Back to Journals » International Journal of Women's Health » Volume 15
Preterm Birth Among Intrapartum Cesarean Deliveries at Public Hospitals in Southern Ethiopia: A Multicenter Retrospective Analysis of Risk Factors
Received 2 December 2022
Accepted for publication 27 May 2023
Published 31 May 2023 Volume 2023:15 Pages 869—879
DOI https://doi.org/10.2147/IJWH.S398830
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Marleen van Gelder
Dereje Zewdu,1 Temesgen Tantu2
1Department of Anesthesia, College of Medicine and Health Science, Wolkite University, Wolkite, Ethiopia; 2Department of Obstetrics and Gynecology, College of Medicine and Health Science, Wolkite University, Wolkite, Ethiopia
Correspondence: Dereje Zewdu, Email [email protected]
Purpose: Although the underlying causes for preterm birth are thought to be multifactorial irrespective of delivery mode, no study investigated its risk factors amongst cesarean deliveries (CD). Thus, we aimed to identify potential risk factors for the occurrence of preterm birth (PTB) among intrapartum CD.
Methods: Data from 1659 singleton intrapartum CDs were retrospectively recruited using medical records and an obstetric database. Gestational age was calculated using the last menstrual period (LMP) and ultrasound report of early onset pregnancy. A multivariable logistic regression analysis was performed to identify potential risk factors associated with PTB. Odds ratios (ORs) and 95% confidence intervals (95% CI) were used. Statistical analysis was performed using SPSS version 26.0.
Results: In this study, the prevalence of PTB among intrapartum CD was 6.1% (95% CI: 4.9, 7.2%). In the multivariable logistic regression model; grand parity ≥ 5 (adjusted odds ratio (AOR) = 2.43, 95% CI: 1.72– 4.73), maternal age < 20 years (AOR=2.63, 95% CI, 1.03– 6.71), maternal age ≥ 35 years (AOR=3.83, 95% CI, 1.49– 5.35), cesarean section scar ≥ 2 (AOR=4.86, 95% CI: 2.68– 8.94), antepartum hemorrhage (AOR=4.37, 95% CI: 2.22– 8.63), pregnancy-induced hypertension (AOR=2.92, 95% CI: 1.41– 6.04), and premature rupture of membranes (AOR=4.56; 95% CI: 1.95– 10.65) were significantly associated with PTB.
Conclusion: The current study showed an association between PTB and a multitude of obstetric variables, including grand parity ≥ 5, CS scar ≥ 2, antepartum hemorrhage, pregnancy-induced hypertension, and premature rupture of the membrane. Understanding these factors could help to implement improved quality of obstetric and neonatal care to increase survival and reduce morbidity among preterm birth.
Keywords: preterm birth, cesarean section, risk factors, cesarean section, intrapartum cesarean delivery
Introduction
Addressing the overall burden of preterm birth (delivery before completed 37 weeks of gestation) is crucial to reduce its related perinatal adverse outcomes. Globally in 2014, it is estimated that approximately 14.84 million (10.6%) neonates were born preterm, and 12 million (81.1%) occurred in Asia and sub-Saharan African countries.1,2 Further, preterm birth (PTB) is a leading cause of mortality in children <5 years of age worldwide, contributing to an estimated 16% of all deaths and 35% of deaths among newborn babies.3
Because of the immature organ system, even the surviving preterm babies are at high risk of developing short-term and long-term adverse outcomes and profoundly correlated with increased expenses to health systems and significant psychological consequences to the parents of the preterm babies.4–6
Despite the increased understanding of mechanisms and determinant factors related to PTB and the implementation of various medical interventions to decrease its prevalence and negative impacts, PTB remains a growing public and clinical health concern.7–9
In Ethiopia, the prevalence of PTB has ranged between 4.4% and 25.9%; however, these results may not represent the entire Ethiopian population because of differences in socio-demographic characteristics, the level of hospital, and the quality of healthcare.10–13
The alarmingly increased rate of CD observed in recent years has paralleled the scaled-up in the prevalence of PTB.14 Several potential risk factors were reported to be associated with PTB, including extreme maternal age,15–23 grand parity,24–28 previous CD history,23,29,30 antepartum hemorrhage,18,26,31 pregnancy-induced hypertension,10,12,20,26,32,33 and premature rupture of the membrane.20,26,33,34
However, the underlying causes vary between studies, and it is unclear whether the outcomes linked to PTB in vaginal deliveries are identical to CD. While some studies35 have reported a positive correlation between CD and improved outcomes of preterm babies, others36,37 found less risk of neonatal death in VB and no correlation between the delivery modes. Given these facts, the preferred mode of delivery for mothers to be in preterm labor is a matter of scrutiny.35–37
Unfortunately, in Ethiopia, there is insufficient data concerning the etiology of PTB and the impact of having the previous CD in subsequent pregnancies. The intrapartum CD might result from a failed induction to terminate the pregnancy when there are obstetric complications such as antepartum hemorrhage and pregnancy-induced hypertension during the pre-labor stage.
Identification of potential risk factors, particularly among intrapartum CD, would be even more important for low-income countries, where the burden of PTB-related neonatal morbidity and mortality is profoundly high.38,39 In the same way, translating those findings into clinical practice offers opportunities for providing stepwise and appropriate decision-making help in decreasing the rate of PTB and its associated complications.
To our knowledge, no study examined the prevalence and risk factors of PTB amongst intrapartum CD in low-income countries, including Ethiopia. Similarly, the underlying factor that raises the likelihood of preterm CDs, such as a prior CS scar and obstetrics indications throughout labor, may be an oddity that warrants further research because it is yet unknown whether or not the PTB risk factors for CS and vaginal deliveries are comparable.40 We therefore sought out to examine the prevalence of PTB and its associated risk factors amongst mothers who underwent intrapartum C-sections in four hospitals in Southern Ethiopia.
Methods
Study Design and Study Participants
In this hospital-based retrospective cohort study, all women who underwent intrapartum CD were studied over 2-years period, from September 2020 to August 2022 at public hospitals located in Gurage Zone, Southern Ethiopia: Wolkite University Specialized Hospital (WKUSH), Gunchure General Hospital (GGH), Atat Hospital (ATH) and Mehal Amba Hospital (MAH).
During the study period, all moms who underwent intrapartum CD for 1704 singleton live births were included. Of these, 1659 women (726 in WKUSH, 413 in GGH, 358 in ATH, and 162 in MAH) who had intrapartum CD and their newborns were subsequently examined for the study’s final analysis (Supplementary Data 1). We excluded 45 study participants who had (1) multiple pregnancies (n=21), (2) insufficient documentation (n=13), and (3) mothers whose gestational ages were unknown (n=11). The four hospitals were the main obstetric centers proving cesarean deliveries in the Gurage Zone during the study period.
This study was conducted under the Declaration of Helsinki Ethical Principles for Medical Research involving human subjects protocol. The study was approved by the Ethical Review Board of Wolkite University. Because of the retrospective nature of the study design and the data being anonymized, informed written consent was exempted.
Data Collection
We retrospectively collected data from medical records and an obstetric database, which includes baseline maternal sociodemographic characteristics, obstetrics, and neonatal and intraoperative data using a standard checklist adapted from previous studies, by trained three data collectors. The data collectors received two days of training for the study before data collection. The completeness of data was checked daily by the principal investigators.
The sociodemographic characteristics included maternal age, residency area, monthly family income, maternal medical illness, antenatal care (ANC) visits, and educational level. Obstetrics variables included parity, adverse obstetric history (previous history of abortion and preterm birth), previous history of cesarean delivery, pregnancy-induced hypertension (PIH), antepartum hemorrhage (APH), premature rupture of membrane (PROM), induced/spontaneous labor, fetal presentation, and other obstetrics complications and intraoperative data included types of anesthesia, incision type, and neonatal sex.
Study Outcome
The main outcome measure was determining the prevalence of preterm birth (PTB), which was defined as intrapartum CD occurring after 28 weeks and before 37 completed weeks of gestation. We estimated the gestational age based on the last menstrual period (LMP) and the results of the earliest ultrasound assessment before 20 weeks of gestation. The secondary objectives were the identification of potential risk factors for PTB among intrapartum CD.
Statistical Analysis
Data were cleaned, coded, and transported into SPSS (Statistical Package for the Social Sciences for Windows version 26.0, SPSS Inc., Chicago, Illinois, USA) for final analysis. Results were converted into categorical data and displayed as frequency tables. Comparison of the categorical data between groups was analyzed using Pearson Chi-square or Fisher exact test. Bivariate analysis was used to check the association between potential predictors and outcome variables. We checked the outliers and multi-collinearity using standardized residual tests, and VIF and tolerance, respectively. For controlling confounding factors, multivariable logistic regression analysis was utilized among significantly associated potential independent variables by univariate analysis. The Hosmer–Lemeshow test was utilized to check model fitness. Odds ratio (OR) with a 95% confidence interval (CI) was used to check the strength of the association.
Results
Sociodemographic Characteristics of Study Participants with and without Preterm Birth Among Intrapartum Cesarean Delivery
During the study period, 1657 intrapartum cesarean deliveries were included for final analysis (Supplementary Data 1). The overall prevalence of preterm birth was 101 (6.1%) (95%: CI 4.9–7.2%). Results for sociodemographic characteristics are displayed in Table 1. Around 85% of maternal age lies between 20 and 34 years, with 6.0% (n=100) and 7.2% (n=119) being aged <20 and ≥35 years, respectively. Most study participants were urban residents (66.2%), married (97.7%), and Muslim by their religion (39.1%). Obesity was rare, with only 4.6% of the study participants having a BMI of >30 kg/m2.
 |
Table 1 Sociodemographic Characteristics of Study Participants with and without Preterm Birth Among Intrapartum Cesarean Delivery |
Obstetrics and Neonatal Characteristics of Study Participants with and without Preterm Birth Among Intrapartum Cesarean Delivery
Results for obstetrics and neonatal characteristics are displayed in Table 2. According to the study, 38% of the participants were primigravida, and 34% of the women had five or more deliveries. Concerning previous delivery, 19.3% of women who had prior delivery had one CD and 8.7% had undergone at least two CDs. Premature rupture of membranes (4.2%), antepartum hemorrhage (5.1%), and pregnancy-induced hypertension (7.8%) were the most frequently identified obstetric problems during the preoperative period. When preterm births and abortions (spontaneous or medically induced) were compared, 2.3% of women had a record of preterm deliveries, whereas 7.0% had at least one abortion.
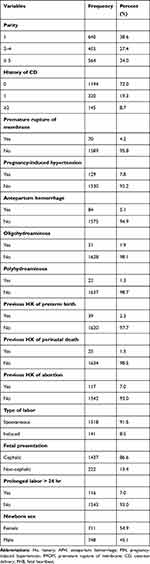 |
Table 2 Obstetrics and Neonatal Characteristics of Study Participants with and without Preterm Birth Among Intrapartum Cesarean Delivery |
Multivariable Logistic Regression Analysis Showing Risk Factors Associated with PTB Among Intrapartum Cesarean Deliveries
The bivariate analysis observed that the sociodemographic variables including maternal age, residence area, and maternal medical illness, and obstetric factors including parity, previous history of CD, APH, PIH, PROM, and labor onset were significantly associated with an increased risk of PTBs. The risk factors found to be associated with PTB at bivariate analysis were further analyzed to control possible confounders.
Multivariable logistic regression analysis demonstrated that the risk of having PTB was 2.63 times (AOR=2.63, 95% CI, 1.03–6.71) and 3.83 times (AOR=3.83, 95% CI, 1.49–5.35) higher in women <20 and ≥35 years compared to women aged 20–34 years, respectively. Grand parous (≥5 parity) mothers were 2.43 times (AOR=2.43, 95% CI: 1.72–4.73) as likely to have PTB compared to mothers with less parity. Mothers who had a previous history of CD ≥ 2 were 4.86 times (AOR=4.86, 95% CI: 2.68–8.94) as likely to have PTB compared to those without a history of cesarean delivery. Women presented with APH were 4.37 times (AOR=4.37, 95% CI: 2.22–8.63) more likely to have PTB than those without APH. The likelihood of having PTB was 2.92 times (AOR=2.92, 95% CI: 1.41–6.04) higher among mothers presented with PIH compared to those without PIH. Furthermore, PROM increases the risk of having PTB > 4-fold (AOR=4.56; 95% CI: 1.95–10.65) compared to mothers presented without PROM (Table 3).
 |
Table 3 Multivariable Logistic Regression Analysis Showing Risk Factors Associated with PTB Among Intrapartum Cesarean Deliveries |
Discussion
The current study demonstrated that the overall PTB prevalence in neonates delivered by emergency cesarean section was 6.1%. This finding is similar to previous studies conducted in other parts of Ethiopia; 4.4% and 8.1% were reported in Gondar10 and Northern Ethiopia,11 respectively. Contrarily, other studies12,13 conducted in Ethiopia showed the PTB rate ranging between 12.8% and 25.9%, which is significantly higher than our findings.
This significant difference is one of the manifestations showing the existing erroneous in determining the gestational age. Surprisingly, despite the reported agreement across the studies on the PTB definition (<37 weeks of complete gestation), in developing countries, the assessment procedures used to determine gestational age have not been reported in 65% of the cases.41 Notably, an accurate estimation of gestational age is challenging in low-income countries because of poor health-seeking behavior, limited access to ultrasound, and the shortage of expertise within and across the hospitals. As a result, a higher chance of considering low birth weight as PTB might be possible, which leads to an overestimation of its prevalence rate. Certainly, predictors and interventions to reduce the prevalence of low birth weight might differ from PTB: therefore, high-quality research using standardized assessment tools for low-income countries is urgently needed. Moreover, the wide variations in the level of healthcare facilities, inclusion criteria, and management strategies between clinical setups could explain the observed difference.10–13
Surprisingly, there is not little that can be accomplished to lessen preterm birth using available techniques.8,9 Standardizing the neonatal intensive care health system would be crucial to minimizing the morbidity and mortality of preterm newborns in low-income areas where expertise and medical resources are scarce and it is not realistic to adopt the modeled therapies.24 Future research will also be necessary to create preventive interventions that can be scaled up and implemented within the current healthcare system.
In this study, the significant risk factors found to be associated with PTB were extreme maternal age (<20 and ≥35 years), premature rupture of membrane (PROM), CS scar ≥2, antepartum hemorrhage (APH), preeclampsia, and grand parity (≥5 parity).
The current study found that extreme maternal age (<20 and ≥35 years) was associated with an increased risk of PTB. Compared to mothers aged between 20 and 34 years, those mothers aged <20 and ≥35 years had a 2.63 and 3.83-fold increased risk of PTB, respectively. Our findings are consistent with previous reports15–17 that found a U-shaped relationship between the risk of PTB and maternal age. Other studies also found that younger <20 years18–20 and older ≥35 years mothers21–23,28 were at a significantly increased risk of PTB. Contrarily, other studies31,42 have observed a higher risk of PTB among mothers aged between 20 and 30 years. This difference may associate with variations in the geographical area, inclusion criteria, sociodemographic characteristics, and management protocols.
We also found that high parity ≥5 increased the risk of preterm birth. Grand parity (parity ≥5) mothers were 2.43 times more at risk of having a PTB. This finding is consistent with other studies24–28 that demonstrate an association between PTB and high parity. This could be because grand parity mothers are more likely to have a chronic medical illness (diabetes mellitus, chronic anemia, and hypertension) and physiologic risk factors (placenta previa, abruption placenta, malpresentation, and hemorrhagic complications), which are known to impact fetal and maternal conditions, and may predispose to preterm CD.43
Moreover, our study upheld the hypothesis for the previous history of CD; mothers with cesarean scar ≥2 were 4.86 times more likely to have preterm CD when compared to those without a history of cesarean deliveries. Consistently, other studies23,29,30,40 also demonstrated the likelihood of increased risk of PTB in mothers who had previous CS scars. These indicate that giving birth by CD has been associated with an increased risk of PTB CD in subsequent pregnancies. In a systematic review of ten cohort studies involving more than 10 million participants, those with previous CD histories had a significantly increased risk of PTB in the subsequent pregnancy, even after adjusting confounding factors.44 Thus, previous CS scars conferred a substantially increased risk of PTB CD in the following pregnancy in those mothers. Thus, efforts to minimize the possibility of a cesarean section may decrease the risk of PTB in the subsequent pregnancy.
Mothers presented with antepartum hemorrhage (APH) were associated with increased odds of PTB. The explanation is that APH, especially with active bleeding, may be indicated for intrapartum CD, irrespective of gestational age, which increases the risk of PTB. In agreement with our findings, others18,26,31 also illustrated that APH was significantly associated with an increased risk of PTB. Depending on the type and severity of APH, women may undergo vaginal delivery/elective CS in non-emergency conditions, whereas those mothers with life-threatening bleeding promptly required immediate CD even at preterm age, which might explain the observed relationship in our study.45,46
PIH (pregnancy-induced hypertension) was associated with a significantly increased risk of PTB CD compared to mothers indicated without PIH. This finding is similar to previous studies conducted in Ethiopia,10,12,20,32 and elsewhere.26,31,33,34 PIH leads both mother and fetus at high risk. The decision to deliver babies in women with PIH needs a balancing approach between the risks of its worsening and PTB. However, in cases of fetal distress and maternal complications, the possibility of undergoing intrapartum CD increases irrespective of gestational age, which may increase the risk of PTB.47 Thereby, proper management of HIP is imperative to reduce its negative impact on PTB.
Evidence also shows that the decision to terminate the pregnancy in PROM, whether with cesarean or vaginal delivery considering the risk/benefit ratio, is based on maternal and fetal conditions.48,49 These indicate that despite the optimal timing and mode of delivery is controversial: pregnancy-related complications such as fetal distress in labor, maternal fever, placental abruption, and infection are common in preterm PROM, resulting in an increased risk of intrapartum CD. Consistent with previous reports,20,26,33,34 our study also found that mothers with prolonged PROM were 4.56 times more at risk for PTB than their counterparts.
These findings suggest that it is essential to comprehend the pathogenesis of placental inflammation and the impact of pathologies related to the placenta on preterm labor to develop more efficient strategies for early detection and lowering the prevalence of PTB and its associated overall burden.50
This study has several strengths. First, it is the first to investigate the risk factors for PTB, particularly for intrapartum cesarean deliveries in low-income countries, using a relatively larger sample size in multiple hospitals. Second, the calculation of gestational age using either known LMP or early ultrasound reports scanned by senior obstetricians confers certain advantages over non-obstetricians. Nonetheless, it is not without some limitations. Because of the retrospective nature of the study design, some important risk factors are not included, which may explain the need for future prospective studies at the national level to extrapolate the results to the entire population.
Conclusion
One in 16 intrapartum cesarean deliveries was associated with PTB. Maternal age (<20 and ≥35 years), grand parity, CS scar ≥2, APH, PIH, and PROM were the best predictors for PTB among intrapartum CD. Clinicians who provide obstetric care must identify potential risk factors to improve the quality of antenatal, obstetric and neonatal care to increase the survival and reduce related morbidity of preterm birth. Further prospective investigations are required to extend these results and establish future clinical recommendations.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge Wolkite University, data collectors, and medical record office staff for their invaluable support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Butler AS, Behrman RE. Preterm Birth: Causes, Consequences, and Prevention. National academies press; 2007.
2. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modeling analysis. Lancet Glob Health. 2019;7(1):e37–46. doi:10.1016/S2214-109X(18)30451-0
3. Li Z, Hsiao Y, Godwin J, Martin BD, Wakefield J, Clark SJ; with Support from the United Nations Inter-Agency Group for Child Mortality Estimation and Its Technical Advisory Group. Changes in the spatial distribution of the under-five mortality rate: small-area analysis of 122 DHS surveys in 262 subregions of 35 countries in Africa. PLoS One. 2019;14(1):e0210645. doi:10.1371/journal.pone.0210645
4. Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(1):1–4. doi:10.1186/1742-4755-10-S1-S2
5. Lakshmanan A, Agni M, Lieu T, et al. The impact of preterm birth< 37 weeks on parents and families: a cross-sectional study in the 2 years after discharge from the neonatal intensive care unit. Health Qual Life Outcomes. 2017;15(1):1–3. doi:10.1186/s12955-016-0578-4
6. Hodek JM, Von der Schulenburg J, Mittendorf T. Measuring economic consequences of preterm birth-methodological recommendations for the evaluation of personal burden on children and their caregivers. Health Econ Rev. 2011;1(1):1. doi:10.1186/2191-1991-1-6
7. Di Tommaso M, Berghella V. Cervical length for the prediction and prevention of preterm birth. Expert Rev Obstet Gynecol. 2013;8(4):345–355. doi:10.1586/17474108.2013.811932
8. Lamont RF. Advances in the prevention of infection-related preterm birth. Front Immunol. 2015;6:566. doi:10.3389/fimmu.2015.00566
9. Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C. Prevention of preterm birth: harnessing science to address the global epidemic. Sci Transl Med. 2014;6(262):262sr5. doi:10.1126/scitranslmed.3009871
10. Gebreslasie K, Hofoss D, Kirkevold M, Bjørk IT, Foss C. Preterm birth and associated factors among mothers who gave birth in Gondar town health institutions. Adv Nurs. 2016;15:5. doi:10.1155/2016/4703138
11. Mengesha HG, Lerebo WT, Kidanemariam A, Gebrezgiabher G, Berhane Y. Pre-term and post-term births: predictors and implications on neonatal mortality in Northern Ethiopia. BMC Nurs. 2016;15:48. doi:10.1186/s12912-016-0170-6
12. Bekele I, Demeke T, Dugna K. Prevalence of preterm birth and its associated factors among mothers delivered in Jimma University specialized teaching and referral hospital, Jimma Zone, Oromia Regional State, South West Ethiopia. J Womens Health Care. 2017;6:356.
13. Mekonen DG, Yismaw AE, Nigussie TS, et al. The proportion of preterm birth and associated factors among mothers who gave birth in Debretabor town health institutions, Northwest, Ethiopia. BMC Res Notes. 2019;12(1):2. doi:10.1186/s13104-018-4037-7
14. Ananth CV, Vintzileos AM. Trends in cesarean delivery at preterm gestation and association with perinatal mortality. Am J Obstet Gynecol. 2011;204(6):505–e1. doi:10.1016/j.ajog.2011.01.062
15. Jiang M, Mishu MM, Lu D, Yin X. A case-control study of risk factors and neonatal outcomes of preterm birth. Taiwan J Obstet Gynecol. 2018;57:814e818.
16. Lawlor DA, Mortensen L, Andersen AM. Mechanisms underlying the associations of maternal age with adverse perinatal outcomes: a sibling study of 264 695 Danish women and their firstborn offspring. Int J Epidemiol. 2011;40(5):1205–1214. doi:10.1093/ije/dyr084
17. Martius JA, Steck T, Oehler MK, Wulf KH. Risk factors associated with preterm (< 37+ 0 weeks) and early preterm birth (< 32+ 0 weeks): univariate and multivariate analysis of 106 345 singleton births from the 1994 statewide perinatal survey of Bavaria. Eur J Obstetr Gynecol Reprod Biol. 1998;80(2):183–189.
18. Pusdekar YV, Patel AB, Kurhe KG. Rates and risk factors for preterm birth and low birth weight in the global network sites in six low‑ and low-middle-income countries. Reprod Health. 2020;17(Suppl3):187. doi:10.1186/s12978-020-01029-z
19. Silveira MF, Victora CG, Barros AJ, Santos IS, Matijasevich A, Barros FC. Determinants of preterm birth: Pelotas, Rio Grande do Sul state, Brazil, 2004 birth cohort. Cad Saude Publica. 2010;26:185–194. doi:10.1590/S0102-311X2010000100019
20. Adugna DG. Prevalence and associated risk factors of preterm birth among neonates in referral hospitals of Amhara Region, Ethiopia. PLoS One. 2022;17(10):e0276793. doi:10.1371/journal.pone.0276793
21. Fuchs F, Monet B, Ducruet T, Chaillet N, Audibert F. Effect of maternal age on the risk of preterm birth: a large cohort study. PLoS One. 2018;13(1):e0191002. doi:10.1371/journal.pone.0191002
22. Waldenström U, Cnattingius S, Vixner norman M, Norman M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: a population‐based register study. BJOG. 2017;124(8):1235–1244. doi:10.1111/1471-0528.14368
23. Leal MD, Esteves-Pereira AP, Nakamura-Pereira M, et al. Prevalence and risk factors related to preterm birth in Brazil. Reprod Health. 2016;13(3):163–174. doi:10.1186/s12978-016-0230-0
24. Sheiner E, Shoham-Vardi I, Hadar A, Hallak M, Hackmon R, Mazor M. Incidence, obstetric risk factors and pregnancy outcome of preterm placental abruption: a retrospective analysis. J Maternal Fetal Neonatal Med. 2002;11(1):34–39. doi:10.1080/jmf.11.1.34.39
25. Koullali B, Van Zijl MD, Kazemier BM, et al. The association between parity and spontaneous preterm birth: a population-based study. BMC Pregnancy Childbirth. 2020;20(1):1–8. doi:10.1186/s12884-020-02940-w
26. Wagura P, Wasunna A, Laving A, Wamalwa D, Ng’ang’a P. Prevalence and factors associated with preterm birth at Kenyatta national hospital. BMC Pregnancy Childbirth. 2018;18(1):1–8. doi:10.1186/s12884-018-1740-2
27. Shaikh K, Premji SS, Rose MS, Kazi A, Khowaja S, Tough S. The association between parity, infant gender, higher level of paternal education and preterm birth in Pakistan: a cohort study. BMC Pregnancy Childbirth. 2011;11(1):1. doi:10.1186/1471-2393-11-88
28. Rahman A, Rahman M, Pervin J, et al. Time trends and sociodemographic determinants of preterm births in pregnancy cohorts in Matlab, Bangladesh, 1990–2014. BMJ Glob Health. 2019;4(4):e001462. doi:10.1136/bmjgh-2019-001462
29. Williams C, Fong R, Murray SM, Stock SJ. Cesarean birth and risk of subsequent preterm birth: a retrospective cohort study. BJOG. 2021;128(6):1020–1028. doi:10.1111/1471-0528.16566
30. Di Renzo GC, Giardina I, Rosati A, Clerici G, Torricelli M, Petraglia F; Italian Preterm Network Study Group. Maternal risk factors for preterm birth: a country-based population analysis. Eur J Obstetr Gynecol Reprod Biol. 2011;159(2):342–346. doi:10.1016/j.ejogrb.2011.09.024
31. Fernandes SF, Chandra S. A study of risk factors for preterm labor. Int J Reprod Contracept Obstet Gynecol. 2015;4(5):1306. doi:10.18203/2320-1770.ijrcog20150701
32. Mulualem G, Wondim A, Woretaw A. The effect of pregnancy-induced hypertension and multiple pregnancies on preterm birth in Ethiopia: a systematic review and meta-analysis. BMC Res Notes. 2019;12(1):1–7. doi:10.1186/s13104-019-4128-0
33. Alijahan R, Hazrati S, Mirzarahimi M, Pourfarzi F, Hadi PA. Prevalence and risk factors associated with preterm birth in Ardabil, Iran. Iran J Reprod Med. 2014;12(1):47.
34. Aseidu EK, Bandoh DA, Ameme DK, et al. Obstetric determinants of preterm delivery in a regional hospital, Accra, Ghana 2016. BMC Pregnancy Childbirth. 2019;19(1):1–8. doi:10.1186/s12884-019-2404-6
35. Thanh BY, Lumbiganon P, Pattanittum P, et al. Mode of delivery and pregnancy outcomes in preterm birth: a secondary analysis of the WHO Global and Multi-country Surveys. Sci Rep. 2019;9(1):1–8. doi:10.1038/s41598-019-52015-w
36. Blue NR, Van Winden KR, Pathak B, et al. Neonatal outcomes by mode of delivery in preterm birth. Am J Perinatol. 2015;32(14):1292–1297. doi:10.1055/s-0035-1562931
37. Kuper SG, Sievert RA, Steele R, Biggio JR, Tita AT, Harper LM. Maternal and neonatal outcomes in indicated preterm births based on the intended mode of delivery. Obstet Gynecol. 2017;130(5):1143–1151. doi:10.1097/AOG.0000000000002320
38. Muhe LM, McClure EM, Nigussie AK, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Health. 2019;7(8):e1130–8. doi:10.1016/S2214-109X(19)30220-7
39. Elias S, Wolde Z, Tantu T, Gunta M, Zewudu D. Determinants of early neonatal outcomes after emergency cesarean delivery at Hawassa University comprehensive specialized hospital, Hawassa, Ethiopia. PLoS One. 2022;17(3):e0263837. doi:10.1371/journal.pone.0263837
40. Yasseen AS, Bassil K, Sprague A, Urquia M, Maguire JL. Late preterm birth and previous cesarean section: a population-based cohort study. J Maternal Fetal Neonatal Med. 2019;32(14):2400–2407. doi:10.1080/14767058.2018.1438397
41. Beck S, Wojdyla D, Say L. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. World Health Organ. 2010;88:31–38. doi:10.2471/BLT.08.062554
42. Klemetti R, Gissler M, Sainio S, Hemminki E. At what age does the risk for adverse maternal and infant outcomes increase? Nationwide register-based study on first births in Finland in 2005±2014. Acta Obstet Gynecol Scand. 2016;95(12):1368–1375. doi:10.1111/aogs.13020
43. Aliyu MH, Jolly PE, Ehiri JE, Salihu HM. High parity and adverse birth outcomes: exploring the maze [review]. Birth. 2005;32:45–59. doi:10.1111/j.0730-7659.2005.00344.x
44. Zhang Y, Zhou J, Ma Y, et al. Mode of delivery and preterm birth in subsequent births: a systematic review and meta-analysis. PLoS One. 2019;14(3):e0213784. doi:10.1371/journal.pone.0213784
45. Sekiguchi A, Nakai A, Kawabata I, Hayashi M, Takeshita T. Type and location of placenta previa affect preterm delivery risk related to antepartum hemorrhage. Int J Med Sci. 2013;10(12):1683. doi:10.7150/ijms.6416
46. Balachandar K, Melov SJ, Nayyar R. The risk of adverse maternal outcomes in cases of placenta praevia in an Australian population between 2007 and 2017. Australian NZ J Obstetr Gynaecol. 2020;60(6):890–895. doi:10.1111/ajo.13172
47. Mammaro A, Carrara S, Cavaliere A, et al. Hypertensive disorders of pregnancy. J Prenat Med. 2009;3(1):1.
48. Chandra I, Sun L. Third trimester preterm, and term premature rupture of membranes: is there any difference in maternal characteristics and pregnancy outcomes? J Chin Med Assoc. 2017;80(10):657–661. doi:10.1016/j.jcma.2016.12.006
49. Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323. doi:10.1097/AOG.0b013e3182255999
50. Green ES, Arck PC. Pathogenesis of preterm birth: bidirectional inflammation in mother and fetus. Semin Immunopathol. 2020;42(4):413–429. doi:10.1007/s00281-020-00807-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
