and its determinants. We also compared the rates noted in the literature to study differences in altitude on DED.
Methods
Ethical Issues
The Scientific Research Ethics Committee at King Faisal Medical Complex in Taif approved this study. (2022-A-29). The participants were informed about the purpose of the study, in accordance with the Declaration of Helsinki.
Study Site
Abha, Taif, Al-Baha, and Khamis Mushait (1.3 million as per census 2020) cities were study sites. These cities are situated more than 6000 feet above sea level. The average humidity is 30% (range 27% to 53%).14
Inclusion and Exclusion Criteria
The 18 year and older population of four cities invited to participate in this study.14 Those agreeing to participate were surveyed. Those declining or responding to less than two questions regarding symptoms of dry eye were excluded from the analysis.
Sample Size Calculation
To calculate the sample size, we assumed that the prevalence of DED in study areas is like the global prevalence of 50% in the adult population.15 To achieve a 95% confidence interval and 8% accepted error margin to the study and clustering effect of 2, we need to survey randomly selected at least 301 participants. To compensate for the nonparticipation, we added a 10% sample. Thus, the final sample was 330. We used Openepi software to calculate the sample for a cross-sectional study.16
Sampling and Recruitment
Four investigators visited a central mosque and mall on weekends and a university on weekdays to recruit participants. The stratification of sample was done by population of four cities. However, the response in different cities was variable with a high number of participants recruited from Abha city. Therefore, age-sex adjusted rates were calculated for better representation. The information on the survey for the adult population was distributed through mosque announcements, social media, and emails. For university students E-mails and whatsApp groups were used to inform about survey and request for participation.
Survey Tools and Validity
We used a validated Dry Eye Syndrome Questionnaire.2,17 The questions were translated into Arabic. Reverse translation was carried out to ensure the quality of the questions. The steps of quality assurance for using Arabic OSDI questionnaire were like those used by Bakker et al and Aljarousha et al.18,19 The questionnaire and responses were tested for reliability. The Cronbach’s Alpha of the Ocular Surface Disease Index (OSDI) of 12 questions was 0.923.
Data Management
The demographic information included age group, gender, education, city of residence, and occupation. Participants replied to principal comorbidity and known risk factors from offered options. Subgroup 1 of the OSDI questionnaire had five questions. Subgroup 2 had four questions and Subgroup 3 had three questions. The frequency of symptoms was given a score from 0 to 4. 0 = not at all, 1 = some of the time, 2 = half of the time, 3 = most of the time, and 4 = all the time in the last week of the survey. To account for partial responses to the questions the following formula was used to calculate the OSDI score:
The DED was graded as “Absent” if the score was “0 to 12”, mild if the score is “13 to 22” moderate DED if the score is “23 to 32” and severe DED if the score is 33 and more. To calculate the age-sex-adjusted prevalence of DED, we used a population, examined sample, persons with different grades of DED subgroup of gender, age group, and city of residence. We projected the number of persons with DED in the smallest subgroup and then estimated adjusted prevalence using the total population as the denominator (Supplementary Tables 1–5).
Statistical Analysis
The data was entered into a spreadsheet of Microsoft XL®. After consistency check, the data was transferred into a spreadsheet of Statistical Package for Social Studies (SPSS 25) (IBM, NY, USA). The qualitative variables were presented as numbers and percentage proportions. The quantitative variables were presented as median and interquartile range. To compare the OSDI score (outcome variable) in two subgroups, we used the Mann–Whitney U-test to estimate Z and two-sided P values. For more than 2 subgroup comparisons, we used the Kruskal–Wallis H-test to estimate the chi-square value and two-sided p-value. A P value of <0.05 was considered statistically significant. The independent variables that were significantly associated with the OSDI score were included in the regression model to study predictors and estimate adjusted Odds ratio with a 95% confidence interval and P value.
Results
We enrolled 401 participants. Of them, 388 completed more than one question so their responses were included in the analysis.
We compared the study population of males and females in age groups. Table 1. Fifty years and older males were overrepresented in our study, while “18 to 25” and “38 to 50” years old females were overrepresented in the examined sample compared to their population proportion. Therefore, the prevalence of DED was age-sex-adjusted.
 |
Table 1 Comparison of Adult Population and Study Participants of Four Cities of Southwest Saudi Arabia |
The age-sex-adjusted prevalence of mild, moderate, and severe DED was 21.7% (95% CI 21.6; 21.8), 13.1% (95% CI 13.0; 13.2), and 32% (95% CI 31.9; 32.1), respectively. Of the 1.3 M adult population in the study area, 0.88 M (66.6%) are projected to have DED. Of them, 0.42M with severe DED. The prevalence of DED by subgroup is given in Table 2.
 |
Table 2 Prevalence of Symptom-Based Dry Eye Disease (DED) in Adult Population of Four Cities of Southwest Saudi Arabia |
The median OSDI of 388 adult participants was 22.9 (IQR 10.4; 47.9) (Minimum; Maximum 0.0; 100). The OSDI of the examined sample was compared in subgroups. Table 3. The score was significantly higher in females (Mann–Whitney U-test P = 0.038), residents of Taif city (KW P = 0.05), those with primary/middle school education (Kruskal–Wallis P = 0.004), comorbidities like hypertension, asthma and other (KW P < 0.001) risk-like past refractive surgeries, arthritis and other (KW P = 0.013).
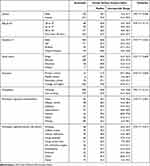 |
Table 3 Ocular Surface Disease Index (OSDI) Score in Adult Population of Four Cities of Southwest Saudi Arabia |
Linear regression analysis suggested that Education status (P <0.001) [B = −9.0 95%] and presence of comorbidity (P = 0.022), [B = −0.823] were significant predictors of DED. Of the 41 contact lens users, DED was present in 14 participants. While among 276 participants who were not using contact lenses, 27 had DED. The risk of DED among CL users was not significant (P = 0.43). Of the 26 participants with a history of refractive surgery in the past, DED was present in 19 persons. While among 276 persons without refractive surgery DED was present in 183 persons. The risk of DED among persons with a history of refractive surgery was not significant (P = 0.23).
We compared the DED rates noted in the present study to other studies of Saudi Arabia and other using OSDI questionnaire in Arabic language from the literature. Table 4.
 |
Table 4 Comparison of Rate, Grades and Risk Factors of Dye Eye Disease in Adult Population of Different Studies |
Discussion
The prevalence of DED was high and one-third of them had a severe grade of DED. Females, less educated, residents of Taif city, history of refractive surgeries, hypertension, dyslipidemia, and asthma were significantly associated with higher OSDI scores. Two-thirds of the adult population was projected to suffer from DED. Of them, one-third with a severe grade of DED would need further evaluation and prompt treatment. There is a strong need for DED preventive measures and counseling for adopting eye health measures among the adult population.
This is perhaps the first study showing the influence of high altitude on DED among adult urban residents of rapidly evolving countries like Saudi Arabia. The validity of the questionnaire used in present study was like that found in Arabic OSDI questionnaire used in Jordan and Palestine.18,19 Reasonably large sample and age sex adjustment of OSDI score-based prevalence of DED enabled to provide of more accurate rates and projections to undertake public health measures to address this fast-developing eye health issue.
In the present study, the prevalence of severe DED was 32%. While 66% had some grade of DED. Aldossari et al used a similar OSDI score to define and grade DED and noted 17% DED prevalence in the adult Saudi population.11 Yasir et al used McCarty Symptom Questionnaire and noted a 45% age-sex-adjusted prevalence of DED among 40 years and older population of Riyadh governorate.13 The age-adjusted prevalence of DED in the adult population of Al Hassa province was assessed using six symptoms-based questionnaires and it was 32%.12 By using Tear Film Ocular Surface Dry Eye Workshop II (TFOS DEWS II) criteria, the researchers noted a 32% prevalence of DED in UK.20 Literature shows a wide variation of DED assessment both using objective and questionnaire methods and noted a high prevalence of DED globally.1,21–26 The variations in magnitude and severity of DES in different studies could be due to differential behavior of using digital devices, humidity, temperatures of the study area and comorbidities.
Increasing age is a known risk factor for DED. In our study, the prevalence of DED was not significantly different in age groups. But mild DED was more in the younger population and severe DED was more in the older population. The ODSI score was less than 20 in 18 to 38 years of age, while the score was >20 in the adult population of more than 38 years of age. Elderly people have a 75% higher risk of DED compared to young adults.1,27 The presence of age-related comorbidities like diabetes, exposure to a dry environment, more screen time, and hormonal changes could be responsible for this observed increased risk of DED by age.
Females had a significantly higher prevalence and severity of DED in our study. Among Asian adult females, the DED rate was higher than males (21.7% vs 16.4%).28 Higher incidence of refractive error, less outdoor activities and more time spent in front of visual display gadgets, higher risk of dyslipidemia and differential hormonal profile could be responsible for this higher risk of DED.1,29
All four cities in our study were at high altitudes (more than 6000 feet above sea level). Our study had high DED prevalence and symptoms of severe grade DED. In contrast, Tandon et al noted that in hilly areas DED prevalence was 24% compared to 41.3% in plains and only 9.9% at places on the seashore.22 Environmental factors like temperatures, humidity, air pollution, and pollens are associated with ocular surface changes including DED.30 These factors are likely to be different at the present study site compared to the places mentioned in other studies from Saudi Arabia.10–13 Higher rate in the present study compared to rates found in the hilly area of India is difficult to explain. Each environmental factor should be associated with DED using a more appropriate study design.
In our study, the OSDI score was 22 among smokers and it was not a significant risk factor for DED. This was also noted in a meta-analysis by Tariq et al.31 However, information on smoking collected in our study was not of desired details. Therefore, further studies with current vs past, extent, and type of nicotine abuse need to be associated with DED before concluding it as a risk factor.
In our study, DED was not associated with contact lens use or a history of refractive surgery. This contrasted with the findings of Li et al.32 Selecting persons without DED for contact lens fitting or performing refractive surgeries in eyes could result in differential DED rate. In our study, subjective definition was used for DED, while objective methods are usually used for selecting patients for CL or surgery.
OSDI score among diabetics was higher but not significantly different from non-diabetic persons in our study. The risk of DED and meibomian gland dysfunction was significantly associated with type 2 diabetes and poor glycemic control.33 An objective assessment of DED is recommended before concluding the association of diabetes with DED.
Among four cities in southwest Saudi Arabia, two-third of the adult population (0.8 Million) are projected to have symptoms of DED. Of them, 0.42 million could be with a severe grade of DED. Therefore, there is an urgent need for a public health approach to address DED. Identified cases with symptoms may be further assessed using objective methods to confirm DED and then offer management to reduce symptoms and visual disabilities.27,34,35 The population at large should be educated to adopt ways to reduce the risk of DED through mass media and social media. Ministry of Health is aware of the negative impact of exposure to devices with visual display terminals especially in reducing screen time in children.36
There were a few limitations in our study. This being the cross-sectional nature of the study, a causal association of the determinants and comorbidities to DED should be done with caution as spatial relationships cannot be established. The DED was entirely symptom-based and needed to be confirmed by objective assessments. Therefore, present study outcomes should be compared with the studies done by a subjective and objective assessment of DED with caution. The sample size calculation was based on 50% prevalence, but our study noted as high as 66%. This could have resulted in calculation error but the sample of 401 instead of 330 could to some extent minimize this error. The examined sample was not recruited by random sampling and stratification. However, age-sex-city adjusted DED rates were carried out to give more reliable projections of DED for the study population.
Conclusions
In the present study adult population of urban areas of high altitude in Saudi Arabia showed a high prevalence of DED. Although the OSDI score is an accepted and widely used method to estimate the magnitude of ocular surface dysfunction, it should be complemented with objective assessments to confirm the presence and severity of DED. Prompt action is needed in the study area to address the DED to reduce its impact on vision-related quality of life. Identified risk factors like female gender and less educated persons should be noted while preparing health education material and selecting the mode of propagating these DED prevention messages to the target population. Primary eye care professionals and family physicians should be periodically trained to diagnose, and suspect DED in patients of systemic comorbidities known to cause DED and provide management to the identified persons at risk.
Acknowledgments
We thank the people of four cities to cooperate and participate in this survey.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
2. American academy of Ophthalmology. Eyewiki. Available from: https://eyewiki.aao.org/Dry_Eye_Syndrome_Questionnaires#cite_note-:2-5.
3. McDonald M, Patel DA, Keith MS, Snedecor SJ. Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review. Ocul Surf. 2016;14(2):144–167. doi:10.1016/j.jtos.2015.11.002
4. Rouen PA, White ML. Dry eye disease: prevalence, assessment, and management. Home Healthc Now. 2018;36(2):74–83. doi:10.1097/NHH.0000000000000652
5. Burgos-Blasco B, Arriola-Villalobos P, Fernandez-Vigo JI, et al. Face mask use and effects on the ocular surface health: a comprehensive review. Ocul Surf. 2023;27:56–66. doi:10.1016/j.jtos.2022.12.006
6. Koh S, Rhee MK. COVID-19 and Dry Eye. Eye Contact Lens. 2021;47(6):317–322. doi:10.1097/ICL.0000000000000797
7. Progress and achievements in VISION 2030 in Kingdom of Saudi Arabia. Available from: https://www.vision2030.gov.sa/v2030/achievements/.
8. Tarawneh QY, Chowdhury S. Trends of climate change in Saudi Arabia: implications on water resources. Climate. 2018;6(1):8. doi:10.3390/cli6010008
9. Bukhari A, Ajlan R, Alsaggaf H. Prevalence of dry eye in the normal population in Jeddah, Saudi Arabia. Orbit. 2009;28(6):392–397. doi:10.3109/01676830903074095
10. Alkhaldi SA, Allam KH, Radwan MA, et al. Estimates of dry eye disease in Saudi Arabia based on a short questionnaire of prevalence, symptoms, and risk factors: the Twaiq Mountain Eye Study I. Cont Lens Anterior Eye. 2023;46(2):101770. doi:10.1016/j.clae.2022.101770
11. Dossari SK, Alkhars AZ, Albaqshi AA, et al. Prevalence of dry eye disease and its risk factors among the general population of Saudi Arabia: a cross-sectional survey. Cureus. 2022;14(12):e32552. doi:10.7759/cureus.32552
12. Alshamrani AA, Almousa AS, Almulhim AA, et al. Prevalence and risk factors of dry eye symptoms in a Saudi Arabian population. Middle East Afr J Ophthalmol. 2017;24(2):67–73. doi:10.4103/meajo.MEAJO_281_16
13. Yasir ZH, Chauhan D, Khandekar R, et al. Prevalence and determinants of dry eye disease among 40 years and older population of Riyadh (except capital), Saudi Arabia. Middle East Afr J Ophthalmol. 2019;26(1):27–32. doi:10.4103/meajo.MEAJO_194_18
14. General Authority for Statistics (stats.gov.sa). Population of four cities is based on General Authority of Statistics. Chapter 01 | Population & Demography; June 03, 2023.
15. Courtin R, Pereira B, Naughton G, et al. Prevalence of dry eye disease in visual display terminal workers: a systematic review and meta-analysis. BMJ Open. 2016;6(1):e009675. doi:10.1136/bmjopen-2015-009675
16. Dean AG, Sullivan KM, Soe MM. OpenEpi: open-source epidemiologic statistics for public health, version. [updated June 04, 2013]. Available from: www.OpenEpi.com.
17. Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
18. Bakkar MM, Qadire MA. Validation of the Arabic version of the ocular surface disease index questionnaire. Int J Ophthalmol. 2021;14(10):1595–1601. doi:10.18240/ijo.2021.10.18
19. Aljarousha M, Badarudin NE, Che Azemin MZ, Aljeesh Y, Amer A, Abdul Rahim MAS. The validity and reliability of the Arabic version of the ocular surface disease index (OSDI) questionnaire in a sample of the Gazan population: a study from Palestine. Int Ophthalmol. 2023;43(4):1303–1316. doi:10.1007/s10792-022-02528-7
20. Vidal-Rohr M, Craig JP, Davies LN, et al. The epidemiology of dry eye disease in the UK: the Aston dry eye study. Cont Lens Anterior Eye. 2023;46(3):101837. doi:10.1016/j.clae.2023.101837
21. Wu J, Wu X, Zhang H, et al. Dry eye disease among Mongolian and Han older adults in Grasslands of Northern China: prevalence, associated factors, and vision-related quality of life. Front Med (Lausanne). 2021;8:788545. doi:10.3389/fmed.2021.788545
22. Tandon R, Vashist P, Gupta N, et al. Association of dry eye disease and sun exposure in geographically diverse adult (≥40 years) populations of India: the SEED (sun exposure, environment and dry eye disease) study - Second report of the ICMR-EYE SEE study group. Ocul Surf. 2020;18(4):718–730. doi:10.1016/j.jtos.2020.07.016
23. Tellefsen Nøland S, Badian RA, Utheim TP, et al. Sex and age differences in symptoms and signs of dry eye disease in a Norwegian cohort of patients. Ocul Surf. 2021;19:68–73. doi:10.1016/j.jtos.2020.11.009
24. Bakkar MM, Shihadeh WA, Haddad MF, et al. Epidemiology of symptoms of dry eye disease (DED) in Jordan: a cross-sectional non-clinical population-based study. Cont Lens Anterior Eye. 2016;39(3):197–202. doi:10.1016/j.clae.2016.01.003
25. Betiku AO, Oduyoye OO, Jagun OO, et al. Prevalence and risk factors associated with dry eye disease among adults in a population-based setting in South-West Nigeria. Niger J Clin Pract. 2022;25(3):354–360. doi:10.4103/njcp.njcp_1598_21
26. Caffery B, Srinivasan S, Reaume CJ, et al. Prevalence of dry eye disease in Ontario, Canada: a population-based survey. Ocul Surf. 2019;17(3):526–531. doi:10.1016/j.jtos.2019.02.011
27. Verjee MA, Brissette AR, Starr CE. Dry eye disease: early recognition with guidance on management and treatment for primary care family physicians. Ophthalmol Ther. 2020;9(4):877–888. doi:10.1007/s40123-020-00308-z
28. Cai Y, Wei J, Zhou J, et al. Prevalence and incidence of dry eye disease in Asia: a systematic review and meta-analysis. Ophthalmic Res. 2022;65(6):647–658. doi:10.1159/000525696
29. Lee SS, Mackey DA. Prevalence and risk factors of Myopia in young adults: review of findings from the Raine Study. Front Public Health. 2022;10:861044. doi:10.3389/fpubh.2022.861044
30. Alves M, Asbell P, Dogru M, et al. TFOS lifestyle report: impact of environmental conditions on the ocular surface. Ocul Surf. 2023;29:1–52. doi:10.1016/j.jtos.2023.04.007
31. Tariq MA, Amin H, Ahmed B, et al. Association of dry eye disease with smoking: a systematic review and meta-analysis. Indian J Ophthalmol. 2022;70(6):1892–1904. doi:10.4103/ijo.IJO_2193_21
32. Li J, Zheng K, Deng Z, et al. Prevalence and risk factors of dry eye disease among a hospital-based population in southeast China. Eye Contact Lens. 2015;41(1):44–50. doi:10.1097/ICL.0000000000000064
33. Yoo TK, Oh E. Diabetes mellitus is associated with dry eye syndrome: a meta- analysis. Int Ophthalmol. 2019;39(11):2611–2620. doi:10.1007/s10792-019-01110-y
34. Al-Saedi Z, Zimmerman A, Bachu RD, et al. Dry eye disease: present challenges in the management and future trends. Curr Pharm Des. 2016;22(28):4470–4490. doi:10.2174/1381612822666160614012634
35. Downie LE, Keller PR. A pragmatic approach to dry eye diagnosis: evidence into practice. Optom Vis Sci. 2015;92(12):1189–1197. doi:10.1097/OPX.0000000000000721
36. Ministry of Health, Saudi Arabia. Screen and children in child health. Available from: https://www.moh.gov.sa/en/HealthAwareness/EducationalContent/BabyHealth/Pages/004.aspx.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.