Back to Journals » Clinical Ophthalmology » Volume 14
Preliminary In Vivo Safety Evaluation of a Tacrolimus Eye Drop Formulation Using Hydroxypropyl Beta Cyclodextrin After Ocular Administration in NZW Rabbits
Authors Mahmoudi A, Malaekeh-Nikouei B, Hanafi-Bojd MY , Toloei M, Hosseini M , Nikandish M
Received 11 September 2019
Accepted for publication 11 March 2020
Published 27 March 2020 Volume 2020:14 Pages 947—953
DOI https://doi.org/10.2147/OPTH.S229405
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Asma Mahmoudi, 1 Bizhan Malaekeh-Nikouei, 2, 3 Mohammad Yahya Hanafi-Bojd, 4, 5 Mojtaba Toloei, 6 Mehran Hosseini, 6 Malihe Nikandish 7
1Department of Pharmaceutical Nanotechnology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran; 2Department of Pharmaceutics, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran; 3Nanotechnology Research Center, Institute of Pharmaceutical Technology, Mashhad University of Medical Sciences, Mashhad, Iran; 4Cellular and Molecular Research Center, Birjand University of Medical Sciences, Birjand, Iran; 5Department of Nanomedicine, Faculty of Medicine, Birjand University of Medical Sciences, Birjand, Iran; 6Cellular and Molecular Research Center, Department of Anatomy, Birjand University of Medical Sciences, Birjand, Iran; 7Ophthalmology Department, Valiasr Hospital, Birjand University of Medical Sciences, Birjand, Iran
Correspondence: Malihe Nikandish
Ophthalmology Department, Valiasr Hospital, Birjand University of Medical Sciences, P. O. Box:9717853577, Birjand, Iran
Tel +9856 32395000
Fax +9856 32430076
Email [email protected]
Aim: Tacrolimus is an immunosuppressive drug with higher potency compared to cyclosporine A (as a useful immunosuppressant). We prepared an ophthalmic solution formulation of Tacrolimus using hydroxypropyl beta cyclodextrin (HP-ßCD). In the present study, safety of this formulation was investigated in rabbits.
Materials and Methods: Formulation containing HP-ßCD, Tacrolimus, Polyvinyl alcohol (PVA) and Benzalkonium Chloride in PBS 7.4 was prepared. Tacrolimus concentration in ophthalmic preparation was 0.05% w/v. Ten male New Zealand white rabbits were housed in clean separated cages. One drop of Tacrolimus prepared formulation and a placebo formulation were applied every 12 hrs in the right and left eyes respectively, for 28 days.
Results: This new aqueous formulation of Tacrolimus could improve Tacrolimus solubility about 42 times. Clinical examinations on the 1st, 3rd, 7th, 14th and 28th days of study showed transient redness and conjunctivitis in some cases of both control and intervention groups that was not persistent. At the end of the study, there were no statistical differences between the two groups in corneal epithelial defect, redness or pathological evaluations.
Conclusion: The results of this study suggest that eye drop formulation of CD-Tacrolimus is safe in preliminary evaluations and can be useful for further studies.
Keywords: Tacrolimus, hydroxypropyl beta cyclodextrin (HP-BCD), animal study, ocular administration, safety
Introduction
Tacrolimus (FK506), is a macrolide with immunomodulatory function and derived from streptomyces tsukubaensis.1 First oral and parenteral Tacrolimus preparations were approved for preventing rejection following organ transplantation.2,3 Tacrolimus acts through mechanisms similar to cyclosporine A; and it is 10 to 100 times more potent.1 Both of them are calcineurin inhibitors and block T cells activation.2 It was found that Tacrolimus inhibits histamine release from mast cells3 and also inhibits the release of inflammatory cytokine (e.g., interleukin-2, IL-3, IL-4, IL-5, IL-8, interferon-gamma, tumor necrosis factor-α) and through this suppresses the immune system response1,4 It has been indicated to be an effective immunosuppressant in vivo and in vitro.
Tacrolimus has been used in various ophthalmic inflammatory disorders including anterior segment diseases,5 severe atopic blepharoconjunctivitis,6 vernal keratoconjunctivitis,7 atopic keratoconjunctivitis,8 keratoconjunctivitis sicca (KCS),9 and giant papillary conjunctivitis.10
Previous studies on the use of topical Tacrolimus have shown good levels of effectiveness in the improvement of ocular symptoms, with no significant adverse effects.4 Preparing an ophthalmic formulation of Tacrolimus is limited by its poor water solubility (about 5–8 µg/mL).11 This low water solubility along with relatively high molecular weight (804.02 g/mol) leads to poor corneal epithelium penetration of FK506.12
KCS, commonly called Dry Eye Disease (DED), is a growing public health concern especially in the elderly and female population.9,13,14
Dry eye as a result of reduced tear production (KCS) or excessive tear evaporation is a multifactorial disease of the tears and ocular surface and results in eye discomfort and visual disturbance. It has the potential of causing damage to the ocular surface and/or bacterial keratitis.9,13 The first-line treatment is frequent administration of artificial tears which helps to prevent the development of severe KCS.14
There are well known connections between dry eye syndrome and several auto-immune and inflammatory systemic diseases such as SS, rheumatoid arthritis, scleroderma, polymyositis, and systemic lupus erythematosus, among others.9 Although not fully understood, both etiological reasons (reduced tear production or increased tear evaporation) induces an inflammation response on the ocular surface and exacerbates annoying symptoms.13 So controlling the inflammation as a major factor in DED is key in the treatment of this condition.9
Long-term administration of topical glucocorticoids induces ocular complications. Use of topical immunosuppressants was considered as an alternative and increased their application. They decrease the inflammation and also in some cases increase the production of tears.1
Using cyclodextrins (CDs) is one of the important practical approaches to improve the solubility of low water soluble drugs.15 They are a group of biocompatible cyclic oligosaccharides with a hydrophilic outer surface and a hydrophobic interior cavity and can incorporate hydrophobic drugs in their central cavity to form a water soluble inclusion complex.15–20 There are three natural CDs with different ring size and solubility. α-, β-, and γ-CDs have 6, 7, or 8 glucose units respectively.17,20 The small cavity size of α-CD makes it inappropriate for many drugs and γ-CD costs expensive. β-CD is readily available and its cavity size is suitable for a wide range of drugs.17
Several kinds of CD derivatives have been prepared in efforts to improve their hydrophilicity (the methylated, hydroxypropylated, and sulfobutyl ether derivatives, among others).18
In this study we tried to increase water solubility of Tacrolimus using hydroxypropyl beta CD (HP-ßCD) in order to reach an ophthalmic drop formulation. Then we evaluated the in vivo local tolerance and safety of the final formulation in rabbits.
Materials and Methods
Materials
HP-ßCD was purchased from Shandong Binzhou Zhiyuan Biotechnology Co., Ltd (Shandong, China). Tacrolimus monohydrate powder was prepared from Aburaihan Pharmaceutical Co. (Tehran, Iran). Benzalkonium Chloride and PVA 72,000 were obtained from Merck (Germany). Methanol and Acetonitrile HPLC grade were obtained from Duksun pure chemicals company (Korea). Phosphate buffered saline tablets were prepared from Sigma-Aldrich.
Male New Zealand white rabbits (weighing 2.5–3 kg) were purchased from Razi Vaccine and Serum Research Institute (Mashhad, Iran), and housed under standard conditions. All procedures performed in this study on live rabbits were organized according to guidelines for animal experimentation of the Institutional Animal Care and approved by local ethical research committee in Birjand University of Medical Sciences.
Methods
Preparation of Formulation
A formulation containing 20% W/V HP-ßCD, Tacrolimus (3mg/mL), PVA (14 mg/mL) and Benzalkonium Chloride (0.13 mg/mL) in PBS 7.4 was mixed and rotated on a solar panel rotator for 24 hrs in room temperature. The final solution was centrifuged (14,000 rpm, 10 min) and supernatant was filtrated through 0.22 um syringe filter and transferred into sterile containers under a laminar flow hood.
The placebo formulation was prepared in the same procedure and contained all excipient but no Tacrolimus.
Evaluation of Loading Efficiency
A High Performance Liquid Chromatography (HPLC) method was used to reach a standard curve and determine the concentration of Tacrolimus in the final product.
Tacrolimus concentration range of 70–1000 µg/mL was determined by HPLC-UV/Vis method at 215 nm and 60ºC. Samples were chromatographed through a 4.6 mm × 250 mm reverse phase C18 column (Knauer, Germany). Mobile phase composed of filtrated deionized water: acetonitrile: phosphoric acid (30:70:0.2 v/v %) (pH 3.5). The flow rate was adjusted on 1.0 mL/min and the volume of each injection was 20µL.21 Standard solutions were diluted in filtrated deionized water: HPLC grade acetonitrile (50:50). The retention time was 14.1 min (Figure 1).
 |
Figure 1 Chromatogram of Tacrolimus in eye drop formulation. |
A sample of prepared formulation was diluted to the range of the standard curve in filtrated deionized water: HPLC grade acetonitrile (50:50) and the concentration was determined through the described HPLC method.
In vivo Animal Study
10 male New Zealand white rabbits (weighing 2.5–3 Kg) were housed in clean separated cages in 12 hr phase shifts of the light/dark cycle. They had accessto water and food and the room temperature and humidity were set at 22–23 °C and 40±5%.
One drop of prepared theTacrolimus formulation and the placebo formulation were applied every 12 hrs in the right and left eyes respectively, for 28 days.
Examination Protocol
Eyes were evaluated macroscopically for any symptoms of redness while receiving both treatments. Anterior segment examination with slit lamp including fluorescein-dye staining of cornea was done at 4 time points during the study (days 1, 7, 14 and 28).
Histopathological Evaluations
All animals were anesthetized on day 30 with an intramuscular injection of a mixture of ketamine hydrochloride (40 mg/kg) and xylazine (10 mg/kg) with adherence to the declaration of Helsinki. Three rabbits were randomly chosen, their eyeballs separated and fixed in formalin and tissue pathological assessments including corneal epithelial defects and follicular inflammations and also corneal stem cells evaluations were performed by Pathologist on separated tissues. The protocol of the animal study proceeded according to the procedure described by the Ethics Committee of Birjand University of Medical Sciences.
Statistical Analysis
Statistical analyses were performed using SPSS v. 22.0. Comparisons of categorical variables were done through the chi-square test or Fisher exact test as appropriate.
Results
Drug Loading Efficiency
The HPLC standard curve was linear over evaluated concentrations (r2=0.9912) and 0.503 mg/mL Tacrolimus was determined in 20% HP-ßCD solution.
Relative Frequency of Alteration in Epithelial Cells
A fluorescein test was done on 20 eyes and in 4 times checkup of each. According to the results, only one rabbit showed an epithelial defect in both eyes on days 7 and 14 but the damage had disappeared by day 28. Clinical examinations on the other 18 eyes were normal. The Fisher exact test between intervention and control groups showed no significant differences in terms of corneal epithelial defect (p value = 1) (Table 1).
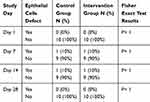 |
Table 1 Relative Frequency of Alteration in Epithelial Cells |
Relative Frequency of Conjunctiva Redness
First day of clinical examination showed no eye redness. On day 7, one rabbit had mild redness in both eyes and the others showed a normal pattern. On day 14, redness was still evident in the previously mentioned rabbit’s eyes. Also, mild redness in the left eyes of 5 rabbitswho received the placebo formulation and a moderate redness in one rabbit’s left eye was obvious. In the last clinical examination (day 28) none of the right eyes (which received the Tacrolimus formulation) or left eyes (even those presenting with redness in previous clinical examinations) showed any signs of redness.
Statistical analysis using the Fisher exact test revealed no significant differences in terms of redness (p value>0.05) between the intervention and control groups in any of the clinical examinations(Table 2).
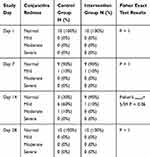 |
Table 2 Relative Frequency of Conjunctiva Redness |
Relative Frequency of Pathological Changes
Pathological evaluations were done in 6 eyes (3 eyes received the placebo formulation and 3 eyes received the Tacrolimus formulation).
In Placebo-Receiving Eyes
Chronic conjunctivitis was detected in one eye and Follicular conjunctivitis was observed in another two eyes (Figure 2).
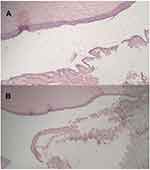 |
Figure 2 Pathological pictures of a right eye with mild chronic conjunctivitis (A and B). |
In Tacrolimus-Receiving Eyes
One eye with chronic conjunctivitis and one eye with follicular conjunctivitis were recognized. A third eye had pathologically normal features (Figure 3).
 |
Figure 3 Pathological pictures of different left eyes with (A) pathologically normal features, (B) follicular conjunctivitis, and (C) chronic conjunctivitis. |
Statistical analysis on the Fisher exact test revealed no significant statistical differences between the intervention and control groups in terms of pathological evaluations (p value = 1) (Table 3).
 |
Table 3 Relative Frequency of Pathological Changes |
Discussion
Tacrolimus as a macrolide has immunomodulatory effects and is about 10–100 times more potent than cyclosporine A in the same mechanism of action;1 however poor aqueous solubility is the main limiting factor for its application.22 Systemic uses of Tacrolimus in treatment of ophthalmic immune-mediated problems such as uveitis, eyes dryness following graft-versus-host disease, corneal transplants and ocular pemphigoid is routine.1 In case of topical eye drop formulations; Tacrolimus 0.03% in olive oil was introduced and showed to be effective in improving the symptoms of severe allergic conjunctivitis.23 Also an almond oil formulation (containing 0.03% Tacrolimus) in humans24 and a suspension form (containing 0.02% Tacrolimus) in dogs25 were newly established and showed good efficacies. Topical Suspension formulations could not provide an impressive tissue permeation and drug availability the for target organ.26 In order to have a good and fast tissue permeation, preparing a solution formulation of low water soluble drugs would be important.
In this study we aimed to produce a new aqueous formulation of Tacrolimus using HP-ßCD and we could improve Tacrolimus solubility about 42 times based on CD intrinsic characteristics. We have evaluated the safety of the final product (containing 0.05% Tacrolimus) in a rabbit in vivo model.
Data analysis through the Fisher exact test showed no significant differences between the control and intervention groups in terms of corneal epithelial defect.
In a previous study Balicki and Trbolova evaluated the efficacy and safety of 0.02% Tacrolimus eye drops (formulated in 0.9% NaCl) in chronic superficial keratitis. For this purpose they followed up 14 dogs in 5 weeks and 0.02% Tacrolimus eye drop was compared to 0.9% NaCl eye drop. Tacrolimus induced reduction of eye redness and inflammation and besides that no sign of ocular irritation was observed.27 These results confirm our safety results of Tacrolimus eye formulation.
Rare incidence of ocular irritation and redness in the control and intervention groups could be due to environmental pollutants.
In a publication released in 2017, the safety and side effects of Tacrolimus eye drop were evaluated in human vernal keratoconjunctivitis and the Tacrolimus eye drop alleviated the clinical symptoms of limbal inflammatory activity and keratitis during the course of treatment.7 In this study there were no statistical differences between the group receiving Tacrolimus eye drops and the control group in terms of limbal inflammation incidence and other pathological changes.
A newly published paper reported the results of a pilot clinical usage of a formulation composed of 0.03% Tacrolimus in 1.4% PVA, Sodium chloride, Sodium phosphate dibasic, Sodium phosphate monobasic, Benzalkonium chloride, Edetate disodium and Purified water on 8 patients (16 eyes) and they observed no adverse effects.28
There is low and limited evidence in literature about Tacrolimus eye drop toxicity topic, and as described above, the available formulated forms have different vehicles but all studies on ocular formulations of Tacrolimus indicated almost the same pattern and revealed no significant toxicity when compared to the control groups. The results obtained from our study also followed the same pattern.
The main advantage of our prepared formulation was using a biocompatible carrier to reach higher concentrations of Tacrolimus in the aqueous phase. As previously mentioned, improving water solubility could provide better corneal epithelium penetration and subsequently better clinical efficacies as a result.
This study was a preliminary safety evaluation and further studies of long-term safety of CD-Tacrolimus eye drop are needed.
Conclusion
In this research we prepared a 0.05% Tacrolimus-CD eye drop formulation and safety studies on male New Zealand white rabbits which indicated no microscopic and macroscopic adverse effects. Also no differences between tissue pathological changes in the control and intervention groups were observed. It can be concluded that this formulation passed the preliminary safety evaluations and can be used for future studies in efficacy and in vivo biodistribution assays.
Disclosure
The authors declare that there is no conflict of interests in this study.
References
1. Moscovici BK, Holzchuh R, Chiacchio BB, Santo RM, Shimazaki J, Hida RY. Clinical treatment of dry eye using 0.03% tacrolimus eye drops. Cornea. 2012;31(8):945–949. doi:10.1097/ICO.0b013e31823f8c9b
2. Ebihara N, Ohashi Y, Fujishima H, et al. Blood level of tacrolimus in patients with severe allergic conjunctivitis treated by 0.1% tacrolimus ophthalmic suspension. Allergol Int. 2012;61(2):275–282. doi:10.2332/allergolint.11-OA-0349
3. Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma. 2013;13(3):308–314. doi:10.1007/s11882-013-0345-0
4. Wakamatsu TH, Tanaka M, Satake Y, et al. Eosinophil cationic protein as a marker for assessing the efficacy of tacrolimus ophthalmic solution in the treatment of atopic keratoconjunctivitis. Mol Vis. 2011;17:932.
5. Joseph MA, Kaufman HE, Insler M. Topical tacrolimus ointment for treatment of refractory anterior segment inflammatory disorders. Cornea. 2005;24(4):417–420. doi:10.1097/01.ico.0000151507.49565.6e
6. Virtanen HM, Reitamo S, Kari M, Kari O. Effect of 0.03% tacrolimus ointment on conjunctival cytology in patients with severe atopic blepharoconjunctivitis: a retrospective study. Acta Ophthalmol Scand. 2006;84(5):693–695. doi:10.1111/j.1600-0420.2006.00699.x
7. Müller EG, Santos MS, Freitas D, Gomes JÁP, Belfort R
8. García DP, Alperte JI, Cristóbal JA, et al. Topical tacrolimus ointment for treatment of intractable atopic keratoconjunctivitis: a case report and review of the literature. Cornea. 2011;30(4):462–465. doi:10.1097/ICO.0b013e3181d83875
9. Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9(2):240.
10. Kymionis GD, Goldman D, Ide T, Yoo SH. Tacrolimus ointment 0.03% in the eye for treatment of giant papillary conjunctivitis. Cornea. 2008;27(2):228–229. doi:10.1097/ICO.0b013e318159afbb
11. Borhade V, Nair H, Hegde D. Design and evaluation of self-microemulsifying drug delivery system (SMEDDS) of tacrolimus. AAPS PharmSciTech. 2008;9(1):13–21. doi:10.1208/s12249-007-9014-8
12. Dai Y, Zhou R, Liu L, Lu Y, Qi J, Wu W. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int J Nanomedicine. 2013;8:1921. doi:10.2147/IJN.S37465
13. Sy A, O’Brien KS, Liu MP, et al. Expert opinion in the management of aqueous Deficient Dry Eye Disease (DED). BMC Ophthalmol. 2015;15(1). doi:10.1186/s12886-015-0122-z
14. Fox RI, Chan R, Michelson JB, Belmont JB, Michelson PE. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984;27(4):459–461. doi:10.1002/(ISSN)1529-0131
15. Zhu Q, Guo T, Xia D, et al. Pluronic F127-modified liposome-containing tacrolimus-cyclodextrin inclusion complexes: improved solubility, cellular uptake and intestinal penetration. J Pharm Pharmacol. 2013;65(8):1107–1117. doi:10.1111/jphp.12074
16. Tirucherai GS, Mitra AK. Effect of hydroxypropyl beta cyclodextrin complexation on aqueous solubility, stability, and corneal permeation of acyl ester prodrugs of ganciclovir. AAPS PharmSciTech. 2003;4(3):124–135. doi:10.1208/pt040345
17. Challa R, Ahuja A, Ali J, Khar R. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6(2):E329–E357. doi:10.1208/pt060243
18. Arima H, Yunomae K, Miyake K, Irie T, Hirayama F, Uekama K. Comparative studies of the enhancing effects of cyclodextrins on the solubility and oral bioavailability of tacrolimus in rats. J Pharm Sci. 2001;90(6):690–701. doi:10.1002/jps.1025
19. Lin H, Chean C, Ng Y, Chan S, Ho P. 2-hydroxypropyl-beta-cyclodextrin increases aqueous solubility and photostability of all-trans-retinoic acid. J Clin Pharm Ther. 2000;25(4):265–269. doi:10.1046/j.1365-2710.2000.00285.x
20. Pitha J, Milecki J, Fales H, Pannell L, Uekama K. Hydroxypropyl-β-cyclodextrin: preparation and characterization; effects on solubility of drugs. Int J Pharm. 1986;29(1):73–82. doi:10.1016/0378-5173(86)90201-2
21. Shi Q, Li J, Ding F. Development and validation of method for the determination of related substances of tacrolimus in tacrolimus capsules and degradation studies. Int J Chemtech Res. 2012;4(4):1543–1552.
22. Ha J-M, Kang S-Y, Park C-W, et al. Effect of poloxamer on physicochemical properties of tacrolimus solid dispersion improving water solubility and dissolution rate. J Pharm Investig. 2012;42(4):171–176. doi:10.1007/s40005-012-0025-4
23. Ohashi Y, Ebihara N, Fujishima H, et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2010;26(2):165–174. doi:10.1089/jop.2009.0087
24. Moscovici BK, Holzchuh R, Sakassegawa-Naves FE, et al. Treatment of Sjögren’s syndrome dry eye using 0.03% tacrolimus eye drop: prospective double-blind randomized study. Cont Lens Anterior Eye. 2015;38(5):373–378. doi:10.1016/j.clae.2015.04.004
25. Radziejewski K, Balicki I. Comparative clinical evaluation of tacrolimus and cyclosporine eye drops for the treatment of canine keratoconjunctivitis sicca. Acta Vet Hung. 2016;64(3):313–329. doi:10.1556/004.2016.030
26. Siegl C, König-Schuster M, Nakowitsch S, et al. Pharmacokinetics of topically applied tacrolimus dissolved in Marinosolv, a novel aqueous eye drop formulation. Eur J Pharm Biopharm. 2019;134:88–95. doi:10.1016/j.ejpb.2018.11.015
27. Balicki I, Trbolova A. Clinical evaluation of tacrolimus eye drops for chronic superficial keratitis treatment in dogs. Bull Vet Inst Pulawy. 2010;54:251–258.
28. Luaces-Rodríguez A, Touriño-Peralba R, Alonso-Rodríguez I, et al. Preclinical characterization and clinical evaluation of tacrolimus eye drops. Eur J Pharm Sci. 2018;120:152–161. doi:10.1016/j.ejps.2018.04.038
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
