Back to Journals » The Application of Clinical Genetics » Volume 11
Prenatal diagnosis and molecular cytogenetic characterization of a de novo duplication of 15q24.3-q26.1
Authors Ochando I, Alonzo Martínez MC , Serrano AM, Urbano A, Cazorla E , Calvo D, Rueda J
Received 9 December 2017
Accepted for publication 15 March 2018
Published 3 July 2018 Volume 2018:11 Pages 77—80
DOI https://doi.org/10.2147/TACG.S159377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Martin Maurer
Isabel Ochando,1,2 Melanie Cristine Alonzo Martínez,3 Ana María Serrano,3 Antonio Urbano,1 Eduardo Cazorla,3 Dolores Calvo,4 Joaquín Rueda1,2
1Genetics Unit, Unidad de Genética, Hospital Clínica Vistahermosa, Alicante, Spain; 2Departamento de Histología y Anatomía, Universidad Miguel Hernández, Alicante, Spain; 3Department of Obstetrics and Gynecology, Servicio de Ginecología y Obstetricia, Hospital Universitario de Torrevieja, Alicante, Spain; 4Emergency Laboratory, Laboratorio Urgencias, Hospital Clínico Universitario, Valladolid, Spain
Abstract: Reported cases of distal 15q interstitial duplications are uncommon and do not result in a recognizable pattern of abnormalities. Some studies report prenatal overgrowth, while others describe growth retardation. We present molecular cytogenetic characterization of a 14 Mb interstitial duplication, encompassing 81 Online Mendelian Inheritance in Man (OMIM) genes, in a fetus with single umbilical artery and short limbs. We propose that growth restriction, previously described and present in our patient, may be due to duplication of a gene or genes contained in the 15q24 region.
Keywords: distal 15q trisomy, prenatal diagnosis, short limbs
Introduction
Reported cases of distal 15q duplications are very uncommon; in fact, there are ~100 cases reported, and even less de novo duplications as in this case report. Previous authors have described a distal 15q trisomy syndrome characterized by prenatal and postnatal overgrowth, developmental delay, craniofacial and skeletal malformations, and genital abnormalities, particularly in affected males.1 There are only few reported cases of de novo single duplication of 15q24-qter region.2–7 The type and severity of reported anomalies depend on the length and location of the duplicated region of chromosome 15q, but there is a common phenotype consisting of minor craniofacial anomalies (downslanting palpebral fissures and ptosis, large prominent nose, facial asymmetry, and micrognathia), arachnodactyly and camptodactyly, heart defects (septal communications, patent ductus arteriosus, pulmonary artery stenosis), hypogonadism and cryptorchidism, scoliosis, severe developmental delay, and anencephaly.3
Reported patients with trisomy of 15q25/q26qter have presented prenatal and postnatal overgrowth.1,2,4–6 Overgrowth has been attributed to overexpression of the IGF1R gene, which is located on 15q26.3. On the contrary, patients with trisomy of 15q24qter region exhibit growth restriction/intrauterine growth restriction (IUGR) and developmental delay.2,7–10
In this report, we present a prenatal diagnosis of a fetus with single umbilical artery, short limbs, and a de novo duplication of 15q24.3q26.1.
Clinical report
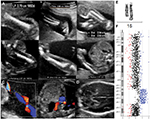
A 30-year-old patient had a routine anomaly scan at 21+1 weeks gestation. Although her first trimester combined screening risk was low, the ultrasound scan showed a male fetus with short long bones (-2.5 standard deviation [SD]), according to Chitty and Altman11 (femur length [FL]: 27–27 mm, tibia: 25–26 mm, fibula: 25–25 mm, humerus: 25-26 mm, ulna: 25–26 mm, radius: 25–25 mm) but with normal morphology and mineralization signs, corresponding to a 19+5 gestation week fetus, with an estimated weight of 289 g (according to Hadlock tables, biparietal diameter [BPD] 45 mm, head circumference [CC] 174 mm, abdominal circumference [AC] 147 mm) (Figure 1A and B). The remaining anatomy was normal except for a single umbilical artery (Figure 1C). At Week 24, the fetus still showed 2-week-younger measurements, and the transverse cerebellar diameter was 23 mm, below the 5th centile, according to Snijders and Nicolaides12 (Figure 1D). After ruling out an error in pregnancy dating, an amniocentesis was performed to find out the origin of the growth delay. The fetus karyotype showed a 15q duplication, visible by conventional G-banding (Figure 1E). The duplication was considered de novo because the parents displayed normal karyotypes on testing. Comparative genomic hybridization (CGH) array (KaryoNIM® Prenatal 60K; NIMGenetics, Madrid, Spain) was carried out to determine the size and extent of the duplication (Figure 1F). It was an interstitial duplication of 14 Mb: 46,XY,dup(15)(q24.3q26.1).arr15q24.3q26.1(76223116-90340143)×3. The duplicated region contains 257 genes in the Genes on Sequences National Center for Biotechnology Information (NCBI) map, of which 81 are in the Online Mendelian Inheritance in Man (OMIM) database. The patients underwent genetic counseling and opted for a termination of pregnancy. They declined postmortem examination.
Written informed consent has been provided by the patient to have the case details and any accompanying images published.
Discussion
The fetus described in this report exhibited single umbilical artery, had short limbs, and had a de novo duplication of 15q24.3q26.1. Based on the cases reported to date, it is not clear whether the duplication of 15q24-qter results in a recognizable pattern of abnormalities.
Zollino et al1 reported a total of 32 patients with 15q duplications and divided them in two groups: one group had trisomy for 15q21-24qter, showing microcephaly and normal prenatal growth; and the other group showed trisomy 15q25-26qter, characterized by prenatal overgrowth, macrocephaly, and craniosynostosis. The cause of overgrowth has been thought to be related to a dosage excess of the IGF1R gene, located in 15q26.3.1,2,4–6 This gene is not contained in the region duplicated in the fetus we report herein.
Roggenbuck et al7 reported two unrelated cases with single 15q24q26.3 duplication showing small size. O’Connor et al6 reported a patient with a single 15q24qter trisomy and normal sizes/measures at birth. Genesio et al8 described the case of a multiple-malformed newborn with IUGR and a de novo inverted duplication of 15q21q26.3, with three copies of the IGF1R gene. The authors hypothesized that the IUGR depends on global transcription dysregulation more than the impairment of a single gene specifically correlated to the malformation.8 El-Hattab et al13 reported a case with short stature and developmental delay and a 15q24 microduplication that contained the OMIM genes SCAPER, ISL2, EFTA, NRG4, FBXO22, and UBE2Q2. All these genes, except UBE2Q2, are duplicated in the fetus reported here. It is tempting to speculate that dosage excess of genes located in 15q24 leads to short limbs/IUGR instead of tall stature.
In the case of a fetal ultrasonogram displaying short long bones (<5th percentile), it is advisable to analyze their morphology and mineralization, as well as rule out a possible error in gestational dating and femur–humerus confusions. Then, differential diagnoses should include constitutional short stature, IUGR, skeletal dysplasia such as achondroplasia, and chromosomal abnormalities such as deletion of 15q24, similar to this case report.
This study underscores the utility of the CGH array in the characterization of the size and nature of rearrangements, as well as in predicting the severity of phenotypes, which depends on the length and location of the duplicated region. Associations between phenotypes and copy number variations of small chromosome regions provide valuable information about clinically important genes or regulatory elements and allow the investigation of their role in the phenotypes. Systematic characterization of newly reported patients provides useful information for clinicians and patients.
Acknowledgments
The authors wish to thank Miss Jéssica Calvo for technical support. This work was supported by Citolab and Cátedra de Biomedicina Reproductiva Vistahermosa. The abstract of this work was presented at the 10th European Cytogenetics Conference, Strasbourg, France, July 2015.
Disclosure
The authors report no conflicts of interest in this work.
References
Zollino M, Tiziano F, Di Stefano C, Neri G. Partial duplication of the long arm of chromosome 15: confirmation of a causative role in craniosynostosis and definition of a 15q25-qter trisomy syndrome. Am J Med Genet. 1999;87(5):391–394. | ||
Cannarella R, Mattina T, Condorelli RA, et al. Chromosome 15 structural abnormalities: effect on IGF1R gene expression and function. Endocr Connect. 2017;6(7):528–539. | ||
Chen CP, Chen CY, Chern SR, et al. Molecular cytogenetic characterization of a duplication of 15q24.2-q26.2 associated with anencephaly and neural tube defect. Taiwan J Obstet Gynecol. 2017;56(4):550–553. | ||
Gutiérrez-Franco Mde L, Madariaga-Campos Mde L, Vásquez-Velásquez AI, Matute E, Guevara-Yáñez R, Rivera H. A girl with 15q overgrowth syndrome and dup(15)(q24q26.3) that included telomeric sequences. Korean J Lab Med. 2010;30(3):318–324. | ||
Kim EY, Kim YK, Kim MK, et al. A case of de novo duplication of 15q24-q26.3. Korean J Pediatr. 2011;54(6):267–271. | ||
O’Connor R, Al-Murrani A, Aftimos S, et al. Pure duplication of the distal long arm of chromosome 15 with ebstein anomaly and clavicular anomaly. Case Rep Genet. 2011;2011:5. | ||
Roggenbuck JA, Mendelsohn NJ, Tenenholz B, Ladda RL, Fink JM. Duplication of the distal long arm of chromosome 15: report of three new patients and review of the literature. Am J Med Genet. 2004;126A(4):398–402. | ||
Genesio R, De Brasi D, Conti A, et al. Inverted duplication of 15q with terminal deletion in a multiple malformed newborn with intrauterine growth failure and lethal phenotype. Am J Med Genet A. 2004;128A(4):422–428. | ||
Miller MS, Rao PN, Dudovitz RN, Falk RE. Ebstein anomaly and duplication of the distal arm of chromosome 15: report of two patients. Am J Med Genet. 2005;139A(2):141–145. | ||
Roetzer KM, Schwarzbraun T, Obenauf AC, Hauser E, Speicher MR. Further evidence for the pathogenicity of 15q24 microduplications distal to the minimal critical regions. Am J Med Genet A. 2010;152A(12):3173–3178. | ||
Chitty LS, Altman DG. Charts of fetal size: limb bones. BJOG. 2002;109(8):919–929. | ||
Snijders RJ, Nicolaides KH. Fetal biometry at 14-40 weeks´ gestation. Ultrasound Obstet Gynecol. 1994;4:34–48. | ||
El-Hattab AW, Smolarek TA, Walker ME, et al. Redefined genomic architecture in 15q24 directed by patient deletion/duplication breakpoint mapping. Hum Genet. 2009;126(4):589–602. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.