Back to Journals » Clinical Ophthalmology » Volume 10
Presence or absence of ocular surface inflammation directs clinical and therapeutic management of dry eye
Authors Sambursky R
Received 8 September 2016
Accepted for publication 3 November 2016
Published 24 November 2016 Volume 2016:10 Pages 2337—2343
DOI https://doi.org/10.2147/OPTH.S121256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Robert Sambursky
Coastal Eye Institute, Cornea and Comprehensive Ophthalmology, Bradenton, FL, USA
Background: The presence of clinically significant inflammation has been confirmed in the tears of 40%–65% of patients with symptoms of dry eye. Ocular surface inflammation may lead to tear film instability, epithelial cell irregularities, and permeability, resulting in chronic symptomatic pain and fluctuating vision as well as negative surgical outcomes.
Patients and methods: A retrospective single center medical chart review of 100 patients was conducted. All patients were tested with the InflammaDry test to determine if patients exhibited elevated levels of matrix metalloproteinase 9 (MMP-9). InflammaDry-positive patients were started on a combination of cyclosporine 0.05% twice daily, 2,000–4,000 mg oral omega-3 fatty acids, and frequent artificial tear replacement. InflammaDry-negative patients were started on 2,000–4,000 mg of oral omega-3 fatty acids and frequent artificial tear replacement. Each patient was retested at ~90 days. A symptom questionnaire was performed at the initial visit and at 90 days.
Results: 60% of the patients with dry eye symptoms tested positive for elevated MMP-9 at the initial visit. 78% of all patients returned for follow-up at ~90 days including 80% (48/60) of the previously InflammaDry-positive patients and 75% (30/40) of the previously InflammaDry-negative patients. A follow-up symptom questionnaire reported at least 75% symptomatic improvement in 65% (31/48) of the originally InflammaDry-positive patients and in 70% (21/30) of the initially InflammaDry-negative patients. Symptomatic improvement of at least 50% was reported in 85% (41/48) of previously InflammaDry-positive patients and 86% (26/30) of previously InflammaDry-negative patients. Following treatment, 54% (26/48) of previously InflammaDry-positive patients converted to a negative InflammaDry result.
Conclusion: Identifying which symptomatic dry eye patients have underlying inflammation may predict patient responses to treatment and influence clinical management strategies.
Keywords: dry eye, inflammation, MMP-9, cyclosporine, diagnosis, treatment
Introduction
Dry eye is a multifactorial disease that is related to the relationship between the amount of tears produced, rate of tear evaporation, goblet cell density, and the presence or absence of inflammation. The discordance between symptoms, clinical signs, and diagnostic test results makes the clinical management and treatment of this condition challenging.1 Only 40%–65%, or approximately half of patients with symptoms of dry eye have clinically significant inflammation, with or without the presence of meibomian gland dysfunction (MGD).2–6 The presence of inflammation may lead to chronic symptomatic pain and fluctuating vision as well as negative surgical outcomes. Identifying the presence or absence of ocular surface inflammation helps guide therapeutic decision making.7
Desiccating stress to the ocular surface epithelium activates the mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB pathways, which stimulate production of epithelial-derived inflammatory mediators such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, IL-8, and matrix metalloproteinase (MMP)-9.8–16 MMP-9 is an ideal biomarker for inflammation since it elevates early, is stimulated by IL-1, TNF-α, IL-6, IL-8, IL-17,8–11,13–17 catalyzes further development of IL-1 and TNF-α,13 and accumulates as part of a persistent cycle of inflammation. Moreover, MMP-9 destabilizes the tear film and directly contributes to corneal barrier dysfunction by breaking down tight junctions, causing epithelial cell desquamation, and facilitating inflammatory cell migration, which ultimately leads to corneal staining and rapid tear break up times.8,13,15,16 Elevation in MMP-9 was shown to precede the development of corneal staining and contribute to the instability of the tear film in 30% of patients, resulting in pain and fluctuating vision.6
Downregulation of MMP-9 expression is associated with improvement in ocular surface epithelia.18 Further, MMP-9 knockout mice are resistant to developing dry eye.19 Normal MMP-9 levels range from 3 to 41 ng/mL.20,21 53% of symptomatic dry eye patients have MMP-9 levels elevated to ≥40 ng/mL.6 InflammaDry (RPS Diagnostics; Sarasota, FL, USA) is a rapid in-office test that detects elevated MMP-9 in tears.
Most dry eye testing methods such as tear breakup time (TBUT), Schirmer tear testing, tear osmolarity, and diagnostic imaging methods such as inferometry, meibomography, corneal topography, and ocular coherence tomography provide valuable information to help characterize patients as evaporative or aqueous deficient but cannot predict which patients have clinically significant ocular surface inflammation.22 However, the presence of corneal and/or conjunctival staining with a vital dye is a clinical indicator of inflammation and directly correlates to the levels of inflammatory mediators and MMP-9. Clinical studies based on a positive ocular surface disease index (OSDI) reveal that 8%–57% of symptomatic dry eye is associated with fluorescein or lissamine green conjunctival or corneal staining.23–25 Staining has been shown to occur in only 65% of patients with moderate-to-severe dry eye.26 Moreover, outside the clinical trial setting, staining and TBUT are not typically performed accurately in busy clinical practices. Staining and TBUT must be measured prior to the use of topical anesthetics, which induce a rapid TBUT and epithelial staining.27,28
Lanza et al enrolled 110 patients with dry eye symptoms and measured Schirmer levels, TBUT, osmolarity, and MMP-9.22 Thirty-nine percent were positive for elevated levels of MMP-9. No statistical difference was found in the symptoms or signs of dry eye patients that tested positive or negative for elevated MMP-9.22 Thus, it is not possible to identify patients who have ocular inflammation based on a profile of their symptoms or signs.
To further investigate the dry eye population, a clinical diagnostic and treatment protocol was implemented within a large multicenter group practice in 2015. The research hypothesis was that targeting anti-inflammatory therapy for patients with confirmed underlying ocular surface inflammation might lead to improved patient symptoms and signs while limiting unnecessary therapy.
Methods
A retrospective single center medical chart review of 100 patients seen over the preceding 180 days was conducted in a large multispecialty private ophthalmology practice in Southwest Florida. A chart review was performed to identify the most recent 100 patients who presented with symptoms of dry eye, were tested with InflammaDry, were started on therapy, and were seen in follow-up ~90 days later. According to the Shulman Institutional Review Board (Cincinnati, OH, USA) exemption classifications, this retrospective study was deemed exempt from institutional review board approval and patient consent.
Data collected included age, gender, InflammaDry test results before and after treatment, and a dry eye symptom questionnaire results before and after treatment.
The InflammaDry test was performed as per the manufacturer’s instructions to determine if patients exhibited elevated levels of MMP-9, which represented clinically significant ocular surface inflammation. The InflammaDry test required an ophthalmic technician to collect a tear sample from the patient’s palpebral conjunctiva. The palpebral conjunctiva was gently dabbed 6–8 times in multiple locations, releasing the lid after every 2–3 dabs to allow the patient to blink. Then, the sampling fleece was allowed to rest against the conjunctiva for an additional 5 seconds until the sampling fleece was saturated with tears (10 μL) and either demonstrated a pink color or appeared glistening. After obtaining the sample, the sample collector was assembled onto the test cassette. The assembled test was activated by dipping the absorbent pad into a buffer solution for ~20 seconds. After 10 minutes had elapsed, the test was interpreted. The presence of 1 blue line and 1 red line in the test’s result window was indicative of a positive test result (MMP-9 ≥40 ng/mL). The intensity of the red line is directly related to the amount of MMP-9 present; thus, mild dry eye is associated with fainter result lines than more severe dry eye. The presence of a red line of any intensity confirmed the presence of elevated MMP-9. One blue control line indicates a negative test result (MMP-9 <40 ng/mL).21
Each patient who tested InflammaDry-positive was started on topical cyclosporine 0.05% (Restasis; Allergan, Irvine, CA, USA), oral omega-3 fatty acids twice daily for a total of 2,000–4,000 mg, and frequent artificial tears. All patients who tested negative were recommended to use oral omega-3 fatty acids at a dose of 2,000–4,000 mg twice daily and frequent artificial tears.
All patients completed a symptom questionnaire derived from the OSDI at the initial visit and at the follow-up visit ~90 days later. All symptomatic patients identified with dry eye symptoms were included so that a wide range of severity was included in the treatment analysis. Each patient was tested with the InflammaDry test at the initial visit and follow-up visit. The patients were asked to provide an overall grade to their clinical symptomatic improvement since starting therapy compared to their initial visit from no improvement to complete resolution of symptoms based on 25% improvement increments (eg, none or minimal symptomatic improvement of ≤5%, modest symptomatic improvement of 6%–24%, moderate symptomatic improvement of 25%–49%, significant improvement of 50%–74%, and dramatic symptomatic improvement of 75%–100%). Patients treated with topical cyclosporine for conditions other than dry eye were excluded. Patients using artificial tears more than twice daily prior to the initial visit were excluded from the review.
Results
A total of 100 charts were examined. Seventy-two percent (72/100) of patients were females with a mean age of 64 years and age range was from 17 to 89 years. Sixty percent of the patients with dry eye symptoms tested positive with the InflammaDry test for elevated MMP-9 at the initial visit.
Only 78% (78/100) of the patients returned for follow-up at ~90 days including 80% (48/60) of the previously InflammaDry-positive patients and 75% (30/40) of the previously InflammaDry-negative patients. Repeat InflammaDry testing and symptom questionnaires were performed at the follow-up visit. Eight percent (5/60) of patients started on cyclosporine could not tolerate it because of stinging and were excluded from calculations.
Sixty percent (29/48) of the initially InflammaDry-positive patients and 63% (19/30) of the initially InflammaDry-negative patients reported at least 75% improvement in symptoms. Eighty-five percent (41/48) of the initially InflammaDry-positive patients reported at least 50% improvement, while 86% (26/30) of the initially InflammaDry-negative patients reported at least 50% improvement. The remainder of the patients reported between 6% and 49% improvement in symptoms (Table 1). The follow-up symptom questionnaire reported that at least modest symptomatic improvement was achieved in 100% (78/78) of follow-up patients.
  | Table 1 90-day follow-up symptom analysis |
Following treatment, 54% (26/48) of previously InflammaDry-positive patients converted from a positive to a negative InflammaDry result. Of the patients who converted to negative, 88% (23/26) reported at least 75% improvement in symptoms, while the remaining 12% (3/26) reported at least 50% improvement in symptoms. Of the 46% (22/48) of initially InflammaDry-positive patients who remained positive at the 90-day follow-up visit, 36% (8/22) reported 6%–49% symptomatic improvement. In symptomatically improved patients who remained InflammaDry-positive at 90 days, most demonstrated a less intense result line on the InflammaDry test, suggesting that the treatment was helpful but not adequate to lower the level of MMP-9 antigen below the cutoff threshold.29 Six percent (2/30) of the previously negative patients became InflammaDry-positive at 90 days.
Discussion
Identifying symptomatic dry eye patients with underlying inflammation may predict patient response to treatment and influence patient management strategy recommendations including artificial tear replacement, punctal occlusion, or anti-inflammatory therapeutics such as a short course of corticosteroids, oral doxycycline, or long-term maintenance treatment with cyclosporine and/or lifitegrast (Xiidra; Shire, Lexington, MA, USA). As evidenced by prevalence studies using staining or point-of-care testing for MMP-9, clinically significant inflammation occurs in ~50% of symptomatic dry eye patients with or without the presence of meibomian gland dysfunction.2–6 Because staining is often performed incorrectly and MMP-9 is induced by all of the primary mediators including IL-1β, TNF-α, IL-6, IL-8, IL-17, and tumor growth factor, early and throughout the inflammatory cascade, MMP-9 represents an ideal marker for ocular surface inflammation.8–14,17,30
The presence of ocular surface inflammation allows for a more targeted clinical management and therapeutic approach. Artificial tears provide palliative relief of eye irritation in patients with aqueous tear deficiency.31 However, artificial tears do not significantly reduce MMP-9 levels,31,32 prevent underlying inflammation, or reverse conjunctival squamous metaplasia in chronic dry eye.31 Tong et al showed that MMP-9 levels are unchanged 3 weeks after punctal occlusion.33 Punctal occlusion should be reserved for patients without ocular surface inflammation or performed after the inflammation is under control.34,35
Patients with confirmed inflammation benefit from chronic anti-inflammatory therapy.36,37 Treatment with anti-inflammatory medications such as topical corticosteroids and cyclosporine decreases the production of inflammatory cytokines.30,32,38–41 Moreover, both corticosteroids42 and topical cyclosporine43 lead to increased goblet cell density in tear dysfunction associated with non-Sjögren’s and Sjögren’s diseases. Treatment of dry eye with methylprednisolone and doxycycline was shown to preserve the tight junction network, increase corneal smoothness, preserve corneal barrier function, and lead to a reduction in the production and activity of MMP-9.44 Doxycycline has been found to inhibit MMP-9 activity in human corneal epithelial cells9 and may be used to treat MMP-mediated ocular surface diseases, such as rosacea, recurrent epithelial erosion, and sterile corneal ulceration.45,46 Lifitegrast reduces the overall inflammatory response resulting from T-cell adhesion to endothelial cells before trans-endothelial migration to inflamed tissues as well as at the point of T-cell interaction with antigen-presenting cells.47 These processes should result in decreased levels of pathogenic mediators and less inflammation on the ocular surface.47 Similarly, elevated MMP-9 should identify populations that enhance liftegrast’s therapeutic efficacy.
Eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha linolenic acid (ALA) are the three omega-3 fatty acids that cannot be synthesized in the body and have to be supplemented in diet. EPA and DHA modulate prostaglandin metabolism toward anti-inflammatory prostaglandin synthesis due to competitive inhibition of the arachidonic acid pathway.48 Omega-3 supplementation demonstrates an anti-inflammatory effect, inhibiting creation of omega-6 prostaglandin precursors, preventing apoptosis of the secretory epithelial cells in the lacrimal gland, and clearing meibomitis, which allows for a healthier lipid layer to protect the tear film and cornea.49
Therapeutic responses from topical anti-inflammatory agents do not result in significant clinical improvement in more than half the patients treated.35,50 This is evidenced by moderate, or complete, regression of symptoms in only 43%, or 57%, respectively, of dry eye patients with delayed clearance following treatment with loteprednol.51 Similarly, treatment with topical cyclosporine 0.1% for 6 months showed only moderate response to treatment in 39% of symptomatic dry eye patients.50
In contrast, in this retrospective study, significant clinical responses approached 85%–87% for >50% clinical improvement, which is significantly higher than previous studies. Enriching the treatment population by targeting only those patients with confirmed ocular surface inflammation may have led to improved outcomes. It is likely that symptomatic dry eye patients who do not respond to anti-inflammatory agents are the same populations that do not have ocular surface inflammation present.
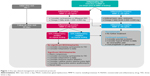
However, 46% of patients demonstrated elevated levels of MMP-9 despite targeted therapies. Patients with chronically elevated MMP-9 were associated with patients reporting less clinical benefit. Similar to managing systemic diseases such as hypertension, diabetes mellitus, and hypertriglyceridemia, it is likely that these patients require an additional anti-inflammatory therapy and not substitution with a different anti-inflammatory treatment. Additional treatments may also include targeting any meibomian gland dysfunction or blepharitis. One option is to increase the frequency of cyclosporine 0.05% to 3–4 times daily.52 Alternatively, a low potency steroid such as fluorometholone or loteprednol may be added to the cyclosporine regimen to achieve adequate inflammatory control. The addition of the steroid to the cyclosporine, rather than the replacement of the cyclosporine therapy, allows for decreased daily steroid dosing, which may reduce the risk for steroid-induced side effects. In the future, it is possible that cyclosporine and lifitegrast may be combined in patients refractory to a single topical agent (Figure 1). It is unlikely that liftegrast will work on cyclosporine failures as both medications inhibit similar inflammatory mediators.
Topical steroids may have the most potent and rapid anti-inflammatory action, which may be ideal prior to initiating cyclosporine to reduce the sting53 and for ocular surface optimization prior to obtaining kerotometry, aberrometry, and biometry. Long-term steroid treatment is not advisable because of the risk for cataract formation and increased intraocular pressure.54 Cyclosporine has minimal side effects compared with steroids and may be used chronically without significant risk.50,55,56 Coupled with oral omega-3 therapy, cyclosporine is effective and low risk as chronic anti-inflammatory therapy.
This study has several limitations. First, this was not a prospective randomized controlled therapeutic study and retrospective studies have inherent weakness. A future study should compare the efficacy of cyclosporine in both the InflammaDry-negative and -positive patients. Rigid periodic therapeutic monitoring throughout the study was not performed. In addition, 22% of patients did not return for the 90-day follow-up period, which could influence the reported clinical success rates. Finally, the omega-3 therapy was not standardized and its efficacy is influenced by bioavailability and absorption. Despite its limitations, this is the first study to demonstrate improved clinical outcomes based on treating a targeted symptomatic dry eye patient population with confirmed ocular surface inflammation.
Conclusion
MMP-9 is induced by key cytokines in the early stages of the inflammatory cascade and therefore is an ideal biomarker because its elevation confirms the presence of clinically significant ocular surface inflammation. Clinically significant inflammation is present only in approximately half of the patients with symptomatic dry eye. This is evidenced by <57% of all comer’s studies revealing conjunctival or corneal staining23–25 and the demonstration of only 39%–57%50–51 symptomatic improvement found in clinical trials evaluating the efficacy of cyclosporine and loteprednol, respectively. Coupled with the results from this study, it suggests that identifying symptomatic dry eye patients with underlying inflammation may help predict patient responses to treatment and influence clinical patient management strategies.
Acknowledgments
The author thanks Maria Geis, OD (Coastal Eye Institute; Bradenton, FL, USA), for her assistance in preparing the data for this study and Laura Sambursky, MA (Saranova, LLC; Lakewood Ranch, FL, USA), for editing this manuscript.
Disclosure
The author is an employee of RPS Diagnostics, the manufacturer of the InflammaDry test, and is a consultant to Allergan, the manufacturer of Restasis. The author reports no other conflicts of interest in this work.
References
Wilson SE, Stulting RD. Agreement of physician treatment practices with the international task force guidelines for diagnosis and treatment of dry eye disease. Cornea. 2007;26(3):284–289. | ||
Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198–205. | ||
Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011;52(10):7725–7730. | ||
Enríquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873. | ||
Afonso AA, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL1-alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999;40(11):2506–2512. | ||
Sambursky R, Davitt WF 3rd, Friedberg M, Tauber S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea. 2014;33(8):812–818. | ||
Kaufman HE. The practical detection of mmp-9 diagnoses ocular surface disease and may help prevent its complications. Cornea. 2013;32(2):211–216. | ||
Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47(8):3293–3302. | ||
Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45(12):4302–4311. | ||
Li DQ, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82(4):588–596. | ||
Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28(9):1023–1027. | ||
De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47(7):2847–2856. | ||
Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50(7):3203–3209. | ||
Na KS, Mok JW, Kim JY, Rho CR, Joo CK. Correlations between tear cytokines, chemokines, and soluble receptors and clinical severity of dry eye disease. Invest Ophthalmol Vis Sci. 2012;53(9):5443–5450. | ||
Li DQ, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73(4):449–459. | ||
Sobrin L, Liu Z, Monroy DC, et al. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci. 2000;41(7):1703–1709. | ||
De Paiva CS, Chen Z, Koch DD, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006;141(3):438–445. | ||
Lanzini M, Curcio C, Colabelli-Gisoldi RA, et al. In vivo and impression cytology study on the effect of compatible solutes eye drops on the ocular surface epithelial cell quality in dry eye patients. Mediators Inflamm. 2015;2015:Article ID 351424. | ||
Pflugfelder SC, de Paiva CS, Tong L, Luo L, Stern ME, Li DQ. Stress-activated protein kinase signaling pathways in dry eye and ocular surface disease. Ocul Surf. 2005;3(Suppl 4):S154–S157. | ||
Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001;85(2):147–153. | ||
Sambursky R, Davitt WF 3rd, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013;131(1):24–28. | ||
Lanza NL, McClellan A, Batawi H, et al. Dry eye profiles in patients with a positive elevated surface matrix metalloproteinase 9 point-of-care test versus negative patients. Ocul Surf. 2016;14(2):216–233. | ||
Alves M, Reinach PS, Paula JS, et al. Comparison of diagnostic tests in distinct well-defined conditions related to dry eye disease. PLoS One. 2014;9(5):e97921. | ||
Martinez JD, Galor A, Ramos-Betancourt N, et al. Frequency and risk factors associated with dry eye in patients attending a tertiary care ophthalmology center in Mexico City. Clin Ophthalmol. 2016;10:1335–1342. | ||
Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762–770. | ||
Begley CG, Chalmers RL, Abetz L, et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci. 2003;44(11):4753–4761. | ||
Josephson JE, Caffery BE. Corneal staining after instillation of topical anesthetic (SSII). Invest Ophthalmol Vis Sci. 1988;29(7):1096–1099. | ||
Zeev MS, Miller DD, Latkany R. Diagnosis of dry eye disease and emerging technologies. Clin Ophthalmol. 2014;8:581–590. | ||
Brujic M, Kading D. Making matrix metalloproteinase-9 levels more meaningful. Poster session presented at: Global Specialty Lens Symposium (GSLS). 2016 Jan 21–24; Las Vegas, NV, USA. | ||
De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526–535. | ||
Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41. | ||
Aragona P, Aguennouz M, Rania L, et al. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology. 2015;122(1):62–71. | ||
Tong L, Beuerman R, Simonyi S, Hollander DA, Stern ME. Effects of punctal occlusion on clinical signs and symptoms and on tear cytokine levels in patients with dry eye. Ocul Surf. 2016;14(2):233–241. | ||
Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900–907. | ||
Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137(2):337–342. | ||
Rao SN. Topical cyclosporine 0.05% for the prevention of dry eye disease progression. J Ocul Pharmacol Ther. 2010;26(2):157–164. | ||
Rao SN. Reversibility of dry eye deceleration after topical cyclosporine 0.05% withdrawal. J Ocul Pharmacol Ther. 2011;27(6):603–609. | ||
Djalilian AR, Nagineni CN, Mahesh SP, Smith JA, Nussenblatt RB, Hooks JJ. Inhibition of inflammatory cytokine production in human corneal cells by dexamethasone, but not cyclosporin. Cornea. 2006;25(6):709–714. | ||
Gürdal C, Saraç O, Genç I, Kirimlioğlu H, Takmaz T, Can I. Ocular surface and dry eye in Graves’ disease. Curr Eye Res. 2011;36(1):8–13. | ||
Byun YJ, Kim TI, Kwon SM, et al. Efficacy of combined 0.05% cyclosporine and 1% methylprednisolone treatment for chronic dry eye. Cornea. 2012;31(5):509–513. | ||
Turner K, Pflugfelder SC, Ji Z, Feuer WJ, Stern M, Reis BL. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000;19(4):492–496. | ||
Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003;136(4):593–602. | ||
Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27(1):64–69. | ||
De Paiva CS, Corrales RM, Farley W, Li DQ, Stern ME, Pflugfelder SC. Anti-inflammatory therapy preserves corneal barrier function in experimental murine dry eye. Invest Ophthalmol Vis Sci. 2005;46(13):2423. | ||
Akpek EK, Merchant A, Pinar V, Foster CS. Ocular rosacea: patient characteristics and follow-up. Ophthalmology. 1997;104(11):1863–1867. | ||
Seedor JA, Perry HD, McNamara TF, Golub LM, Buxton DF, Guthrie DS. Systemic tetracycline treatment of alkali-induced corneal ulceration in rabbits. Arch Ophthalmol. 1987;105(2):286–271. | ||
Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2010;14(2):207–215. | ||
Pinna A, Piccinini P, Carta F. Effect of oral linoleic and gamma-linolenic acid on meibomian gland dysfunction. Cornea. 2007;26(3):260–264. | ||
Liu Y, Kam WR, Sullivan DA. Influence of omega 3 and 6 fatty acids on human meibomian gland epithelial cells. Cornea. 2016;35(8):1122–1126. | ||
Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631–639. | ||
Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138(3):444–457. | ||
Dastjerdi MH, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28(10):1091–1096. | ||
Sheppard JD, Scoper SV, Samudre S. Topical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye disease. J Ocul Pharmacol Ther. 2011;27(1):23–27. | ||
Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78(3):409–416. | ||
Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf. 2004;2(2):124–130. | ||
Perry HD, Donnenfeld ED. Dry eye diagnosis and management in 2004. Curr Opin Ophthalmol. 2004;15(4):299–304. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.