Back to Journals » Cancer Management and Research » Volume 10
Pretreatment NRS-2002 scores combined with hematologic inflammation markers are independent prognostic factors in patients with resectable thoracic esophageal squamous cell carcinoma
Authors Guo XW , Liu YC, Gao F, Ji SJ , Zhou JY , Ji L, Zhou SB
Received 3 March 2018
Accepted for publication 14 May 2018
Published 2 August 2018 Volume 2018:10 Pages 2409—2418
DOI https://doi.org/10.2147/CMAR.S167179
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Xin-Wei Guo,1,* Yang-Chen Liu,2,* Fei Gao,2,* Sheng-Jun Ji,3 Ju-Ying Zhou,1 Lei Ji,1 Shao-Bing Zhou2
1Department of Radiation Oncology, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 2Department of Radiation Oncology, Affiliated Taixing People’s Hospital of Yangzhou University, Taixing, People’s Republic of China; 3Department of Radiotherapy and Oncology, Nanjing Medical University Affiliated Suzhou Hospital, Suzhou, People’s Republic of China
*These authors contributed equally to this work
Background: The purpose of this study was to investigate the prognostic values of Nutritional Risk Screening 2002 (NRS-2002) and hematologic inflammation markers in patients with esophageal squamous cell carcinoma (ESCC) receiving curative esophagectomy.
Materials and methods: A total of 277 patients with ESCC treated with standard curative esophagectomy were retrospectively analyzed. These patients were grouped for further analysis according to the systemic inflammation score (SIS), the combination of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (CNP) score and NRS-2002 score. The Kaplan–Meier method and log-rank test were adopted to calculate and compare the progression-free survival (PFS) and overall survival (OS) rates with these parameters. The Cox proportional hazards model was used to carry out univariate and multivariate analyses. Receiver operating characteristic (ROC) curves were applied to verify the accuracy of SIS, CNP and NRS-2002 for survival prediction.
Results: In univariate analysis, the following factors were significantly associated with poor PFS and OS: sex, T stage, N stage, TNM stage, SIS, CNP and NRS-2002 (all P<0.05). Furthermore, multivariate Cox regression analysis showed that CNP (hazard ratio [HR]=1.602; 95% confidence interval [CI] 1.341–1.913; P=0.000), NRS-2002 (HR=2.062; 95% CI 1.523–2.792; P=0.000) and TNM stage (HR=1.194; 95% CI 1.058–1.565; P=0.048) were independent prognostic factors for PFS. Correspondingly, CNP (HR=1.707; 95% CI 1.405–2.074; P=0.000), NRS-2002 (HR=2.716; 95% CI 1.972–3.740; P=0.000) and TNM stage (HR=1.363; 95% CI 1.086–1.691; P=0.036) were also independent prognostic factors for OS. Finally, the results of ROC curves indicated that CNP and NRS-2002 were superior to SIS as a predictive factor for PFS or OS in patients with ESCC receiving surgery.
Conclusion: This study demonstrated that CNP combined with NRS-2002 is promising as a predictive marker for predicting clinical outcomes in patients with ESCC receiving surgery.
Keywords: esophageal squamous cell carcinoma, surgery, hematological markers, nutritional risk screening, prognosis
Introduction
Esophageal cancer (EC) is the eighth most common malignancy and the fifth most common cause of cancer death all over the world.1 People’s Republic of China accounts for about half of the world’s total cases of EC,2 and esophageal squamous cell carcinoma (ESCC) is the most lethal pathological type.3 Despite significant improvements in the diagnosis and treatment, the prognosis of ESCC is still poor due to its aggressive biological behavior.4 At present, surgical resection remains the best curative method for non-metastatic EC patients. Nevertheless, most of the patients still develop local relapse or distant metastasis after esophagectomy, and so the 5-year overall survival (OS) rate is still unfavorable and ranges from 26.2% to 49.4%.5 Therefore, it is critical to search some biomarkers for distinguishing patients who are likely to develop recurrence following surgery from patients who are not easy to relapse.
Recently, there is increasing evidence that the survival of cancer patients is determined not only by tumor itself, but also by host-related factors, such as the preoperative nutritional and inflammatory status. Essentially, EC patients have a high risk of being malnourished prior to treatment, and there is accumulating evidence demonstrating that poor nutritional status is associated with inferior clinical prognosis in patients who underwent esophagectomy.6–8 Therefore, pretreatment nutritional condition is important for the prognosis of ESCC in patients receiving surgery. At present, there are many assessment methods applied to nutritional evaluation;9–11 among these, Nutritional Risk Screening 2002 (NRS-2002) was a new evaluation system, published by the European Society for Clinical Nutrition and Metabolism (ESPEN) in 2002 and was based on 128 randomized controlled trials. It was the first system in the world that was developed via evidence-based medicine with a great advantage of predicting malnutrition risk,11 especially in patients with carcinoma. Chen et al12 found that the standard of the NRS-2002 was feasible in China.
In addition, there are several studies demonstrating that the presence of a systemic inflammatory response and malnutrition were associated with a worse prognosis in various malignancies,13–16 and the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte–monocyte ratio (LMR) have been studied in EC.17–19 Recently, systemic inflammation score (SIS), a novel prognostic score consisting of serum albumin and LMR, and CNP (the combination of NLR and PLR) may be better predictive factors for postoperative clinical outcome in malignancies. To the best of our knowledge, SIS and CNP have been well documented in other types of human malignancies, including EC,20–22 but the combination of nutritional status and hematological markers has rarely been studied in ESCC patients. Therefore, we conducted this retrospective study, attempting to investigate the correlations of preoperative NRS-2002, CNP and SIS with their prognostic impacts on progression-free survival (PFS) and OS in ESCC patients.
Materials and methods
Patients
Between January 2010 and December 2013, a total of 277 esophageal carcinoma patients who underwent esophagectomy and lymph node dissections at the Department of Thoracic Surgery, The First Affiliated Hospital of Soochow University, were recruited in this retrospective research. The inclusion criteria were as follows: 1) curative esophagectomy with R0 resection and no presence of preoperative adjuvant therapy; 2) histologically proven ESCC; 3) normal liver and renal function, without severe dysfunction of important organs, and overall performance status of 0 or 1; 4) complete record of pretreatment hematological variables; 5) no presence of distant metastasis; 6) without second primary cancers before or at diagnosis; 7) patients with complete follow-up time; and 8) no presence of infection or inflammatory conditions, such as rheumatologic conditions, connective tissue disorders or heart diseases. Finally, 277 patients were enrolled and analyzed in this study. Clinicopathological features were obtained from the patients’ medical records. The hematological and laboratory parameters were routinely examined in all patients within 1 or 2 weeks prior to surgery. All patients were staged according to the American Joint Committee on Cancer staging manual (seventh edition, 2010).23 This research was approved by the Ethics Committee of The First Affiliated Hospital of Soochow University. Informed written consent was obtained from all individual participants included in this study.
Surgery
Esophagectomy with thoracic and abdominal dissection was required in each surgical procedure, including the left thoracotomy with standard lymphadenectomy and the cervico-thoraco-abdominal approach with extended lymphadenectomy. In this research, 168 patients (61%) underwent two-field lymphadenectomy. In this cohort of patients, thoracoabdominal lymphadenectomy was performed, including the subcarinal, paraesophageal, pulmonary ligament, diaphragmatic and pericardial lymph nodes, as well as those located along the lesser gastric curvature, the origin of the left gastric artery, the celiac trunk, the common hepatic artery and the splenic artery. For 109 (39%) patients, the three-field lymphadenectomy was performed, and in this group, the cervical lymph nodes were thought to be abnormal according to preoperative imaging evaluation.
Nutritional assessment
Nutritional risk was assessed by NRS-2002 within 1 week before surgery.11 NRS-2002 consists of impaired nutritional status (low, moderate or severe) and severity of disease (low, moderate or severe), with an adjustment for age ≥70 years. Nutritional status was evaluated by three variables: body mass index (BMI), recent weight loss and food intake during 1 week before treatment. For severity of disease, as an indicator of stress metabolism and increased nutritional requirements, a score between 1 and 3 was given according to the recommendations. A data collection sheet was used to obtain information about changes in the body weight, food intake and severity of disease according to the ESPEN guidelines.24 A total score exceeding 3 suggested nutritional risk, whereas that below 3 suggested no nutritional risk temporarily.
Hematological parameters calculation and follow-up
The following pretreatment hematological parameters were collected within 1 week prior to the initial treatment: serum albumin, neutrophil count, lymphocyte count, monocyte count and platelet count. NLR, PLR and LMR were calculated by division of the absolute values of the corresponding hematological parameters. The median values of serum albumin, NLR, PLR and LMR were as the optimum cutoff value. Then the SIS was scored as follows: patients with both elevated serum albumin and elevated LMR were assigned a score of 0, patients with either decreased serum albumin or decreased LMR were assigned a score of 1 and patients with both decreased serum albumin and decreased LMR were assigned a score of 2. Correspondingly, the CNP was established based on the combination of NLR and PLR: patients with both an elevated NLR and PLR were allocated a score of 2, and patients showing one or neither were allocated a score of 1 or 0, respectively.
After the completion of treatment, all patients were asked to return to the hospital for examination every 3 months for the first year, every 6 months for the next 2 years and then annually. The duration of follow-up was calculated from the day of treatment to the day of death or March 2018.
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Science program (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL, USA). CNP and SIS were divided into CNP 0, SIS 0 and CNP1/2, SIS 1/2 groups by corresponding score, respectively. The relationships between clinical characteristics and CNP, SIS and NRS-2002 were examined by chi-square test or Fisher’s exact test. The end points for this study were 5-year PFS and 5-year OS. PFS was defined as the length of time after surgery during which the patient survived with no sign of tumor recurrence. OS was calculated from date of surgery to the date of individual’s death or last follow-up. The Kaplan–Meier method and log-rank tests were used for 5-year PFS and 5-year OS analyses. Univariate and multivariate analyses of Cox regression proportional hazard model were used to evaluate the influence of each variable on PFS and OS with the enter method. Hazard ratio (HR) with 95% confidence interval (CI) was used to quantify the strength of the association between predictors and survival. Receiver operating characteristic (ROC) curves were also plotted to verify the accuracy of CNP, SIS and NRS-2002 for survival prediction. A 2-tailed P-value ≤0.05 was considered statistically significant.
Results
Clinicopathological characteristics of patients
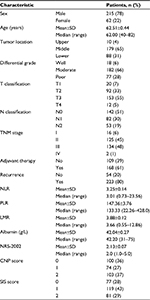
The basic characteristics of the enrolled patients are shown in Table 1. Among the 277 patients, 62 (22%) were females and 215 (78%) were males. The median age prior to surgery was 62 years (range 40–82 years). The location of the tumors mostly occurred in the middle third (179/277, 65%) and the lower third (88/277, 31%) of the esophagus. In our cohort, 109 (39%) patients underwent esophagectomy alone and 168 (61%) received postoperative chemotherapy or radiotherapy. None of these patients received neoadjuvant therapy before surgery. The median follow-up period was 36 months (range 6–72 months). During the follow-up period, 223 (80%) patients had tumor recurrences (48 cases with surgical anastomosis recurrences, 118 cases with locally regional lymph node metastasis and 57 cases with distant metastasis).
Associations of NRS-2002 and inflammation-based markers with clinicopathological characteristics
The relationships of CNP, SIS and NRS-2002 with clinicopathological characteristics are shown in Table 2. We determined the cutoff value of 42.20 g/L for serum albumin, 3.01 for NLR, 133.33 for PLR and 3.66 for LMR according to the corresponding median values. As already mentioned, the CNP was established based on the combination of NLR and PLR and the SIS was established based on the combination of serum albumin and LMR; then, 100 (36%) and 77 (28%) patients were assigned a score of 0 in CNP and SIS, respectively; 74 (27%) and 119 (43%) patients were assigned a score of 1 in CNP and SIS, respectively; and 103 (37%) and 81 (29%) patients were assigned a score of 2 in CNP and SIS, respectively.
As shown in Table 2, we identified a close relationship between CNP, SIS, NRS-2002 and TNM stage (all P<0.05), that is to say, high CNP, SIS and NRS-2002 score, compared with low ones, were significantly correlated with advanced TNM staging. Furthermore, we found that the high scores in SIS and CNP were significantly correlated with more advanced N status (P<0.05). In addition, the NRS-2002 ≥3.0 group was related to advanced T stage and elder age.
PFS and OS according to CNP, SIS and NRS-2002 status
Among the 227 patients,, the median PFS time was 15 months (range: 2–72 months); the PFS rates at the 1-, 3- and 5-year period were 59.6%, 22.0% and 19.5%, respectively; as shown in Figure 1, in the CNP=0 group, the 1-, 3- and 5-year PFS rates were 80.0%, 45.0% and 42.0%, respectively; in the CNP=1 group, the PFS rates were 52.7%, 12.2% and 12.2%, respectively; and in the CNP=2 group, the PFS rates were 44.7%, 6.8% and 2.9%, respectively (Figure 1A; χ2=60.348, P=0.000). In the SIS=0 group, the 1-, 3- and 5-year PFS rates were 75.3%, 39.0% and 33.8%, respectively; in the SIS=1 group, the PFS rates were 56.3%, 17.6% and 16.8%, respectively; and in the SIS=2 group, the PFS rates were 49.4%, 12.3% and 9.9%, respectively (Figure 1B; χ2=19.057, P=0.000). In the NRS-2002 <3.0 group, the 1-, 3- and 5-year PFS rates were 65.8%, 37.3% and 33.5%, respectively, while in the NRS-2002 ≥3.0 group, the PFS rates were 50.9%, 5.20% and 0.00%, respectively (Figure 1C; χ2=48.702, P=0.000).
Correspondingly, in our cohort, the median OS time was 36 months (range: 6–72 months); the OS rates at the 1-, 3- and 5-year time were 96.4%, 47.7% and 29.6%, respectively; the OS grouped according to CNP, SIS and NRS-2002 status. Additionally, the 1-, 3, and 5-year OS rates were 99.0%, 70.0%, and 56.0% in the CNP=0 group, 95.9%, 43.2% and 25.7% in the CNP=1 group, and 94.2%, 29.1% and 6.8% in the CNP=2 group, separately (Figure 2A; χ2=73.982, P=0.000). The 1-, 3- and 5-year OS rates were 98.7%, 66.2% and 53.2% in the SIS=0 group, 95.8%, 47.1% and 26.1% in the SIS=1 group, and 93.8%, 30.9% and 12.3% in the SIS=2 group (Figure 2B; χ2=36.552, P=0.000), respectively. Furthermore, in the NRS-2002 <3.0 group, the 1-, 3- and 5-year OS rates were 98.8%, 63.4% and 49.1% separately, while in the NRS-2002 ≥3.0 group, the OS rates were 92.2%, 25.9% and 2.6% respectively (Figure 2C; χ2=83.427, P=0.000). On a whole, PFS and OS of patients in the CNP=0, SIS=0 and NRS-2002 <3.0 group were obviously improved compared with patients in the CNP=1/2, SIS=1/2 and NRS-2002 ≥3.0 groups.
Univariate and multivariate survival analyses
The results of univariate analysis of the factors related to PFS and OS are shown in Table 3. In univariate analysis, the following factors were significantly associated with poor PFS and OS: sex, T stage, N stage, TNM stage, CNP, SIS and NRS-2002 (all p<0.05). Table 4 shows the results of multivariate Cox regression analysis of the factors related to PFS and OS. This analysis showed that CNP (HR=1.602; 95% CI 1.341–1.913; P=0.000), NRS-2002 (HR=2.062; 95% CI 1.523–2.792; P=0.000) and TNM stage (HR=1.194; 95% CI 1.058–1.565; P=0.048) were independent prognostic factors for PFS in patients with ESCC after surgery. Correspondingly, CNP (HR=1.707; 95% CI 1.405–2.074; P=0.000), NRS-2002 (HR=2.716; 95% CI 1.972–3.740; P=0.000) and TNM stage (HR=2.363; 95% CI 1.086–1.691; P=0.036) were also independent prognostic factors for OS in ESCC patients following surgery (Table 4)
ROC curve for survival prediction
Figure 3 shows the ROC curves analysis of CNP, SIS and NRS-2002 for PFS and OS prediction. As shown in Figure 3A, the area under the curve (AUC) for CNP, SIS and NRS-2002 was 0.788 (95% CI: 0.727–0.850, P=0.000), 0.654 (95% CI: 0.573–0.736, P=0.003) and 0.760 (95% CI: 0.704–0.816, P=0.000), respectively. The results indicated that CNP and NRS-2002 were superior to SIS as predictive factors for PFS in patients with ESCC receiving surgery.
ROC curves for OS were also plotted; as shown in Figure 3B, AUC was 0.774 (95% CI: 0.715–0.832, P=0.000) for CNP, 0.699 (95% CI: 0.632–0.766, P=0.045) for SIS and 0.771 (95% CI: 0.717–0.826, P=0.000) for NRS-2002, indicating that CNP and NRS-2002 were also superior to SIS as predictive factors for OS in patients with ESCC after surgery.
Discussion
Malnutrition and systemic inflammatory response are common in various malignancies and are correlated with poor prognosis. In this clinical research, we explored the importance for the survival prediction of pretreatment NRS-2002, CNP and SIS scores in patients with ESCC receiving surgery. The present study demonstrated that CNP and NRS-2002 were not only the significant risk factors for PFS, but also the independent prognostic factors for OS in ESCC patients following surgery. To the best of our knowledge, this is the first report to demonstrate the clinical significance of CNP and SIS combined with NRS-2002 in patients with ESCC by curative surgery.
Malnutrition has been considered as a significant prognostic factor in cancer patients since 1980, when Dewys et al25 discovered a shorter survival in malnourished patients compared with well-nourished ones. Since then, the correlation between nutritional risk and clinical prognosis has also been demonstrated in a variety of patients, including different types of malignancies.26 Liu et al27 demonstrated that preoperative nutritional status, a novel nutritional-based prognostic score, was independently associated with OS in gastric cancer. Wu et al6 showed that pre-therapeutic serum albumin level was a significant prognostic factor for survival outcomes in patients who underwent esophagectomy. Therefore, nutritional assessment is critical to the efficacy and prognosis of anti-neoplastic therapy, and it should be taken into consideration along with other well-defined prognostic factors for better preoperative assessment and prognostic evaluation.
At present, there are many assessment methods applied to nutritional evaluation; among these, patient-generated subjective global assessment (PG-SGA) is widely used as a golden standard for subjective assessment of nutritional status in cancer patients.28–29 On the other hand, NRS-2002 is a valid method for identifying risk patients and those who will benefit from nutritional treatment.11 A previous study has shown that 28% of patients were at nutritional risk based on NRS-2002, and 34% of patients with head and neck cancer were malnourished according to PG-SGA.30 These results suggested that NRS-2002 seems to be a reliable indicator of malnutrition. Because PG-SGA required specialized nurses to implement and needed long- time evaluation in everyday clinical practice, in contrast, NRS-2002 was the first one developed via evidence-based medicine in the world, with a great advantage of the prediction of malnutrition risk, and it was applicable for a preoperative assessment for patients with ESCC receiving surgery, with the characteristics of non-invasiveness, objective evaluation, convenience and generalization. Therefore, our present study cohort adopts NRS-2002 as nutritional risk assessment tool to stratify patients in malnourished and well-nourished groups. The results showed that PFS and OS of ESCC patients in the NRS-2002 <3.0 group were obviously improved compared with those of patients in the NRS-2002 ≥3 groups. These results indicated that NRS-2002 might be an excellent instrument in predicting the association between nutritional risk and clinical outcome; consequently, preoperative nutritional support is necessary in ESCC patients with a preoperative nutritional score (NRS-2002) ≥3.0.
In the case of hematologic inflammation markers, a high CNP score was significantly associated with poor PFS and OS in our ESCC patients receiving curative esophagectomy with R0 resection. Since the pathologist Rudolf Virchow first discovered leukocytes in malignant tissue specimens about 150 years ago,31 the prognostic values of pretreatment hematologic markers have been highlighted. Compelling evidence suggested that there were statistically significant differences in the survival rates grouped by NLR, PLR and LMR levels for several types of malignancies.16–19 However, the current study also showed that hematologic parameters were controversial in the prediction of prognosis in esophagus carcinoma. Duan et al32 reported that preoperative serum NLR is a useful prognostic marker to complement TNM staging for operable ESCC patients, particularly in patients with stage IIIA disease; on the contrary, Rashid et al33 found that NLR did not prove to be a significant predictor of number of involved lymph nodes, disease recurrence or death. Furthermore, survival time was not significantly different between patients with high (≥3.5) or low (<3.5) NLR (P=0.49). This controversy might result from the optimal cutoff points for NLR and PLR to predict overall survival. In our present study, therefore, the median values of NLR and PLR were as the cutoff point, which were 3.01 and 133.33, respectively, and then the CNP score was established based on the combination of NLR and PLR, consisting of more prognostic information than single NLR or PLR; the results indicated that pretreatment CNP score was an independent risk factor for PFS and OS in ESCC following surgery; nevertheless, there was no prognostic association found for SIS in multivariate analyses.
The limitations of this study are as follows: first, not all hematologic markers of inflammation were used in the analysis, because some biomarkers were not routinely examined, such as C-reactive protein34 and fibrinogen.35 Second, it was a single-institution, retrospective study. Third, relying on recalled weight, height and food intake from the medical record might have caused bias in assessing BMI and weight change, and ultimately had some effect on NRS-2002 rating; finally, 277 patients with ESCC were enrolled in this study and the sample size is relatively small and may be insufficient to strengthen our results. Given these limitations, future larger randomized trials are needed to clarify these results.
In conclusion, this study demonstrated that CNP combined with NRS-2002 is promising as a predictive marker for predicting clinical outcomes in patients with ESCC receiving surgery. However, considering the retrospective nature of this study, large-scaled prospective trials are still warranted to verify our results.
Acknowledgment
This work was supported by the grant from Suzhou Cancer Clinical Medical Center (grant no. Szzx201506).
Disclosure
The authors report no conflicts of interest in this work.
References
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.