Back to Journals » Cancer Management and Research » Volume 11
Pre/Post-Treatment Dynamic of Inflammatory Markers Has Prognostic Value in Patients with Small Hepatocellular Carcinoma Managed by Stereotactic Body Radiation Therapy
Authors Zhuang Y, Yuan BY, Hu Y, Chen GW, Zhang L, Zhao XM, Chen YX , Zeng ZC
Received 20 September 2019
Accepted for publication 19 December 2019
Published 3 January 2020 Volume 2019:11 Pages 10929—10937
DOI https://doi.org/10.2147/CMAR.S231901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Yuan Zhuang, Bao-Ying Yuan, Yong Hu, Gen-Wen Chen, Li Zhang, Xiao-Mei Zhao, Yi-Xing Chen,* Zhao-Chong Zeng*
Department of Radiation Oncology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi-Xing Chen; Zhao-Chong Zeng
Department of Radiation Oncology, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai 200032, People’s Republic of China
Tel +86-139-1605-6575
Fax +86-21-6404-8472
Email [email protected]; [email protected]
Purpose: The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are inflammatory indexes that may reflect immune response to tumors and prognosis. We investigated the prognostic values of pre-treatment and post-treatment NLR and PLR and changes in those ratios in patients with small hepatocellular carcinoma (sHCC) treated with stereotactic body radiation therapy (SBRT).
Patients and methods: Sixty patients who received SBRT were retrospectively reviewed. NLR and PLR were calculated by division of neutrophil and platelet counts, respectively, by lymphocyte counts. Independent factors for progression-free survival (PFS) and overall survival (OS) were determined by the Kaplan–Meier method, log-rank test, and Cox multivariate regression. Hazard ratios (HRs) and 95% confidence intervals (CIs) were also calculated.
Results: The median follow-up was 36.9 (range: 4.1–73.5) months. Median PFS was 21.4 (range: 1.8–66.9) months. The 1-year and 2-year PFS rates were 76.7% and 55.0%, respectively. The 1-year and 2-year OS rates were 95.0% and 78.3%, respectively. In multivariate analysis, post-treatment PLR ≥263.0 indicated both poor PFS (HR: 3.70; 95% CI: 1.07–12.76, p=0.038) and OS (HR: 3.23; 95% CI: 1.01–9.11, p=0.043) for sHCC patients treated with SBRT. In addition, the presence of hepatitis infection and a low level of red blood cell count were also proved to be significantly associated with patients’ poor prognosis (pp=0.029).
Conclusion: High post-treatment PLR and change in NLR ≥2.7-fold were associated with poor prognosis in patients treated with SBRT and might be considered as reliable and independent prognostic biomarkers for patients with sHCC.
Keywords: stereotactic body radiation therapy, hepatocellular carcinoma, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, progression-free survival
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies, and its incidence is increasing worldwide.1,2 As diagnostic imaging and screening programs for HCC have progressed, small tumors are now more likely to be detected with high accuracy. Surgical intervention including resection and liver transplant is the first-line therapy for small HCC (sHCC).3,4 Some patients with sHCC are not candidates for surgery at the time of diagnosis because they refuse surgery, their tumor is in a high-risk location, they are of advanced age, or they have other comorbid diseases.5 Those patients can be managed with locoregional treatments3 including radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and radiation therapy (RT). Among the alternative approaches, stereotactic body radiation therapy (SBRT) has emerged as a non-invasive and radical therapeutic option for patients with sHCC who were not candidates for surgery. There is broad consensus among experts that SBRT offers substantial local control, improved overall survival (OS), and low toxicity.6 However, there have been few studies of prognostic factors in patients with sHCC that undergo SBRT, and the mechanism of tumor cell death after SBRT is not completely elucidated.
Emerging evidence showed that systematic inflammation and host immune status are key determinants of disease progression in various malignancies,7,8 which has led to much interest in the relationship between patients’ prognosis and inflammatory hematologic markers. Among the inflammatory indexes, an elevated neutrophil-to-lymphocyte ratio (NLR) or platelet-to-lymphocyte ratio (PLR) has consistently exhibited a strong association with poor outcomes among patients with liver cancer who receive multi-modality therapies including transarterial chemoembolization (TACE),9 targeted therapy,10 RFA,11,12 surgical resection,13,14 and selective internal radiation therapy (SIRT).15 However, the association between inflammation-based biomarkers and oncologic outcomes in patients with sHCC who undergo SBRT is unclear. It is well known that patients receiving RT may experience a marked decline or even depletion of circulating lymphocytes,16,17 and a decreased lymphocyte count can be connected to a weaker anti-tumor immune response and an inferior prognosis.18–20 It is therefore of great clinical significance to investigate the predictive roles of the NLR and PLR both before and after SBRT in patients with sHCC.
This study is the first to explore the prognostic value and determine the optimal cutoff values of the pre-treatment NLR and PLR (pre-NLR and pre-PLR), the post-treatment NLR and PLR (post-NLR and post-PLR), and changes in NLR and PLR in patients with sHCC that receive SBRT.
Patients and Methods
Patient Selection
This research was approved by the Institutional Review Board of the Ethics Committee at our institution and performed in accordance with the principles of the Declaration of Helsinki. Patients diagnosed with HCC in our hospital from November 2012 to February 2017 who were not suitable for surgery received SBRT. Small HCC in our study is defined as a solitary tumor ≤5cm in maximum diameter or as multiple nodules (≤3 total) measuring ≤3 cm in maximum diameter, without vascular invasion/extra-hepatic metastasis and with Child-Pugh A or B hepatic function. The inclusion criteria for our study were as follows: 1) sHCC confirmed by histologic or imaging criteria based on the National Comprehensive Cancer Network guidelines for hepatobiliary cancers, 2) age >18 years, 3) Child-Pugh class A or B disease within 1 month before SBRT, 4) laboratory tests taken within 1 month before and 1 month after SBRT, and 5) one or more radiological evaluations before and after SBRT. Patients with any of the following conditions were excluded from the study: 1) previous history of high-frequency thermal therapy, 2) distant metastasis, 3) double primary malignancy, or 4) no follow-up or follow-up lasting less than 6 months after the completion of SBRT.
We retrospectively collected clinical and laboratory information including lymphocyte count (LC), platelet count (PC), neutrophil count (NC), red blood cell (RBC) count, tumor characteristics, and host factors. NLR and PLR were calculated by dividing the NC and PC, respectively, by the LC. Changes in those ratios were determined by dividing the post-treatment values by the pre-treatment values.
SBRT
All enrolled patients were trained to maintain a slow breathing with respiratory exercise before the implementation of SBRT. The Body Pro-Lok system was used for abdominal compression to reduce the amplitude of liver motion. Patients underwent a contrast-enhanced computed tomography (CT) simulation while immobilized by a customized vacuum body mold in the supine position. The gross tumor volume (GTV) included all tumors detected via dynamic CT. For tumors that were not well visualized by CT scan, a pre-treatment magnetic resonance imaging (MRI) study was registered to the planning CT. Four-dimensional CT simulations were used to generate an internal target volume (ITV). The planning target volume (PTV) was defined as the ITV plus a radial margin of 3 mm. SBRT was planned using the TomoTherapy Planning System (Accuray Inc., Madison, WI). A radiotherapy dose was prescribed to the isodose surface covering 95% of the PTV. Patients received 5–10 fractions, delivered five times per week with a median dose of 48 Gray (gy) and a range of 48 gy to 60 gy.
Patient Follow-Up
Follow-up CT or MRI studies were performed 6–8 weeks after SBRT and tri-monthly thereafter. The recurrence of tumor, an increase in the size of the primary tumor, or the development of regional or distant metastasis was defined as progression. Progression-free survival (PFS) was calculated at the patient level as the interval between the first SBRT treatment and disease progression, last follow-up, or death. OS was calculated as the time from the first SBRT treatment until death from any cause or the last follow-up. Local control was defined as freedom from local progression according to mRECIST guidelines. Toxicity was evaluated by the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4.0).
Statistics and Analysis
Descriptive statistics were summarized as the mean ± standard deviation or the median and interquartile range, depending on whether the data were distributed normally according to the Kolmogorov–Smirnov test. Comparisons between quantitative variables were estimated using the two-sides t-test or the Mann–Whitney test, as appropriate. The primary endpoint was PFS, and the secondary endpoint was OS. Cumulative survival was calculated using the Kaplan–Meier method, and optimal cutoff values of continuous variables for patients’ prognosis were determined with the maximally selected log-rank test.21 Univariate and multivariate analyses were performed using the Cox regression model with hazards ratios (HRs) and 95% confidence intervals (95% CIs). The multivariate analysis was performed using the statistically significant factors identified by univariate analysis. Data were analyzed using SPSS Statistics 23.0 (IBM Corp., Armonk, NY, USA).
Results
Eligible Patients
From November 2012 to February 2017, 64 patients with sHCC underwent SBRT in our hospital. Three of those patients were excluded from our study because of follow-up duration of less than 6 months. Another patient was excluded because of distant metastasis before SBRT. Thus, a total of 60 patients with sHCC were included in the study. The baseline characteristics of the patients are listed in Table 1. Forty-four (73.3%) of the patients had chronic viral hepatitis, whereas the other 16 patients had no hepatitis. Forty-five patients (75.0%) had been treated previously with surgery, TACE, PEI, or RFA at least 6 weeks prior to SBRT. All of the patients had Child-Pugh grade A disease within 1 month before SBRT, and no patients demonstrated radiation-induced liver injury after SBRT. Complete blood counts were available for 50 patients both before (median 7 days prior to) and after (median 10 days since) SBRT.
 |
Table 1 Patient and Tumor Characteristics |
Patient Outcomes and Treatment-Related Toxicity
The median follow-up duration was 36.9 (range: 4.1–73.5) months. The 1-year and 2-year local control rates after SBRT were 95.1% and 90.0%, respectively. At the time of our analysis, 42 patients (70.0%) had developed actual progression, and 21 patients (35.0%) were deceased or lost to follow-up. Overall, the median PFS was 21.4 (range: 1.8–66.9) months, and the 1-year and 2-year PFS rates were 76.7% and 55.0%, respectively. The 1-year and 2-year OS rates were 95.0% and 78.3%, respectively.
Three patients exhibited SBRT-related toxicity. Two patients who experienced gastric ulcer or duodenal ulcer were identified as having grade 2 toxicity. One patient was determined to have grade 3 toxicity attributed to gastrointestinal hemorrhage. No patient experienced grade 4 or 5 treatment-related toxicity. Most episodes of toxicity were self-limiting and could be managed on an outpatient basis.
Factors Associated with Patient Outcomes at the Univariate Level
Factors including age; gender; tumor size; GTV; biologically effective dose; presence or absence of chronic hepatitis and previous treatments; pre-treatment RBC count and alpha-fetoprotein (AFP) level; and pre-treatment and post-treatment inflammation-based markers were analyzed at the univariate level. According to the results of the univariate analysis (Table 2), the presence of chronic hepatitis, tumor size ≥1.5 cm, pre-treatment RBC <4.5×1012/l and AFP ≥20.0 ng/mL, post-NLR ≥6.5, post-PLR ≥263.0, and change in NLR ≥2.7-fold were significantly associated with poor PFS (p<0.05 for each). There was no significant association between PFS and change in PLR. The presence of chronic hepatitis, tumor size ≥1.5 cm, pre-treatment RBC <4.5×1012/l and AFP ≥20.0 ng/mL, post-NLR ≥6.5, post-PLR ≥263.0, increase in NLR ≥2.7-fold, and increase in PLR ≥2.5-fold were identified as predictors of poor OS (p<0.05 for each). Pre-treatment NLR and PLR exhibited no significant association with patients’ PFS and OS (p>0.05 for each).
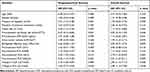 |
Table 2 Factors Associated with Patient Outcomes at the Univariate Level |
Independent Variables Influencing Patient Outcomes
After adjustment for the covariates, the association of inflammatory factors with PFS and OS was analyzed using the Cox regression model. First, Spearman's analysis showed that NLR and PLR did not correlate with other relevant prognostic factors (p>0.05). Then, multivariate analysis demonstrated that post-PLR ≥263.0 was significantly associated with both inferior PFS (HR: 3.70; 95% CI: 1.07–12.76, p=0.038) and OS (HR: 3.23; 95% CI: 1.01–9.11, p=0.043), whereas there was no significant relationship between high post-NLR and patients’ prognosis (p>0.05; Table 3). In addition, the presence of hepatitis infection and a low value of RBC were also proved to be independent adverse factors for patients’ outcomes (p<0.05 for each; Table 3). As for OS, an increase in NLR ≥2.7-fold was further identified as an independent predictor of worse OS (HR: 3.43; 95% CI: 1.14–10.38, p=0.029; Table 4).
 |
Table 3 Multivariate Analysis of Post-Treatment NLR and PLR for Patient Outcomes |
 |
Table 4 Multivariate Analysis of Change in NLR for Patient Outcomes |
In addition, in a comparison between patients with post-NLR <6.5 and those with post-NLR ≥6.5, there was a difference of 6.8 months in median PFS (22.0 months vs 15.2 months, p<0.05; Table 5). Furthermore, there was a difference of 6.6 months in PFS between patients with post-PLR <263.0 and those with post-PLR ≥263.0 (23.6 months vs 17.0 months, p<0.05). The Kaplan–Meier PFS and OS curves for post-NLR and post-PLR are shown in Figure 1.
 |
Table 5 Median PFS After SBRT According to Different Levels of Post-Treatment NLR and PLR |
Discussion
The prognosis of patients with HCC can be affected by a diverse set of clinical factors involving tumor biological characteristics, hepatic function, and the host immunology status. Emerging evidence7,8,22 shows that cancer-associated inflammation is significantly associated with clinical outcomes in most malignant tumors. Because NLR and PLR can reflect the systemic inflammation status of the host, the relationship between these two biomarkers and the prognosis of patients with HCC has gained great interest.
Recently, a meta-analysis comprising 6318 patients revealed that increased pre-treatment NLR and PLR were predictive of poor OS among patients with HCC, with HRs of 1.54 (95% CI: 1.34–1.76) and 1.63 (95% CI: 1.34–1.98), respectively.23 According to Ei Uchinaka et al24 and Lin et al25 the significant cutoff values of pre-treatment NLR and PLR that predict poor patient survival range from 1.28 to 2.81 and from 15.0 to 150.0, respectively. Many studies9–15 have found that high NLR and PLR are adverse prognostic factors for patients with HCC who received TACE, targeted therapy, RFA, or surgical resection. Investigating the application of inflammation-based markers in RT, D’Emic et al15 reported that pre-PLR >78 was a predictor of poor OS and PFS in patients with liver cancer who undergo SIRT. In another study, pre-NLR <2.1 was associated with improved PFS and OS in patients with locally advanced HCC treated with RT.26 Our results clearly show that low post-treatment PLR, and/or less change in NLR, were independent predictive factors for improved PFS and OS after SBRT in patients with sHCC, whereas the pre-treatment values of those inflammatory indexes showed no significant association with patients’ survival. The discrepancies might be due to differences in the composition of the study populations. All of the patients in our study had Child-Pugh class A small HCC. By contrast, most of the patients in D’Emic et al’s15 study has unresectable liver metastases (68.1%), and all of the patients in Son et al’s26 study had locally advanced HCC. Besides, the tumor size of most patients in our study was less than 3cm, which may indicate the low profile of inflammation that further explain the lack of prognostic value of pre-treatment NLR/PLR. Furthermore, Dan et al27 showed that the change in NLR after RFA, but not the preoperative NLR value alone, in patients with sHCC was an independent prognostic factor for both OS (HR: 2.39; 95% CI: 1.53–3.72) and relapse-free survival (HR: 1.69; 95% CI: 1.87–8.24), which is consistent with our results. In addition, a research focused on non-small cell lung cancer (NSCLC) receiving chemoradiotherapy also proved that post-treatment and change in inflammatory biomarkers may have more prognostic significance than baseline measurements.28
It is well known that as the most radiosensitive cells of the hematopoietic system, lymphocytes circulating through the radiation portal are frequently depleted.17 Patients receiving SBRT may, therefore, experience a marked decrease in peripheral lymphocyte count and even lymphopenia. Lower numbers of circulating lymphocytes after RT were associated with oncogenic immunosuppression and worse survival outcomes in patients with various solid tumors.18–20 Therefore, elevated post-SBRT inflammatory markers, which can reflect a decline in lymphocyte numbers, may be indicative of the complex factors contributing to patient survival and the immunologic response of liver tumors. Currently, there is no consensus on the cutoff value of post-treatment PLR and NLR for poor prognosis, and various methods including the receiver operating characteristics curve (ROC) and median values have been used to determine the optimal segregation points.29,30 In a retrospective study with a relatively small population, using the median value to determine a cutoff point and to stratify patients into distinct groups might bias the results. The ROC method is a better predictor of tumor response than of patient survival. Therefore, we calculated the cutoff values using a maximally selected log-rank test, which can represent a direct association with survival analysis.21 We found that post-PLR ≥263.0 was a prognostic factor of inferior PFS and OS in sHCC patients treated with SBRT. Besides, our study also indicated that patients with a relatively small change in inflammatory biomarkers after SBRT (an increase in NLR <2.7-fold) showed favorable OS. The results are in line with previous observations of the predictive role of post-treatment and change in inflammation markers in different malignancies.15,28 For example, an elevated post-PLR >290.0 was significantly associated with inferior OS (HR: 3.45; 95% CI: 1.17–10.17; p<0.05) in patients with liver cancer treated with SIRT.15 The delta in NLR >2.24 after chemoradiotherapy in NSCLC patients had a significantly worse OS and PFS (p<0.05 for each).28 Together with those findings, our study adds to the growing body of literature supporting post-treatment and change in inflammatory factors as predictors of clinical outcomes in patients treated with SBRT.
The molecular mechanisms underlying the relationships between high PLR and large change in NLR with poor prognosis in patients with HCC are not fully understood. What is known is that increased neutrophil levels are associated with the systemic release of chemokines and interleukins (e.g., interleukins 1 and 6) and vascular endothelial growth factor which can promote cancer growth, angiogenesis, invasion, and metastasis in HCC.31 Moreover, neutrophils in intratumoral regions may increase autophagic activity and sustain the pro-tumorigenic characteristics of HCC.32 Platelets have the potential to recognize and kill invading pathogens and to release various mediators that modify responses of immune and endothelial cells.33 HCC patients with a high platelet count may have a high risk of extrahepatic metastasis and immune evasion by tumor cells.34,35 As crucial components of the immune surveillance system, functional lymphocytes identify and destroy cancerous cells and mediate anti-tumor responses, which play a vital role in tumor control.36 The known associations between cancer and neutrophils, platelets, and lymphocytes may partially explain why high PLR and change in NLR are adverse prognostic factors for patients with sHCC that undergo SBRT.
However, the results of this study should be carefully interpreted because of the small number of patients and the retrospective design. The population of patients with sHCC that undergo SBRT might be more heterogeneous than our study sample, so there was a potential for selection bias. Another potential limitation of our study is that the blood neutrophil, platelet, and lymphocyte counts might have been influenced by infection, cirrhosis-associated hypersplenia, or medications taken before HCC treatment. Furthermore, the treatments that the patients received prior to SBRT were not uniform; some patients had previously been treated with TACE, RFA, PEI, or surgical resection, which might have biased the results. Beyond those limitations, our study has several strengths. First, previous studies of liver cancers focused primarily on the pre-treatment NLR or PLR. Our study is the first to investigate the value of post-SBRT inflammatory indexes for the evaluation of treatment efficacy and the prediction of survival in patients with sHCC. Additionally, in contrast to pre-treatment indexes, the change in NLR or PLR after SBRT can reflect the dynamic changes in the host inflammatory and immune responses that occur during treatment. Furthermore, the inflammation-based variables are convenient and easy to acquire during routine clinical practice.
Conclusions
Elevated post-treatment PLR as well as a large increase in NLR after SBRT were associated with poor outcomes in patients with sHCC and may be considered as reliable predictors of patients’ survival. Further large-scale validation studies will be needed to confirm the effectiveness of these inflammatory markers in patients with sHCC who undergo SBRT.
Abbreviations
AFP, alpha-fetoprotein; CI, confidence interval; CT, computed tomography; GTV, gross tumor volume; HCC, hepatocellular carcinoma; HR, hazard ratio; LC, lymphocyte count; NC, neutrophil count; NLR, neutrophil-to-lymphocyte ratio; NSCLC, non-small cell lung cancer; OS, overall survival; PC, platelet count; PEI, percutaneous ethanol injection; PFS, progression-free survival; PLR, platelet-to-lymphocyte ratio; RBC, red blood cell; RFA, radiofrequency ablation; ROC, receiver operating characteristics curve; RT, radiation therapy; SBRT, stereotactic body radiation therapy; SIRT, selective internal radiation therapy; sHCC, small hepatocellular carcinoma.
Ethics Approval and Informed Consent
The ethics committee of Zhongshan Hospital, Fudan University approved the study protocols (B2018-272), and written informed consent was obtained from all patients.
Acknowledgments
The abstract of this paper was presented at the 10th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2019) as a conference talk with interim findings. The poster’s abstract was published in “Poster Abstracts” in Liver Cancer 2019;8(suppl 1):1-204. See link below to the published abstract: https://www.karger.com/Article/Abstract/502497.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
3. Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer. 2018;7(3):235–260. doi:10.1159/000488035
4. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370.
5. Truty MJ, Vauthey JN. Surgical resection of high-risk hepatocellular carcinoma: patient selection, preoperative considerations, and operative technique. Ann Surg Oncol. 2010;17(5):1219–1225. doi:10.1245/s10434-010-0976-5
6. Zeng ZC, Seong J, Yoon SM, et al. Consensus on stereotactic body radiation therapy for small-sized hepatocellular carcinoma at the 7th Asia-Pacific Primary Liver Cancer Expert Meeting. Liver Cancer. 2017;6(4):264–274. doi:10.1159/000475768
7. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi:10.1038/nrc3611
8. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi:10.1016/S1470-2045(14)70263-3
9. Fan W, Zhang Y, Wang Y, Yao X, Yang J, Li J. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS ONE. 2015;10(3):e119312.
10. Da FL, Barroso-Sousa R, Bento AS, et al. Pre-treatment neutrophil-to-lymphocyte ratio affects survival in patients with advanced hepatocellular carcinoma treated with sorafenib. Med Oncol. 2014;31(11):264. doi:10.1007/s12032-014-0264-5
11. Chu MO, Shen CH, Chang TS, et al. Pretreatment inflammation-based markers predict survival outcomes in patients with early stage hepatocellular carcinoma after radiofrequency ablation. Sci Rep. 2018;8(1):16611. doi:10.1038/s41598-018-34543-z
12. Tajiri K, Baba H, Kawai K, et al. Neutrophil-to-lymphocyte ratio predicts recurrence after radiofrequency ablation in hepatitis B virus infection. J Gastroenterol Hepatol. 2016;31(7):1291–1299. doi:10.1111/jgh.13287
13. Liao W, Zhang J, Zhu Q, et al. Preoperative neutrophil-to-lymphocyte ratio as a new prognostic marker in hepatocellular carcinoma after curative resection. Transl Oncol. 2014;7(2):248–255. doi:10.1016/j.tranon.2014.02.011
14. Li C, Wen TF, Yan LN, et al. Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res. 2015;198(1):73–79. doi:10.1016/j.jss.2015.05.003
15. D’Emic N, Engelman A, Molitoris J, et al. Prognostic significance of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients treated with selective internal radiation therapy. J Gastrointest Oncol. 2016;7(2):269–277. doi:10.3978/j.issn.2078-6891.2015.108
16. Raben M, Walach N, Galili U, Schlesinger M. The effect of radiation therapy on lymphocyte subpopulations in cancer patients. Cancer-Am Cancer Soc. 1976;37(3):1417–1421.
17. Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiat Oncol. 2018;3(4):512–519. doi:10.1016/j.adro.2018.08.014
18. Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. doi:10.1016/j.critrevonc.2018.01.003
19. Maehata Y, Onishi H, Kuriyama K, et al. Immune responses following stereotactic body radiotherapy for stage I primary lung cancer. Biomed Res Int. 2013;2013:731346. doi:10.1155/2013/731346
20. Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi:10.1158/1078-0432.CCR-11-0774
21. Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE. 2012;7(12):e51862. doi:10.1371/journal.pone.0051862
22. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
23. Zheng J, Cai J, Li H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem. 2017;44(3):967–981. doi:10.1159/000485396
24. Uchinaka E, Amisaki M, Morimoto M, et al. Utility and limitation of preoperative neutrophil lymphocyte ratio as a prognostic factor in hepatocellular carcinoma. Yonago Acta Med. 2018;61(4):197–203. doi:10.33160/yam.2018.12.002
25. Lin WF, Zhong MF, Zhang YR, et al. Prognostic role of platelet-to-lymphocyte ratio in hepatocellular carcinoma with different BCLC stages: a systematic review and meta-analysis. Gastroenterol Res Pract. 2018;2018:5670949. doi:10.1155/2018/5670949
26. Son SH, Park EY, Park HH, Kay CS, Jang HS. Pre-radiotherapy neutrophil-to-lymphocyte ratio as an independent prognostic factor in patients with locally advanced hepatocellular carcinoma treated with radiotherapy. Oncotarget. 2017;8(10):16964–16971. doi:10.18632/oncotarget.v8i10
27. Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS ONE. 2013;8(3):e58184. doi:10.1371/journal.pone.0058184
28. Guo M, Li W, Li B, et al. Prognostic value of delta inflammatory biomarker-based nomograms in patients with inoperable locally advanced NSCLC. Int Immunopharmacol. 2019;72:395–401. doi:10.1016/j.intimp.2019.04.032
29. Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000;19(1):113–132. doi:10.1002/(ISSN)1097-0258
30. Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163(7):670–675. doi:10.1093/aje/kwj063
31. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283–287. doi:10.1023/B:AGEN.0000029415.62384.ba
32. Li XF, Chen DP, Ouyang FZ, et al. Increased autophagy sustains the survival and pro-tumourigenic effects of neutrophils in human hepatocellular carcinoma. J Hepatol. 2015;62(1):131–139. doi:10.1016/j.jhep.2014.08.023
33. Kim SJ, Davis RP, Jenne CN. Platelets as modulators of inflammation. Semin Thromb Hemost. 2018;44(2):91–101. doi:10.1055/s-0037-1607432
34. Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–1300.
35. Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi:10.1200/JCO.2010.30.5425
36. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi:10.1016/j.immuni.2004.07.017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

