Back to Journals » Clinical Ophthalmology » Volume 16
Preliminary Surgical Outcomes of a Trimmed-Plate Aurolab Aqueous Drainage Implant (AADI) in Eyes at High Risk of Hypotony
Authors AlJaloud A , AlHilali S, Edward DP, Ahmad K , Malik R
Received 12 November 2021
Accepted for publication 4 March 2022
Published 13 May 2022 Volume 2022:16 Pages 1487—1496
DOI https://doi.org/10.2147/OPTH.S343378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ahmad AlJaloud,1,2 Sara AlHilali,1 Deepak P Edward,1,3 Khabir Ahmad,4 Rizwan Malik1,5
1Glaucoma Division, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia; 2King Abdullah bin AbdulAziz University Hospital, Riyadh, Saudi Arabia; 3University of Illinois Eye and Ear Infirmary Chicago, Chicago, IL, USA; 4Research Department, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia; 5Department of Ophthalmology and Visual Sciences, University of Alberta, Edmonton, AB, Canada
Correspondence: Khabir Ahmad, Research Department, King Khaled Eye Specialist Hospital, Al Aruba Branch Road, Riyadh, 12329, Saudi Arabia, Tel +966 11 482 1308, Email [email protected]
Purpose: We describe the technique of trimming the 350 mm2 AADI glaucoma shunt plate and report preliminary results that test the hypothesis that the IOP-lowering efficacy of the trimmed AADI glaucoma shunt is comparable to the Baerveldt 250 mm2 glaucoma drainage implant with a comparable safety profile to the standard AADI implant.
Methods: Consecutive patients who had received the modified trimmed-plate AADI, standard AADI and Baerveldt 250 mm2 were included in the study. This included patients with refractory or primary or secondary glaucoma of all ages and eyes with and without previous glaucoma surgery. The decision for trimming the AADI plate was made according to the surgeon’s perceived risk of hypotony. Pre-operative, intraoperative and post-operative data were collected from the hospital electronic medical record system. Surgical success was defined as IOP ≥ 5 mmHg and ≤ 21 mmHg on two consecutive visits after 3 months, whilst maintaining at least LP vision and avoiding re-operation for glaucoma.
Results: The sample consisted of 69 eyes (19 with trimmed-plate AADI implant; 36 eyes with the standard AADI implant and 14 eyes who received a BGI-250). The mean IOP reduction at 1 year was 15 mmHg for the Baerveldt-250, 10 mmHg for the AADI and 13 mmHg for the trimmed-plate AADI. The surgical success rate of the implants over 1 year was 85.7% (95% CI, 53.9– 96.2%) for BGI-250, 81.5% (62.6– 91.5%) for standard AADI and 78.2% (51.7– 91.3%) for the trimmed AADI.
Conclusion: Trimming the plate of the AADI manually may provide a safe and low-cost method of obtaining a successful surgical outcome in eyes at high risk of hypotony.
Keywords: refractory glaucoma, non-valved glaucoma drainage device, AADI, trimmed-plate implant, hypotony
Introduction
Glaucoma drainage devices (GDD) are typically reserved for glaucomas refractory to filtering procedures and following failure of a primary filtering surgery, although there is an increasing trend towards primary GDD surgery.1 GDD are broadly classified by the presence or absence of a valve to prevent early post-operative hypotony. Examples of valved GDDs include the Ahmed Glaucoma valve (New World Medical Inc., Rancho Cucamonga, CA) and the Krupin valve model (Eagle Vision, Inc. Memphis, TN, USA); and non-valved devices include the Baerveldt Glaucoma Implant (Advanced Medical Optics, Inc., Santa Ana, CA, USA), the Molteno GDD (IOP, Inc., Costa Mesa, CA, USA, and Molteno Ophthalmic Limited, Dunedin, New Zealand) and the Aurolab Aqueous Drainage Implant (Aurolab, Madurai, India).
The Aurolab Aqueous Drainage Device (AADI) is a non-valved, low-cost glaucoma implant replicating the BGI 350 design with plate size of 350 mm2. It has been commercially available in India since 2013 and gained broader availability since it received the CE marking approval by the European Commission. Whilst the BGI comes in two plate sizes, ie, the 250 mm2 (BGI-250), and 350 mm2 (BGI-350), the AADI is only available in the 350 mm2 size.
Five year outcomes of randomized clinical trial data have demonstrated lower surgical success of valved compared to non-valved glaucoma implants, but with a lower hypotony rate, for the management of adult glaucoma in Western populations.2 Whilst the surgical success rates of non-valved devices are comparable to valved devices in adult populations, non-valved implants may be superior to valved implants in Indian and Middle-Eastern persons who typically have a higher rate of encapsulation.3,4
One of the main safety concerns of non-valved implants, compared to valved GDDs is the risk of hypotony.2 For non-valved implants, the risk of hypotony can be lowered by complete ligation of the GDD at the time of insertion and by decreasing the surface area of the plate.5,6 The amount of aqueous drainage and IOP lowering effect of GDDs is roughly proportional to the size of the plate and the inner surface area of the surrounding fibrovascular capsule bleb.7–9
Manually trimming the plate of the standard AADI provides a low-cost method of producing an implant with a smaller plate which may be more suitable for eyes in high risk of hypotony. In this study, we describe the technique of truncating the AADI plate and test the hypothesis that the IOP-lowering efficacy of a trimmed plate was comparable to the BGI-250 with a similar safety as the standard AADI implant.
Methods
Study Design
This was a retrospective cohort study. We reviewed all patients who underwent surgery with the trimmed AADI implant between April 2016 and December 2018. For comparison, we also identified a group of patients who had received the standard AADI and another group of patients who received the BGI-250 during the same time period.
Ethical Considerations
The study was approved by the Institutional Review Board (IRB) at King Khaled Eye Specialist Hospital (IRB No: 1936-R). Due to the retrospective nature of the study, written informed patient consent was waived by the IRB. All data were anonymized for collection and analysis and this report contains no identifiable information. The study followed the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act regulations.
Inclusion and Exclusion Criteria
All consecutive patients who had received the modified trimmed-plate AADI, standard AADI and BGI-250 were included in the study. This included patients with any refractory or primary or secondary glaucoma of all ages and eyes with and without previous glaucoma surgery. The decision for trimming the AADI plate was made according to the surgeon’s perceived risk of hypotony and based on pre-operative risk factors such as myopia, pseudophakia/aphakia, previous cyclodestruction and history of vitrectomy.
Data Collection
Patients who received AADI or Baerveldt implants were identified from our operation room logbook of implanted devices. The operation notes were then reviewed to discern patients who received the trimmed-plate AADI, standard AADI and BGI-250.
Pre-operative, intraoperative and post-operative data were collected from electronic medical record system (EMR), TrakCare (InterSystems Corporation, 2016). Data collection included age, gender, diagnosis, number and type of previous ocular surgeries, visual acuity (VA), IOP and number of glaucoma medications (AGM), surgical details, time of ripcord removal, complications and need for further surgery, (see below for description of surgical technique and post-operative care). IOP was measured by Goldmann applanation tonometry unless there was corneal opacity or nystagmus, in which case it was measured with contact pneumotonometry (Model 30 Pneumotonometer, Ametek Reichert Technologies, Depew, NY). Surgical details including the type of GDD, quadrant of implantation, type(s) of ligature used, type of patch graft used, and intraoperative complications were recorded.
Surgical Techniques
Our surgical techniques for the AADI was similar to that used for the BGI. Detailed descriptions of the technique are available elsewhere,10 but a brief description is given here. A corneal 7/0 vicryl traction was placed and a fornix-based conjunctival flap was dissected in the desired quadrant and eraser cautery performed. The corresponding recti muscles were identified then non-valved implant was inserted into the exposed quadrant behind muscles. The plate was sutured to the sclera with 9/0 prolene or 9/0 nylon with the anterior edge 9–10 mm posterior to the limbus. The tube was ligated according to surgeon preference. A 4/0 nylon was used as ripcord and then the tube ligated externally with 6/0 vicryl or 7/0 vicryl suture, with an additional a 9/0 nylon suture ligature near the plate to partially occlude the tube. The 9/0 nylon suture provided resistance to flow after the vicryl resorbed. Complete occlusion was verified by flushing the tube with a 27-gauge cannula. The tube was trimmed to an appropriate length beveled up and inserted into the anterior chamber (AC), or rarely the sulcus, after making a 23-gauge sclerostomy. The tube was sutured to the episclera with 9/0 nylon mattress suture and 2–3 fenestrations with a 7/0 needle were done at the discretion of the surgeon. A scleral, pericardium or corneal patch graft was sutured to the episclera with 9/0 nylon to cover the tube and entry site. The 4/0 nylon ripcord was buried inferiorly under the conjunctiva in the fornix for retrieval in a future visit. The conjunctiva was secured with continuous 9/0 vicryl suture and the subconjunctival injections of 100 mg cefazolin and 4 mg dexamethasone administered.
For the trimmed-plate AADI, both lateral wings of the implant were trimmed after marking the plate with a surgical marker aiming for a plate size approximately equivalent to a Baerveldt-250 implant. The plate was trimmed using a curved Steven’s scissors (Figure 1) by the primary surgeon under sterilized conditions. The trimmed edges of the wings were checked visually for any irregularities that could make it unfit for use.
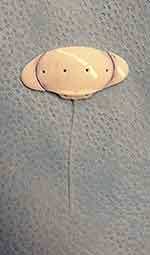 |
Figure 1 The trimmed-plate AADI is shown in relation to the standard plate size. The trimmed-plate AADI implant is achieved by trimming the two lateral wings of the AADI. |
Post-Operative Care
All patients were treated with topical vigamox 0.5% (moxifloxacin ophthalmic solution, Alcon, Fort Worth, Texas) every 6 hours for 2 weeks, prednisolone acetate 1% (Pred Forte, Allergan Inc, NJ) for 8 weeks in a tapering dose starting from every 2 hours and atropine 1% every 12 hours for 2 weeks, then four times a day until the tube ligature vicryl sutures dissolved, then tapered thereafter. Patients were typically seen at 1 day, 1–2 weeks, 1–2 months, 3–4 months and 6–8 months, 12 months, then annually in the post-operative period. Examination during these visits included assessment of VA and IOP measurement with Goldmann applanation tonometry, as well as a complete anterior and posterior segment examination and ultrasound B-scan if needed, to assess the presence of any post-operative complications. Based on glaucoma status and the postoperative course, AGMs were restarted to control IOP when judged to be clinically necessary.
Primary and Secondary Outcome Measures
The primary outcome measure was post-operative IOP for the trimmed-plate AADI relative to the standard AADI and the BGI 250. Secondary outcome measures included number of glaucoma medications in the post operative period needed to control IOP, and surgical complications. Success was defined as IOP ≥ 5 mmHg and ≤ 21 mmHg with or without the need of using AGM on two consecutive visits after 3 months, similar to that reported in RCTs of non-valved implants.12,13 We did not distinguish between complete and qualified success as our routine practice is to commence aqueous suppressants early in the post-operative course, within the first month, to minimise the risk of longer-term encapsulation.11
Failure was defined when on two consecutive visits, IOP measurements was less than 5 mmHg or more than 21 mmHg on AGM after the visit when the ripcord was removed, loss of light perception vision or the need for further glaucoma surgical intervention.12,13 Cyclophotocoagulation was regarded as an additional procedure resembling failure. In the case of re-operation, the type of subsequent surgery was recorded.
Data Analysis
Data were entered in Microsoft Excel 2010 (Microsoft Corporation, Redmond, Washington) and analyzed using STATA 16.1 (StataCorp LLC, College Station, TX, USA). Categorical data (baseline, operative or post-operative) were reported as frequencies and percentages and compared using Chi-squared or Fisher’s exact tests. Mean and standard deviation (SD) were used to describe continuous variables. One-way ANOVA was used to compare the baseline mean age, IOP and the number of AGM among the three implant groups. Levene’s test was used to determine whether the homogeneity of variance assumption was satisfied. The mean IOP and number of glaucoma medications over time, with 95% confidence intervals, were computed and plotted for the trimmed-plate AADI, standard AADI and the Baerveldt-250 groups. Kaplan-Meier curves were plotted to depict surgical survival by implant type and compared using log rank test. Statistical significance was defined as a p-value <0.05.
Results
We studied 19 eyes that had received a trimmed-plate AADI implant during the study period. In addition, there were 36 eyes that received the standard AADI implant and 14 eyes that received BGI-250. Baseline characteristics were similar across the three groups, as shown in Table 1. The mean ± SD of age of the patients at implant placement was 25.1 ± 22.9, 34.2 ± 23.8 and 34.0 ± 26.7 in the trimmed-plate AADI, the standard AADI and the Baerveldt-250 groups, respectively (p=0.384). The proportion of patients less than 16 years of age in group 1, 2 and 3 were respectively: 42.1%, 30.6% and 42.9%. The mean baseline IOP was comparable among the treatment groups (29.3 ± 10.0 for trimmed AADI, 26.7 ± 9.7 for the standard AADI and 30.7 ± 9.1 for BGI-250; p=0.377) as was the mean number of glaucoma medication (3.6 ± 0.9 for trimmed AADI, 3.4 ± 1.2 for standard AADI and 3.8 ± 0.7 for BGI-250; p=0.453).
 |
Table 1 Baseline Characteristics of Eyes/Persons in the Trimmed-Plate AADI, Standard AADI and Baerveldt-250 Groups* |
Most of the eyes (89.5%, 91.7% and 71.4% in the trimmed AADI, standard AADI and BGI-250 groups, respectively) had one or more previous surgeries. The mean number of prior surgeries was 1.9 ± 1.2 for the trimmed-plate AADI implant, 1.7 ± 1.0 for the standard AADI and 1.2 ± 1.1 for the BGI-250 group (p= 0.202). The AADI plate was trimmed in cases with a perceived risk of post-operative hypotony. These included 4 eyes (21.1%) with aphakia, and 6 (31.6%) that had undergone endoscopic or trans-scleral cyclophotocoagulation.
The mean IOP noted over time is shown in Figure 2. The mean reduction of IOP was similar among the groups at all-time points. The mean IOP reduced to 16.5 mmHg (95% CI, 13.1–19.9) for the trimmed-plate AADI, 16.3 (95% CI, 13.5–19.2) mmHg for the standard AADI and 16.0 (95% CI, 11.6–20.4) mmHg for the BGI-250 at 1 year, and 14.8 (95% CI, 8.3–21.2) mmHg for the trimmed-plate AADI, 13.6 (95% CI, 9.7–17.4) mmHg for the standard AADI and 16.9 (95% CI, 10.4–23.4) for the BGI-250 at 18 months. Similarly, the mean number of glaucoma medications for these groups reduced respectively to 1.6 (95% CI, 0.6–2.5), 1.4 (95% CI, 0.9–2.0), 2.1 (95% CI, 1.2–3.0) at 1 year, and to 1.5 (95% CI, −0.6–3.6), 1.7 (95% CI, 0.6–2.8), and 1.6 (95% CI, −0.7–3.9) at 18 months.
 |
Figure 2 Mean IOP for trimmed-plate AADI, standard AADI and the Baerveldt-250 groups over time. |
Survival of implants is manifested by Kaplan-Meier survival curves for each implant during the follow-up period of 1 year is shown in Figure 3. Success of the implants over 1 year was 78.2% (95% CI, 51.7–91.3%) for the trimmed AADI, 81.5% (95% CI, 62.6–91.5%) for the standard AADI and 85.7% (95% CI, 53.9–96.2%) for the BGI-250. The primary causes of failure were: in the trimmed-plate AADI group, there were four failures (one for subsequent glaucoma surgery, two patients underwent tube removal for recurrent tube exposure and one had loss of light perception, phthisis bulbi then tube exposure followed by removal of plate and tube). In the standard AADI group, there were 6 failures (one from loss of LP vision, two from uncontrolled IOP, two for subsequent glaucoma surgery and one for tube removal 1 month after the surgery due to conjunctival dehiscence and tube exposure that could not be repaired). In the BGI-250 group, two implants failed because of uncontrolled IOP.
 |
Figure 3 Kaplan-Meier plots depicting cumulative surgical success over time for the trimmed-plate AADI, standard AADI and the Baerveldt-250 groups. |
There was no intraoperative complication seen with any of the implants. Post-operative complications are shown in Table 2. For the trimmed AADI, there were choroidal effusions in four patients, two of them developed hypotony in the early post-operative period that resolved with conservative treatment (atropine sulfate 1% and prednisolone acetate). The third patient developed choroidal detachment after removing the ripcord that resolved with the same conservative regimen. The fourth patient was a known case of Peter’s anomaly who had undergone cyclophotocoagulation in the past. He developed choroidal detachment and chronic hypotony in the post-operative period and ended up with a phthisical blind eye.
 |
Table 2 Intra- and Post-Operative Complications in the Three Treatment Groups |
In the trimmed AADI group, three patients experienced a tube exposure. The exposure rate was similar to that seen in the other groups (p=0.489). These patients initially were treated with revision of the conjunctival dehiscence which recurred and later resulted in tube removal in two patients. There was one case of endophthalmitis in the BGI-250 group that was secondary to tube erosion. This patient was managed with pars plana vitrectomy (PPV) and removal of the tube. One patient with early hypotony in the BGI-250 was managed by AC reformation which resolved the hypotony.
Post-operative choroidal effusion secondary to transient hypotony occurred in 4 eyes (23.5%) in the trimmed-AADI group. The first patient developed choroidal effusion that was detected by B-scan 5 days post-operatively. This patient was aphakic and had a prior AGV implant. The second was a patient with Peter’s anomaly who had previously undergone transscleral diode CPC twice and then underwent combined surgery (tube, lensectomy, optical iridectomy, PPV).
Subsequent glaucoma surgeries are detailed in Table 3. Tube revision was needed in 12 eyes. In trimmed-AADI group, three eyes needed removal of the tube (two removed due to recurrent exposure, and one removed due to secondary exposure after cataract surgery that was done in other institution). One eye in AADI group had his tube removed for recurrent exposure. One eye in trimmed-AADI group needed an orphan trabeculectomy 11 days post-surgery to overcome the IOP spike not responding to antiglaucoma medications during tube ligation in a patient with end-stage glaucoma. The mean logMAR best corrected visual acuity (BCVA) preoperatively, and at 1 month, and 3, 6, 12, 18 months after surgery in three groups was similar (Table S1).
 |
Table 3 Subsequent Surgeries |
Discussion
In this study, we examined the clinical outcome of trimmed-plate AADI glaucoma implant and compared this with the standard AADI (350 mm2) and the BGI-250, which had a similar plate size to the trimmed AADI implant. Manually trimming the plate provides an alternative to the standard AADI implant in eyes with a high risk of hypotony, without the need for additional equipment or cost and therefore could provide a useful technique for high-risk eyes in the low and middle income countries (LMICs).
Overall, the reduction in IOP and need for glaucoma medication was similar for the trimmed-AADI, the standard AADI and the BGI-250. The surgical survival of the implants was also similar and approximately 75% at one year (Figure 2). This is lower than the surgical success of the Baerveldt implant in the TVT and ABC studies at one year, which were around 94% and 86%.14,15 The lower success in our study can be explained by population characteristics: our study population consisted of patients with secondary glaucomas, who had undergone multiple procedures and a high proportion of patients were children. Moreover, the rate of encapsulation and failure is higher in the colored races. A prospective interventional study in India (n=34 eyes of 31 patients) reported an overall success rate of 82% after AADI glaucoma shunt implantation at 18–24 months in Indian children (aged <16 years with uncontrolled IOP refractory to medical treatment and considered at high risk of failure following trabeculectomy).16 One randomized trial reported a complete success rate of 79% at 6 months in adult Indian eyes.3
The trimmed-plate AADI was used to minimize hypotony risk in eyes with high pre-operative hypotony risk. Such eyes may be predisposed to hypotony because of lack of aqueous production (eg uveitis, post cyclophotocoagulation)17,18 or due to compromise of structural integrity of the eye (eg myopia, aphakia/pseudophakia).19 The amount of aqueous filtration is proportional to the size of the implant plate as the size of the plate determines the size of the capsule and filtration surface. As such, reducing the area of the plate has been proposed as a method of reducing the risk of postoperative hypotony.5,6 However, we still witnessed some hypotony in the trimmed-plate AADI group: four eyes experienced transient choroidal effusion in the trimmed-plate AADI group compared to only one in the standard AADI group and none in the BGI-250 group. Whilst it is difficult to make definite conclusions with this small sample, one explanation is that eyes receiving the trimmed-plate AADI were at high risk for hypotony in the first place and the decision to trim the plate in these cases was undertaken on this premise.
Overall, the number of tube exposures were not different in the trimmed-AADI group (n=3), the standard AADI (n=2) and the BGI-250 group (n=2). Although seemingly higher for the trimmed AADI group, statistically, tube erosion rates were similar among the groups (p=0.489). Had tube exposure been related to the trimming of the plate, we would have expected plate exposures in this group (rather than tube exposures), which was not the case. None of the patients with a trimmed-plate AADI developed exposure of the plate. Thus, it seems that the cut edges of the plate did not contribute to irritation of the conjunctiva and exposure in these eyes. We expect this high rate of exposure is just due to chance, but a larger prospective study is needed to confirm this. One study in Egypt reported erosive conjunctivitis and severe AC inflammation in more than a third of eyes which received the conventional AADI in young children, which they attributed to the tube material.20 We did not encounter any such complications in our study. Studies have reported GDD exposure in around 2 to 6% of patients,21–23 but up to 15% in one study.24 A high number of prior surgeries and a relatively younger population may have been factors that contributed to a higher risk of exposure and subsequent revisions in our patients. We are a tertiary setting and the referrals we get are complex in nature, with patients often having received multiple surgeries prior to presentation and presenting with advanced glaucoma.
There are several limitations of our study. Being a retrospective study, lack of randomization and homogeneity among the groups in terms of primary diagnosis, implant size, and previous treatments or surgeries is a concern. A small sample size, the varying surgical techniques of the multiple surgeons who performed the surgeries, and the short period of follow up are also limitations of our study. We acknowledge that multiple pictures showing the trimmed implant in its position in relation to the extraocular muscles in comparison to the non-trimmed valve would have added value to the understanding of the process, but it was an oversight on our part when designing the study.
However, the study provides some proof of principle regarding reasonable efficacy and comparable safety of the trimmed-plate AADI to the BGI-250 and standard AADI.
In conclusion, trimmed-AADI may be a low-cost alternative to the BGI-250 in eyes at high risk of hypotony, with comparable safety to the standard AADI. Further prospective studies with larger sample size and longer follow up are needed to confirm our findings with, ideally, patients who are at high risk of hypotony randomized to receive the conventional and trimmed-plate AADI.
Acknowledgments
We specially thank Mr. Sultan AlSubaie, Research Assistant I, Research Department, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia, for his excellent assistance with data acquisition and management.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article was submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mandal AK, Senthil S, et al. Childhood Glaucoma Surgery in Developing Countries. In Grajewski A, Bitrian E, Papadopoulos M, editors. Surgical Management of Childhood Glaucoma. Springer, Cham; 2018. 159–171.
2. Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122(2):308–316. doi:10.1016/j.ophtha.2014.08.043
3. Rathi SG, Seth NG, Kaur S, et al. A prospective randomized controlled study of Aurolab aqueous drainage implant versus Ahmed glaucoma valve in refractory glaucoma: a pilot study. Indian J Ophthalmol. 2018;66(11):1580–1585. doi:10.4103/ijo.IJO_427_18
4. Pandav SS, Seth NG, Thattaruthody F, et al. Long-term outcome of low-cost glaucoma drainage device (Aurolab aqueous drainage implant) compared with Ahmed glaucoma valve. Br J Ophthalmol. 2020;104(4):557–562. doi:10.1136/bjophthalmol-2019-313942
5. Mavrommatis MA, Dangda S, Sidoti PA, et al. Downsizing a Baerveldt Glaucoma Implant For the Management of Persistent Postoperative Hypotony: a Case Series. J Glaucoma. 2019;28(11):1019–1022. doi:10.1097/IJG.0000000000001365
6. Chen PP. Truncation of In Situ Baerveldt Glaucoma Drainage Device for Treatment of Late Persistent Postoperative Hypotony. J Glaucoma. 2017;26(2):e113–e14. doi:10.1097/IJG.0000000000000601
7. Britt MT, LaBree LD, Lloyd MA, et al. Randomized clinical trial of the 350-mm2 versus the 500-mm2 Baerveldt implant: longer term results: is bigger better? Ophthalmology. 1999;106(12):2312–2318. doi:10.1016/S0161-6420(99)90532-8
8. Gardiner BS, Smith DW, Coote M, et al. Computational modeling of fluid flow and intra-ocular pressure following glaucoma surgery. PLoS One. 2010;5(10):45. doi:10.1371/journal.pone.0013178
9. Heuer DK, Lloyd MA, Abrams DA, et al. Which is better? One or two? A randomized clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology. 1992;99(10):1512–1519. doi:10.1016/s0161-6420(92)31772-5
10. Minckler DS, Francis BA, Hodapp EA, et al. Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115(6):1089–1098. doi:10.1016/j.ophtha.2008.03.031
11. Rateb MF, Eldaly ZH, Soliman WM. Early weaning versus prolonged administration of aqueous suppressants for prevention of hyperencapsulation in paediatric Ahmed glaucoma valve. Acta Ophthalmol. 2020;98(1):e101–e06. doi:10.1111/aos.14220
12. Gedde SJ, Schiffman JC, Feuer WJ, et al. The tube versus trabeculectomy study: design and baseline characteristics of study patients. Am J Ophthalmol. 2005;140(2):275–287. doi:10.1016/j.ajo.2005.03.031
13. Christakis PG, Tsai JC, Zurakowski D, et al. The Ahmed Versus Baerveldt study: design, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011;118(11):2172–2179. doi:10.1016/j.ophtha.2011.05.003
14. Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology. 2011;118(3):443–452. doi:10.1016/j.ophtha.2010.07.016
15. Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol. 2007;143(1):9–22. doi:10.1016/j.ajo.2006.07.020
16. Kaushik S, Kataria P, Raj S, et al. Safety and efficacy of a low-cost glaucoma drainage device for refractory childhood glaucoma. Br J Ophthalmol. 2017;101(12):1623–1627. doi:10.1136/bjophthalmol-2017-310276
17. Rosentreter A, Gaki S, Lappas A, et al. Previous cyclodestruction is a risk factor for late-onset hypotony and suprachoroidal haemorrhage after glaucoma drainage device surgery. Br J Ophthalmol. 2013;97(6):715–719. doi:10.1136/bjophthalmol-2012-302351
18. Kwon HJ, Kong YXG, Tao LW, et al. Surgical outcomes of trabeculectomy and glaucoma drainage implant for uveitic glaucoma and relationship with uveitis activity. Clin Exp Ophthalmol. 2017;45(5):472–480. doi:10.1111/ceo.12916
19. Balekudaru S, Basu T, Sen P, et al. Risk factors and outcomes of management of delayed suprachoroidal haemorrhage following Ahmed glaucoma valve implantation in children. Br J Ophthalmol. 2020;104(1):115–120. doi:10.1136/bjophthalmol-2018-313804
20. Rateb MF, Abdel Motaal H, Shehata M, et al. Outcome of a Low-Cost Glaucoma Implant versus the Baerveldt Glaucoma Implant for Paediatric Glaucoma in a Tertiary Hospital in Egypt. J Ophthalmol. 2019;2019:5134190. doi:10.1155/2019/5134190
21. Stewart WC, Kristoffersen CJ, Demos CM, et al. Incidence of conjunctival exposure following drainage device implantation in patients with glaucoma. Eur J Ophthalmol. 2010;20(1):124–130. doi:10.1177/112067211002000117
22. Levinson JD, Giangiacomo AL, Beck AD, et al. Glaucoma drainage devices: risk of exposure and infection. Am J Ophthalmol. 2015;160(3):516–21.e2. doi:10.1016/j.ajo.2015.05.025
23. Al-Beishri AS, Malik R, Freidi A, et al. Risk Factors for Glaucoma Drainage Device Exposure in a Middle-Eastern Population. J Glaucoma. 2019;28(6):529–534. doi:10.1097/ijg.0000000000001220
24. Edo A, Jian K, Kiuchi Y. Risk factors for exposure of Baerveldt glaucoma drainage implants: a case-control study. BMC Ophthalmol. 2020;20(1):364. doi:10.1186/s12886-020-01632-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
