Back to Journals » Clinical Ophthalmology » Volume 14
Prevalence and Associated Factors of Pterygium Among Adults Living in Kolla Diba Town, Northwest Ethiopia
Authors Kassie Alemayehu T, Addis Y, Yenegeta Bizuneh Z , Mulusew Tegegne M, Alemayehu AM
Received 25 November 2019
Accepted for publication 7 January 2020
Published 28 January 2020 Volume 2020:14 Pages 245—255
DOI https://doi.org/10.2147/OPTH.S239982
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tibebu Kassie Alemayehu, Yezinash Addis,* Zewdu Yenegeta Bizuneh,* Mebratu Mulusew Tegegne,* Abiy Maru Alemayehu*
Department of Optometry, School of Medicine, College of Medicine and Health Science, University of Gondar, Gondar City, Ethiopia
*These authors contributed equally to this work
Correspondence: Tibebu Kassie Alemayehu Tel +251910606005
Email [email protected]
Background: Pterygium is a disfiguring disease that can potentially lead to blindness. It is more common in warm, windy and dry climates of tropical and sub-tropical regions of Africa. Globally, the prevalence ranging from 0.07% to 53%. Studies conducted on the prevalence of pterygium in developing countries were limited with a wider discrepancy between them. In this study, we aimed to assess the prevalence of pterygium and its associated factors among adults in Kolla Diba town.
Methods: A community-based cross-sectional study was done in Kolla Diba town from May 30-June 16, 2019. A systematic random sampling technique was used to select 627 study participants. The basic ophthalmic examination was performed using portable slit lamp, 3x magnifying loop with torch light and a pretested structured questionnaire was completed. The data entered into EPI INFO version 7 and analyzed using SPSS version 20. Descriptive statistics and binary logistic regression analysis were employed. P-values of < 0.05 were considered statistically significant.
Results: A total of 605 study participants were involved with a response rate of 96.5%. Among them, 317 (52.4%) participants were males. The mean age of the respondents was 38.18 ± 15.56 with a range of (18– 95) in years. The overall prevalence of pterygium was 112 (18.5% (95% CI (15.6– 21.7)). Being widowed (AOR = 7.32 (95% CI: 2.88, 18.57)), outdoor occupation (AOR = 2.50 (95% CI: 1.46, 4.29)), sun exposure (AOR = 2.38 (95% CI: 1.28, 4.43)), wind exposure (AOR = 1.97 (95% CI: 1.04, 3.72)), alcohol drinking (AOR = 2.26 (95% CI: 1.48, 4.63)), and severe blepharitis (AOR = 2.45 (95% CI: 1.48, 4.05)) had statistically significant positive association with pterygium.
Conclusion: The prevalence of pterygium was relatively higher. Being widowed, outdoor occupation, sun exposure, wind exposure, alcohol drinking, and severe blepharitis were significantly associated with the development of pterygium.
Keywords: pterygium, marital status, prevalence, Kolla Diba, Ethiopia
Introduction
Pterygium is a benign, radially oriented, fleshy triangular/wing shaped abnormal fibro-vascular growth of the conjunctiva encroaching towards the central cornea in either one or both eyes.1,2 The main clinical presentations are redness, irritation, foreign body sensation, decreased vision and ocular discomfort.3,4 The severity of pterygium can be graded as grade 1, 2, 3 and 4 based on the location of pterygium head encroachment on the cornea.5,6 Genetics and other environmental factors such as wind, dry atmosphere, dust, chemicals, air pollution, geographical location, and hereditary factors may contribute to the development of pterygium.7 Pterygium is more common in warm, windy and dry climates of tropical and subtropical regions.8,9 The burden of pterygium is higher among individuals who live in rural residence, low economic status, illiterates, and outdoor workers, who spend a greater part of the day outdoors under excessive sun exposure, wind, and dust.10–13
Globally, the prevalence of pterygium ranges from 0.07% to 53% worldwide and 8.0% to 38.7% in Africa.2,3,6,12,14,15 In Ethiopia, the prevalence was 8.8% in Meskan district, Southern Ethiopia and 38.7% in Gondar city, Northwest Ethiopia.2,16
Untreated pterygium may lead to visual impairment, cosmetic problems, restriction of ocular motility, significant socioeconomic problems and reduced quality of life.17–21 Even though the consequence is higher, it is possible to minimize the chances of being afflicted with pterygium by wearing UV protective sunglasses, wide-brimmed hats, caps and using an umbrella to reduce exposure to environmental irritants.2,6,10,15,22,23
Pterygium is a common ophthalmic condition in “pterygium belt” regions, located between latitudes 40° N and 40 ° S of the equator.7,15,24 The exposure to environmental factors is also higher in Africa, including Ethiopia but the evidence is scarce on the prevalence of pterygium in this region.13,25–29 Therefore, this study aimed to determine the prevalence and associated factors of pterygium among adults in Kolla Diba town. The result of this study will be important for planning and implementing health care services and also act as baseline data for further studies.
Methods and Materials
Study Design, Setting, and Sampling
A community-based cross-sectional study was conducted in Kolla Diba town, Northwest Ethiopia, located 712km from the capital city of Ethiopia, Addis Ababa. The altitude of the town is 1827m above sea level. According to statistical data from Dembiya woreda Administrative office, Kolla Diba town has a total population of 25,831 with three kebeles including 5328 households. The total number of adults (≥ 18 years) living in Kolla Diba town was 15,534. In the town, there is one primary hospital and one health center.
A total of 627 sample size was determined by a single proportional formula by considering 10% non-response rate. In the study, 605 study participants were recruited and completed the questionnaire along with basic ophthalmic examinations with a response rate of 96.5%. Households from all kebeles were chosen by using a systematic random sampling technique with a sampling fraction of eight. The first household was selected by the lottery method between, the 1st & 8th household and then continued in every 8th household. Finally, one adult in each participating household age ≥ 18 years old was randomly selected and recruited as a study participant.
The study was conducted under the Declaration of Helsinki and approved by the University of Gondar Ethical Review Board. Under the Ethiopian National Research Ethics Review Guideline, verbal informed consent was obtained from all adults ≥18 years old using an information sheet in the local language “Amharic”. Since the study did not involve invasive eye examination procedures, the university ethical review board approved verbal informed consent. The study participants’ agreements were first obtained verbally before data collection. Then the data were collected by trained senior optometrists. Those adults who had pterygium were prescribed sunglass and referred to the University of Gondar tertiary eye care and training center for further examination and management.
Operational Definition
Sun exposure divided into two groups by crude cut off value of sun exposure duration: a participant who exposed to sunlight for above or equal to 5hrs per day for more than a year considered as exposed, whereas less than 5hrs per day considered as non-exposed.26
Dust exposure: Any exposure to sawdust or wood-dust, chalk dust and sand/soil, dust for more than a year.23
Grading of Pterygium
Grade 1: head of a pterygium at the limbus
Grade 2: head of a pterygium between the limbus and the un-dilated pupil margin
Grade 3: head of a pterygium at the pupil margin
Grade 4: head of a pterygium within the pupil margin30
Data Collection
The pre-tested and structured questionnaire of local language “Amharic” was used to interview adults’ ≥18 years old. (Figure 1). The study tools were pretested in a nearby area of the study site that had not been sampled (Chuahit), on 5% of the sample who fulfilled the sampling inclusion criteria, to validate questions and observations. The collected data from study participants include information’s on socio-demographic characteristics, behavioral/lifestyle-related factors, and environmental factors. Face to face interviews by using standardized questionnaires were conducted with those selected participants. Standard basic ophthalmic examination by using a portable slit lamp, torchlight, and 3x magnifying loop was done by trained senior optometrists to rule out the diagnosis of pterygium. On the fieldwork, the quality of data was cross-checked (5% of the filled questionnaire) for completeness, accuracy, and clarity through supervision daily.
 |
Figure 1 Continued. |
 |
Figure 1 Data extraction form/questionnaire. |
Statistical Analysis
After coding, the data was entered into EPI INFO 7, exported and analyzed by using SPSS version 20. The descriptive statistics were carried out to compute different proportions and summary statistics. Model fitness was checked by using the Hosmer and Lemeshow. Variables were fitted into the model by using the backward stepwise method. The analytical statistics were done by using bivariate and multivariate logistic regression. Those variables with 95% CI and p-value less than 0.05 were considered as statistically significant factors of pterygium.
Results
A total of 605 participants was recruited and completed the questionnaire along with basic ophthalmic examinations with a response rate of 96.5%. Among them, 317 (52.4%) participants were males. The mean age of the respondents was 38.18 ± 15.56 with a range of (18–95) in years. Majority 188 (31.1%) of the respondents completed secondary school and almost half of the participants got married 303 (50.1%). Most of the participants were indoor workers 410 (67.8%). The majority of the participants had an income of ≤ 1000 ETB from the monthly median income of 2075.50 ETB. Table 1.
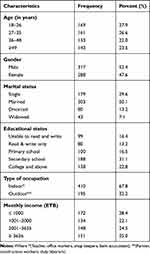 |
Table 1 Socio-Demographic Characteristics Among Adults Living in Kolla Diba Town, Northwest Ethiopia, June 2019 (n=605) |
Study participants who exposed to dust and wind were 337 (55.7%) and 350 (57.9%) respectively. Participants who exposed to sunlight for more than 5hrs per day were 280 (46.3%) and half of the respondents 302 (49.9%) spend most of their time on outdoor activities. Table 2. Of the total study participants only 171 (28.3%) study participants used sunglass or hats. Most of the study participants never smoked cigarettes, any history of dry eye and hypertension with 518 (85.6%), 503 (83.1%) and 558 (92.2%) respectively. Table 3.
 |
Table 2 Environmental Factors Associated with Pterygium Among Adults Living in Kolla Diba Town, Northwest Ethiopia, June 2019 (n=605) |
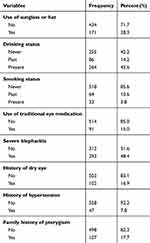 |
Table 3 Behavioral/Lifestyle Factors Associated with Pterygium Among Adults Living in Kolla Diba Town, Northwest Ethiopia, June 2019 (n=605) |
The overall prevalence of pterygium among adults living in Kolla Diba town was 112 (18.5% (95% CI (15.6–21.7)). Among those participants, 65 (58%) had unilateral pterygium. The majority of the participants 87 (77.7%) had pterygium on the nasal side and the rest 12 (10.7%) had temporal and 13 (11.6%) had double pterygium. Most of the study participants had grade 1 pterygium, 63 (56.3%) followed by grade 2 pterygium, 38 (33.9%) and the rest grade 3 and 4 accounts less than 8% in total. Table 4.
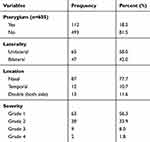 |
Table 4 Prevalence, Laterality, Location and Severity of Pterygium Among Adults Living in Kolla Diba Town, Northwest Ethiopia, 2019 |
From the Bi-variable logistic regression analysis; factors like age, gender, marital status, educational status, occupation, dust exposure, sun exposure, wind exposure, duration of outdoor activities, use of sunglass or hat, drinking status, smoking status, use of traditional eye medication, severe blepharitis, history of dry eye and history of hypertension were independently associated with pterygium with the significant level of p < 0.20.
In multivariable logistic regression analysis factors such as marital status, occupation, sun exposure, wind exposure, drinking status, and severe blepharitis had a statistically significant association with pterygium. This study found that there is a positive association between marital status (being widowed) and the development of pterygium. The odds of developing pterygium among widowed participants were 7 times more likely as compared to single participants (AOR = 7.32 (95% CI: 2.88, 18.57)). Outdoor occupation workers such as farmers, day laborers, taxi drivers, vendors, construction workers, were 2.50 times more likely to develop pterygium as compared to indoor workers such as office workers, teachers and so on (AOR = 2.50 (95% CI: 1.46, 4.29)).
Participants who had exposed to wind were 2 times more likely to develop pterygium than non-exposed (AOR = 1.97 (95% CI: 1.04, 3.72)). Alcohol drinking had a positive association with pterygium and those participants who had currently drink alcohol were 2.62 times more likely to develop pterygium as compared to non-drinkers (AOR = 2.26 (95% CI: 1.48, 4.63)). The odds of developing pterygium on study participants who exposed to the sun were 2.38 times more likely than non-exposed (AOR = 2.38 (95% CI: 1.28, 4.43)). Those participants who had severe blepharitis were 2.45 times higher risk of developing pterygium (AOR = 2.45 (95% CI: 1.48, 4.05)). Table 5.
 |
Table 5 Binary Logistic Regression of Factors Associated with Pterygium Among Adults Living in Kolla Diba Town, Northwest Ethiopia, June 2019(n=605) |
Discussion
The prevalence of pterygium of various studies in the world shows considerable variation, ranging from 0.07% to 53%, depending on the difference in various geographical and environmental factors.2,3,31,32
In this community-based cross-sectional study the prevalence of pterygium among adults living in Kolla Diba town was 112 (18.5% (95% CI; 15.6–21.7)). This finding is lower as compared to the study conducted, in Gondar city (Ethiopia) 38.7%.2 This discrepancy might be due to the difference in socioeconomic status and reference age group. In the study conducted in Gondar city, 71% of the study participants had a low monthly income (< 1000 ETB). But here in this study, only 28.4% of the study participants had low monthly income. As previous studies described as low economic status had positively associated with the prevalence of pterygium.11,26,28,33,34 Another reason might be an age cut off point as the present study involved all available inhabitants aged ≥18 years as compared to the study conducted in Gondar city (age >20 years). In contrast to few other studies, this study also shows lower prevalence than other studies conducted in Ghana 31.0%,18 30.8% in Kumejima (Southwest Japan),35 37.46% in Duomen County (Republic of China)32 and 36.6% in Amazon Basin (Brazil).36 This variation might be due to the difference in the target population, ethnicity and altitudinal variation.
This finding is higher when compared with the study conducted in Meskan district, Southern Ethiopia, which was 8.80%.16 This variation might be due to the difference in the target population and ethnicity (the prevalence of pterygium varied among different ethnic groups).5,9,24,37 The present study shows higher prevalence of pterygium in comparison to other studies conducted in Saudi Arabia (Alkhobar) 0.07%,31 13.0% in Rural agrarian central India,22 10.53% in China (Shandong province),20 4.40% in Japan,1 8.80% in South Korea,26 2.3% in Russia, 8.12% in Brazil (Sao Paulo (Botucato city)),38 11.24% in Hawaii,24 5.90% in Spain,3 and 1.09% in Portugal.39 This discrepancy might be due to the difference in geographical location, climatic change, altitudinal variation, lifestyle and other environmental factors. Most of the above studies were also done on older adults (40 years and above).
The present study finding is laying in between the highest and the lowest prevalence among different epidemiological studies in the world. This finding is in line with the study conducted in Southeastern Nigeria, Central Myanmar and in Riau Archipelago (tropical islands of Indonesia) which was 19.3%, 19.6% and 17.0% respectively.19,23,30 This is because Ethiopia, Nigeria, Central Myanmar and Riau Archipelago (Indonesia) located in a similar geographical region (pterygium belt tropical regions) in the tropics near the equator. These studies also had similar age group reference and study design.
In many works of literature marital status had no direct association with pterygium.2,13,28 But here in this study being widowed, had a positive association with pterygium. The reason may be due to aging since in this study widowed participants were older in age, despite still in debate, many authors revealed that pterygium is a degenerative (aging) disorder of the conjunctival tissue that leads to vascular proliferation.2,11,37,40,41 However; Age had no association with pterygium in this study, which is one part of the debate “It is not just a degenerative disease, but may be a proliferative disorder of the ocular surface”. Therefore, it is due to multifactorial, which includes genetics (which plays an important role in the pathogenesis) and other environmental factors such as wind, dry atmosphere, dust, chemicals, and air pollution may contribute to the development of pterygium.2,7,15,42,43
The outdoor occupation was significantly associated with the increased prevalence of pterygium. This finding was supported by plenty of previous literature as an outdoor occupation is usually associated with many environmental factors like excessive UV radiation and dust exposure that predisposed to the development of pterygium.15,33,44 For instance, Nepalese people are equally involved in outdoor activities like farming, animal rearing, and manual labor to balance their needs, thus increased exposure to potential risk factors like ultraviolet radiation in outdoor jobs.41 Another study showed that people who spend more than three-quarters of their day outside were more likely to develop pterygium, thus outdoor activity is frequently associated with the degree of UVAF and can damage limbal stem cells by activation of matrix metalloproteinase and leads to pterygium.15,42,43,45
This study found that there is a positive association between sun exposure and the development of pterygium. This might be because prolonged ultraviolet exposure causes a biological change in Bowman’s membrane and that altered protein, so formed could then act as an endogenous or “pterygiogenic” factor.24,28,42 Many other previous studies also found a strong positive association between sun exposure and pterygium as excessive sun exposure causes damage to limbal stem cells by activation of matrix metalloproteinase, which leads to pterygium.24–26,33,42,46
There was also a positive association between wind exposure and the prevalence of pterygium. This finding is supported by the study conducted in Hawaii, which showed that increased risk of exposure to wind while surfing and doing outdoor activities may contribute to pterygium development due to the entrance of airborne debris into the eye.24
According to this study, alcohol drinking had a positive association with pterygium. This finding is also supported by previous studies and described as alcohol consumption was another lifestyle-related factor related to a low socioeconomic status that leads to the development of pterygium.15,33 But contrary to this, a study conducted in Xinjiang, China revealed that alcohol consumption had no influence on the development of pterygium.9
A significant association between severe blepharitis and pterygium was found and different authors reported a similar finding. For instance, A study in Israel and others showed that severe blepharitis was significantly associated with pterygium development.13,21,28 In severe blepharitis, the inflammation and oxidative stress may play a putative role in pterygium pathogenesis by increasing the damage caused by UV radiation on the limbal basal stem cells and accelerating the multistep mutation pathway that leads to pterygium formation. Again in severe blepharitis, there is tear film abnormality which may cause a predisposition to the proliferation of fibro-vascular tissue.28
Some of the data were subject to recall bias from study participants. This study did not conduct laboratory investigations to rule out other findings related to pterygium such as OSSN (ocular surface squamous neoplasia) and this may compromise the result. Being a cross-sectional study was also another limitation. It’s difficult to determine causality between the risk factors and pterygium in this study.
Conclusion
The prevalence of pterygium in Kolla Diba town is about 18.5%, which is relatively higher. Marital status (being widowed), outdoor occupation, alcohol drinking, severe blepharitis, excessive sun exposure, and wind exposure had statistical significant associated factors for the development of pterygium.
Acknowledgments
The authors would like to acknowledge the University of Gondar for all the necessary services like diagnostic material support to conduct this study.
The authors appreciation also goes to supervisors, data collectors and study participants for their cooperation in taking part in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tano T, Ono K, Hiratsuka Y, et al. Prevalence of pterygium in a population in Northern Japan: the locomotive syndrome and health outcome in Aizu cohort study. Acta Ophthalmol. 2013;91(3):e232–6. doi:10.1111/aos.2013.91.issue-3
2. Anbesse DH, Kassa T, Kefyalew B, Tasew A, Atnie A, Desta B. Prevalence and associated factors of pterygium among adults living in Gondar city, Northwest Ethiopia. PLoS One. 2017;12(3):e0174450. doi:10.1371/journal.pone.0174450
3. Viso E, Gude F, Rodríguez-Ares MT. Prevalence of pinguecula and pterygium in a general population in Spain. Eye. 2011;25(3):350–357. doi:10.1038/eye.2010.204
4. Mahar PS, Manzar N. Pterygium recurrence related to its size and corneal involvement. J Coll Physicians Surg Pak. 2013;23(2):120–123.
5. Pan C-W, Niu Z, Zhong H, et al. Ethnic variations in pterygium in a rural population in Southwestern China: the Yunnan Minority Eye Studies. Ophthalmic Epidemiol. 2016;23(2):116–121. doi:10.3109/09286586.2015.1099685
6. Zhong H, Cha X, Wei T, et al. Prevalence of and risk factors for pterygium in rural adult Chinese populations of the Bai nationality in Dali: the Yunnan minority Eye Study. Investig Opthalmol Vis Sci. 2012;53(10):6617. doi:10.1167/iovs.11-8947
7. Saw S-M, Tan D. Pterygium: prevalence, demography, and risk factors. Ophthalmic Epidemiol. 2003;6(3):219–228. doi:10.1076/opep.6.3.219.1504
8. Fotouhi A, Hashemi H, Khabazkhoob M, Mohammad K. Prevalence and risk factors of pterygium and pinguecula. Tehran Eye Study. 2009;23(5):1125–1129.
9. Chen T, Ding L, Shan G, Ke L, Ma J, Zhong Y. Prevalence and racial differences in pterygium: a cross-sectional study in Han and Uygur adults in Xinjiang, China. Invest Ophthalmol Vis Sci. 2015;56(2):1109–1117. doi:10.1167/iovs.14-15994
10. Padha A, Koul P, Sharma S. Study of prevalence and socio- determinants of pterygium in Sub Himalayan region, India. Int J Res Med Sci. 2018;6(12):3916. doi:10.18203/2320-6012.ijrms20184882
11. Rim TH, Kang MJ, Choi M, Seo KY, Kim SS. The incidence and prevalence of pterygium in South Korea: a 10-year population-based Korean cohort study. PLoS One. 2017;12(3):e0171954. doi:10.1371/journal.pone.0171954
12. Hashemi H, Khabazkhoob M, Yekta A, Jafarzadehpour E, Ostadimoghaddam H, Kangari H. The prevalence and determinants of pterygium in rural areas. J Curr Ophthalmol. 2017;29(3):194–198. doi:10.1016/j.joco.2016.09.002
13. Parviz M, Esfandiari H, Behnaz N, Fatemeh J, Sima A, Javadi MAKM. Risk factors for pterygium in Ilam Province, Iran Parviz. J Ophthalmic Vis Res. 2017;12(3):270–274. doi:10.4103/jovr.jovr_85_16
14. Li Z, Wu S, Mai J, et al. Prevalence of and risk factors for Pterygia in a Rural Northern Chinese population. Ophthalmic Epidemiol. 2014;21(6):378–383. doi:10.3109/09286586.2014.967359
15. Rezvan F, Khabazkhoob M, Hooshmand E, Yekta A, Saatchi M, Hashemi H. Prevalence and risk factors of pterygium: a systematic review and meta-analysis. Surv Ophthalmol. 2018;63(5):719–735. doi:10.1016/j.survophthal.2018.03.001
16. Meseret A, Bejiga A, Ayalew M. Prevalence of pterygium in the rural community of Meskan District, Southern Ethiopia. Ethiop J Heal Dev. 2008;22(2):191–194.
17. Olatunji F, Owoeye J, Ayanniyi A, Sanni T, Badmos K. Blindness caused by pterygium – a case report. Sierra Leone J Biomed Res. 2011;3(1):1–4.
18. Kumah DB, Oteng-Amoako AO, Apio H. Prevalence of pterygium among kitchen staff in senior high schools in the Kumasi metropolis, Ghana. Ghana J Sci. 2011;13(2):83–88.
19. Durkin SR, Abhary S, Newland HS, Selva D, Aung T, Casson RJ. The prevalence, severity and risk factors for pterygium in central Myanmar: the Meiktila Eye Study. Br J Ophthalmol. 2008;92(1):25–29.
20. Jiao W, Zhou C, Wang T, et al. Prevalence and risk factors for pterygium in rural older adults in Shandong Province of China: a cross-sectional study. Biomed Res Int. 2014;205(4):27–36.
21. Modenese A, Gobba F. Occupational exposure to solar radiation at different latitudes and pterygium: a systematic review of the last 10 years of scientific literature. Int J Environ Res Public Health. 2017;15(1):37. doi:10.3390/ijerph15010037
22. Nangia V, Jonas JB, Nair D, Saini N, Nangia P, Panda-Jonas S. Prevalence and associated factors for pterygium in rural agrarian central India. The central India eye and medical study. PLoS One. 2013;8(12):e82439. doi:10.1371/journal.pone.0082439
23. Achigbu E, Ezepue UF. Prevalence and severity of pterygium among commercial motorcycle riders in southeastern Nigeria. Ghana Med J. 2014;48(3):153–157. doi:10.4314/gmj.v48i3.6
24. Lin AD, Miles K, Brinks MV. Prevalence of pterygia in Hawaii: examining cumulative surfing hours as a risk factor. Ophthalmic Epidemiol. 2016;23(4):264–268. doi:10.3109/09286586.2015.1119284
25. Lee KW, Choi YH, Hwang SH, et al. Outdoor air pollution and pterygium in Korea. J Korean Med Sci. 2017;32(1):143–150. doi:10.3346/jkms.2017.32.1.143
26. Pyo E-Y, Mun GH, Yoon KC. The prevalence and risk factors for pterygium in South Korea: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Epidemiol Health. 2016;38:e2016015. doi:10.4178/epih.e2016015
27. Shah SIA, Shah SA, Rai P. Factors associated with pterygium based on history and clinical examination of patients in Pakistan. J Curr Ophthalmol. 2016;28(2):91–92. doi:10.1016/j.joco.2016.03.005
28. Nemet AY, Vinker S, Segal O, Mimouni M, Kaiserman I. Epidemiology and associated morbidity of pterygium: a large, community-based case-control study. Semin Ophthalmol. 2016;31(5):446–451. doi:10.3109/08820538.2014.962169
29. Bikbov MM, Zainullin RM, Kazakbaeva GM, et al. Pterygium prevalence and its associations in a Russian population: the ural eye and medical study. Am J Ophthalmol. 2019;205:27–34. doi:10.1016/j.ajo.2019.02.031
30. Tan CCSH, Lim TH, Koh WP, et al. Epidemiology of pterygium on a tropical island in the Riau Archipelago. Eye. 2006;20(8):908. doi:10.1038/sj.eye.6702046
31. Alqahtani J. The prevalence of pterygium in Alkhobar: a hospital-based study. J Fam Community Med. 2013;20(3):159. doi:10.4103/2230-8229.121980
32. Wu K, He M, Xu J, Li S. Pterygium in the aged population in Doumen County, China. Eye Sci. 2012;18(3):181–184.
33. Marmamula S, Khanna RC, Rao GN. Population-based assessment of prevalence and risk factors for pterygium in the South Indian state of Andhra Pradesh: the Andhra Pradesh eye disease study. Investig Ophthalmol Vis Sci. 2013;54(8):5359–5366. doi:10.1167/iovs.13-12529
34. West S, Muñoz B. Prevalence of pterygium in Latinos: proyecto VER. Br J Ophthalmol. 2009;93(10):1287–1290. doi:10.1136/bjo.2008.152694
35. Shiroma H, Higa A, Sawaguchi S, et al. Prevalence and risk factors of pterygium in a Southwestern Island of Japan: the Kumejima STUDY. Am J Ophthalmol. 2009;148(5):766–771.e1. doi:10.1016/j.ajo.2009.06.006
36. Coutts SJ, Coombes A. Pterygium: prevalence and severity in an Amazonian ophthalmic setting, Brazil. Rev Bras Oftalmol. 2012;71(6):372–376. doi:10.1590/S0034-72802012000600006
37. Pan Z, Cui J, Shan G, et al. Prevalence and risk factors for pterygium: a cross-sectional study in Han and Manchu ethnic populations in Hebei, China. BMJ Open. 2019;9(2):e025725. doi:10.1136/bmjopen-2018-025725
38. Shiratori CA, De Barros JC, De Matos Lourenço R, Padovani CR, Cordeiro R, Schellini SA. Prevalência de pterígio no município de botucatu - estado de são paulo, Brasil. Arq Bras Oftalmol. 2010;73(4):343–345. doi:10.1590/S0004-27492010000400008
39. Rezvan F, Khabazkhoob M, Hooshmand E, Yekta A, Saatchi M, Hashemi H. Prevalence of and racial differences in pterygium: a multiethnic population study in Asians. Surv Ophthalmol. 2018;63(5):719–735. doi:10.1016/j.survophthal.2018.03.001
40. Song P, Chang X, Wang M, An L. Variations of pterygium prevalence by age, gender and geographic characteristics in China: a systematic review and meta-analysis. PLoS One. 2017;12:e0174587. doi:10.1371/journal.pone.0174587
41. Shrestha P, Kaiti R. A hospital based study of pterygium in tertiary care hospital of Nepal. Kathmandu Univ Med J. 2016;55(3):192–197.
42. Chun YH, Paik JS, Oh JH, Kim HS, Na KS. Association between pterygium, sun exposure, and serum 25-hydroxyvitamin in a nationally representative sample of Korean adults. Lipids Health Dis. 2018;17(1):260. doi:10.1186/s12944-018-0902-6
43. Wong W, Saw S-M, Tan DTH, et al. Prevalence of and racial differences in pterygium. Ophthalmology. 2012;119(8):1509–1515. doi:10.1016/j.ophtha.2012.02.009
44. Jonas P, Ocampo F, Lee A, Agahan D. Profile of pterygium cases seen at a tertiary referral hospital in the Philippines. J Ophthalmic Eye Res. 2016;1(1):1–9.
45. Sherwin JC, Mackey DA, Coroneo MT, et al. The association between pterygium and conjunctival ultraviolet autofluorescence: the norfolk island eye study. Acta Ophthalmol. 2011;91(4):363–370. doi:10.1111/j.1755-3768.2011.02314.x
46. Liu L, Wu J, Geng J, Yuan Z, Huang D. Geographical prevalence and risk factors for pterygium: a systematic review and meta-analysis. BMJ Open. 2013;3(11):3787. doi:10.1136/bmjopen-2013-003787
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
