Back to Journals » Patient Preference and Adherence » Volume 18
Preferences, Adherence, and Satisfaction: Three Years of Treatment Experiences of People with Multiple Sclerosis
Authors Hoffmann O, Paul F, Haase R, Kern R, Ziemssen T
Received 1 December 2023
Accepted for publication 13 February 2024
Published 22 February 2024 Volume 2024:18 Pages 455—466
DOI https://doi.org/10.2147/PPA.S452849
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Olaf Hoffmann,1– 3 Friedemann Paul,3,4 Rocco Haase,5 Raimar Kern,6 Tjalf Ziemssen5
1Department of Neurology, Alexianer St. Josefs-Krankenhaus Potsdam, Potsdam, Germany; 2Medizinische Hochschule Brandenburg Theodor Fontane, Neuruppin, Germany; 3Experimental and Clinical Research Center, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität Zu Berlin, Berlin, Germany; 4Max Delbrueck Center for Molecular Medicine, Berlin, Germany; 5Center of Clinical Neuroscience, Department of Neurology, University Hospital Carl Gustav Carus, TU Dresden, Dresden, Germany; 6MedicalSyn GmbH, Stuttgart, Germany
Correspondence: Olaf Hoffmann, Alexianer St. Josefs-Krankenhaus Potsdam, Department of Neurology, Allee nach Sanssouci 7, 14471 Potsdam, Brandenburg, Germany, Tel +49 331 9682 6000, Fax +49 331 9682 6009, Email [email protected]
Background: To reduce the risk of long-term disability in people with Multiple Sclerosis (pwMS), an increasing number of disease-modifying immune therapies (DMT) are available, involving diverse mechanisms of action, levels of efficacy, treatment risks, and tolerability aspects. Including patient preferences and expectations in shared decision-making may improve treatment satisfaction, adherence, and persistence.
Purpose: To investigate long-term alignment of individual preferences and expectations of pwMS with their actual DMT and its effect on treatment satisfaction, health-related quality of life (HRQoL), adherence, and treatment discontinuation.
Methods: A total of 401 pwMS beginning a new DMT were enrolled from 2015 to 2018 in a non-interventional study at three German MS centres. Patient preferences regarding DMT, TSQM-9, SF36, and self-reported adherence as well as relapses and EDSS were recorded at baseline and every 3 to 6 months for up to 3 years.
Results: Efficacy and tolerability were the highest-ranking preferences at baseline. Actual selection of DMT corresponded more closely to safety than efficacy, tolerability, or convenience preferences. Participants reported excellent adherence throughout the study. DMT persistence was 69.0%, with earlier discontinuation for injectable vs oral or infusion therapies. Breakthrough disease, rather than patient-reported outcomes, was the main driver of DMT discontinuation. For all routes of administration, global treatment satisfaction increased over time despite lower satisfaction with convenience. Several patterns of changing preferences were observed.
Conclusion: This study provides insight into the interaction of DMT preferences of pwMS with their actual treatment experience. Treatment decisions should be aligned with long-term expectations of pwMS to promote continuous adherence.
Keywords: multiple sclerosis, treatment adherence and compliance, treatment discontinuation, patient preference, health-related quality of life
Introduction
Multiple sclerosis (MS) is the most common autoimmune demyelinating disorder of the central nervous system, affecting 2.8 million worldwide.1 In most people with MS (pwMS), the disease is initially characterised by recurring relapses, separated by phases of complete or partial remission of symptoms (relapsing-remitting MS, RRMS), whereas about 10% experience progressive deterioration from onset (primary progressive MS, PPMS).2 Individuals affected by RRMS, typically young adults, are at risk of accumulating neurological deficits and severe disability from clinical relapses and neurodegeneration.3,4 Physical, cognitive, and psychiatric symptoms, as well as effects on everyday life develop in a highly individual manner.
In the absence of a cure, disease-modifying therapy (DMT) aims to reduce harmful adaptive immune responses by modulating or depleting pro-inflammatory lymphocytes subsets or restricting their access to the CNS.5 Effective control of inflammatory disease activity, ie, preventing relapses and formation of new lesions on MRI, slows the accumulation of disability and reduces the risk of transitioning from RRMS to a secondary chronic progressive course (SPMS) that is much less amenable to DMT.6
Over the past decade, immunomodulatory therapy options for pwMS have expanded significantly, involving different routes of application (oral, infusion, subcutaneous or intramuscular injection) and frequencies of treatment.5 Diverse mechanisms of action translate into moderately vs highly efficient therapies (MET, HET) as well as drug-specific safety and tolerability aspects.5,7 In parallel, individual prognostication has improved, considering personal co-factors as well as MRI findings and liquid biomarkers.8–10 Moreover, diagnostic criteria and treatment strategies have evolved significantly. Data from randomized clinical trials, open-label extension studies, as well as large real-world registries provide clear evidence that treatment effectiveness is dependent on initiating DMT as early as possible, and optimizing treatment early in case of persistent disease activity.11,12 Some controversy remains on the criteria for using HET first line vs following an escalation approach.13
Non-adherence to DMT increases the risk of relapses and more pronounced disease severity.14–16 Frequent switching of DMT may involve cumulative safety risks from sequential immune therapies.17 Shared decision-making is key to supporting treatment adherence and persistence in chronic diseases including MS, regarding not only DMT use, but also drug-specific risk mitigation strategies, and non-pharmacological aspects of therapy.18 From the patient’s perspective, decision-making in early RRMS involves weighing an assumed treatment burden against an unclear risk of future disability, often at a time of little or no subjective impairment.19 Physicians can provide an assessment of disease severity and risk indicators as well as a rational recommendation of treatment choices, including contraindications.20,21 For effective counselling, individual treatment preferences and expectations are an equally important point of consideration.22–24 Beyond the initial choice of treatment, however, it is likely that treatment preferences are subject to change, eg based on the actual treatment experience and health-related outcomes. In the multicentre longitudinal ANTHEMS study, we therefore investigated how therapeutic preferences of pwMS influence the selection of a DMT, their relationship with clinical outcomes, treatment satisfaction, health-related quality of life (HRQoL), adherence to DMT and persistence, as well as changes during the first two to three years of a newly initiated immunotherapy.
Materials and Methods
Participants
We conducted an observational multi-centre study in three large neurological centres specializing in MS in Germany between 2015 and 2020. People with confirmed RRMS, SPMS, or a clinically isolated syndrome (CIS) aged 18 years or above were eligible to enrol in the study. Participants had to start their first disease-modifying therapy (DMT) or switch from a prior DMT at the baseline visit (visit 0, V0). During follow-up, pwMS were assessed after three (V1) and six months (V2) and every six months (V3 to V7) thereafter for up to 36 months, resulting in up to seven follow-up visits. The study protocol was implemented as an extension to routine clinical visits at the participating centre. During the study, patients had access to extensive counselling by the study physician and nurses on all aspects of the disease, treatment, and drug safety. The ethics committee at the Brandenburg Chamber of Physicians approved the study protocol and materials (S4a/2014). The study was conducted in compliance with the Declaration of Helsinki. All participants provided written informed consent prior to entering the study.
Measures
Sociodemographic and anamnestic data were assessed at study entry. At each visit, participants were asked by the study physicians about relapses that have occurred in the meantime, increase in disability, changes in therapy, and compliance with the risk management plan.
In addition, we applied patient-reported outcomes including the Treatment Satisfaction Questionnaire for Medication (TSQM-9, 9-item version), the 36-Item Short Form Survey (SF-36, version 1), a 20-item questionnaire on treatment preferences as well as additional items on attitudes and decisions regarding DMTs via tablet.25,26 Electronic implementation of TSQM was not reviewed or certified by the developer IQVIA. Participants rated their treatment preferences on a 20-item list using a 4-level scale ranging from “very important” to “less important”. Self-reported adherence was assessed on a numerical analog scale from 0 (“very low”) to 10 (“very high”). For the SF-36, data provided by Greiner et al were used to calculate normalized values.27
Like the other instruments, the Expanded Disability Status Scale (EDSS) was administered on a regular basis during study visits to document MS-related progression.28 All data were collected using the Multiple Sclerosis Documentation System 3D (MedicalSyn GmbH, Stuttgart, Germany).29
Statistical Analysis
Normal distribution of data was visually assessed using quantile–quantile plots. Results are presented as mean, standard deviation (SD), or 95% confidence intervals (CI). Data were analysed applying generalized linear mixed models (GLMM) with linear link function for normally distributed data and gamma distribution and log link function for right-skewed data. For pairwise comparisons, contrast tests with Sidak correction were applied. Time-to-event data were evaluated by Kaplan–Meier-estimators and analysed using Cox regression. T-tests, ANOVA, χ2 tests, and median tests were used for baseline comparisons. For the explorative analysis of initial treatment choices, an Exhaustive χ2 Automatic Interaction Detection (CHAID) analysis with multiple Bonferroni adjusted χ2 tests was used in a decision tree model with 10-fold cross validation and complete regrouping of predictor variables.30 Estimates for treatment preferences were recalculated from averaged absolute ranks to relative ranks using the highest rated criterion as anchor. P-values <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS software (version 29.0, IBM Corporation, Armonk, NY).
The target sample size of n = 400 was originally calculated for a fixed model linear regression analysis including up to 8 predictors with a p-value of 0.05 and a power of 0.8, presuming a small effect size (f2 = 0.05) and a drop-out rate of up to 25%. Feasibility and available funding were additional points of consideration.
Results
Patient Characteristics
A total of 401 pwMS were enrolled in the study. Of these, 379 provided sufficient data to be included in our analyses (Table 1). At baseline, patients presented with a mean age of 40.2 years, a mean disease duration of 6.01 years, and a median EDSS of 2.0. About 95.2% were people with RRMS, 2.0% with SPMS, and 2.8% with CIS. About 26.1% of pwMS started on an injection regimen, 22.2% on infusions, and 51.7% on an oral treatment.
 |
Table 1 Patient Characteristics by Type of Treatment Application (N = 379) |
Preferences for Choosing a New Therapy
In an explorative decision tree analysis of the influence of individual preferences on the choice of treatment (N = 379), more people opted for an infusion therapy (20.1% vs 57.1%, p < 0.001) at baseline when high efficacy was less important. Within the majority of people stating a high importance of efficacy (94.5%), younger pwMS (age <42 years) were more likely to start an infusion therapy (25.9% vs 11.3%) instead of an injection therapy (21.3% vs 37.3%, p = 0.009). For pwMS younger than 42 years, a present wish for pregnancy increased the frequency of injection therapies (37.2% vs 17.3%) and reduced that of infusion therapies (7.0% vs 30.6%, p = 0.004). PwMS who had one or more relapses in the previous 12 months were more likely to choose infusion therapies (27.1% vs 12.9%) and less likely to choose oral therapies (45.3% vs 63.6%, p = 0.009). In this group, a high valuation of available long-term safety information resulted in more injection therapies (30.9% vs 10.0%) and fewer infusion therapies (23.2% vs 47.5%, p = 0.017).
Adherence and Time on Treatment
Treatment adherence saw a steady increase over time (from 8.7 to 9.9, p < 0.001), which did not differ between the subgroups. The median time of participation in the study was 18 months with a final dropout rate of 27.1%. Within the study period of up to 36 months, 69.0% of pwMS continued their new DMT, while 31.0% stopped their treatment at least once. With Kaplan–Meier estimation, the median time to discontinuation was lower in pwMS on injection therapies (30 months) than on oral or infusion therapies (36 months, both, p < 0.001) (Figure 1). In an additional Cox regression, men had a slightly longer time on treatment than women (B = 0.450, p < 0.001). Age and disease duration were not related to the continuation of treatment. In addition, discontinuation was not significantly associated with a closely preceding relapse (p = 0.130), disability worsening (p = 0.409), or adverse event (p = 0.446). Considering the entire study period after treatment initiation, the annual relapse rate was higher in pwMS who discontinued their treatment (0.29 vs 0.18, p = 0.048). At the same time, the average portion of relapse free (81.0%, p = 0.051) and adverse-event-free pwMS did not differ (68.6%, p = 0.241) between oral, injection, and infusion therapies.
Treatment Satisfaction, Adherence, and Quality of Life
No differences were found for the efficacy aspect of treatment satisfaction (71.05 [68.82, 73.29]; mean and CI) over time and between subgroups. For the global treatment satisfaction scale of the TSQM, a general trend of increasing satisfaction over time was reported in all subgroups (p = 0.005) (Table 2). This trend did not differ between people discontinuing or staying on treatment (p = 0.104).
 |
Table 2 Global Satisfaction with Treatment by Application Type of Treatment Over Three Years (N = 272) |
Over the study period, a rise in the interest in oral therapies was associated with an increase in satisfaction with efficacy (r = 0.461), convenience (r=0.315), and global treatment satisfaction (r = 0.402). With an increase in global satisfaction, a low number of treatment-related appointments became less important (r = −0.378).
Higher satisfaction with convenience was reported by pwMS on oral therapies compared to those on infusion therapies (79.30 [76.50, 81.65] vs 73.25 [68.95, 77.56], p = 0.027) (Table 3). In the first year of treatment, convenience was generally higher than in the second year (p < 0.001). Again, those who discontinued the treatment did not provide different ratings (p = 0.194).
 |
Table 3 Satisfaction with Treatment Convenience by Application Type of Treatment Over Three Years (N = 273) |
The SF-36 was used to assess HRQoL. Younger age was associated with higher scores in the physical (B = −0.139, p < 0.001) and psychological subscales (B = −0.056, p = 0.024). Physical and psychological QoL increased over the first two years (p < 0.001, respectively) (Figure 2), but increases did not last to up to 36 months. An improving psychological HRQoL was especially associated with an increased preference for a good overall safety profile (r = 0.373) and an increased preference for stable MRI results (r = 0.370). An increase in physical HRQoL correlated with a higher interest in low interference of treatment with the personal lifestyle (r = 0.322) and a lower importance of physical side effects (r = −0.315).
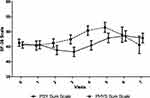 |
Figure 2 Health-related quality of life over three years (N=286). Physical (PHYS) and psychological (PSY) sum scales of the SF-36 are display as mean and 95% confidence interval. |
Changes in Treatment Preferences Over Time
PwMS were repeatedly asked about their preferences for DMT to assess shifts in decision-related categories while on treatment. We found several changes over time (Table 4).
 |
Table 4 Importance of Treatment Aspects for Choice of Treatment Over the Course of Up to 36 Months After Start of Treatment in People with Relapsing-Remitting Multiple Sclerosis (N = 197) |
Many decision drivers, such as high efficacy in terms of relapse prevention and low risk of long-term complications, remained highly relevant throughout the study period. The aspect of causing only little interference with personal lifestyle gained importance throughout the treatment experience. On the other hand, application frequency and convenience, overall tolerability, number of appointments, physical and psychological side effects, interference with occupation, availability of long-term safety information, prevention of disability progression, and improvement of MS-related symptoms were rated less important during the first 36 months of treatment.
As part of the model analyses, we estimated the effects of gender, disease duration, route of application (oral, injection, and infusion), and the interaction of time and application route. Regarding the importance of ease of application, pwMS on infusion therapy gave lower ratings already after six months on treatment, whereas other participants joined them after two years (p = 0.018). High overall efficacy was rated more important by men than women (92% vs 81%, p = 0.009). For pwMS on infusion therapies, a low frequency of administration was more important than for those on oral treatments (63% vs 52%, p = 0.022). PwMS on injection therapies preferred a lower number of appointments compared to people on oral treatments (63% vs 53%, p = 0.028). A low number of psychological side effects were especially welcomed in pwMS on injection therapies (81%) compared to oral (72%, p = 0.041) and to infusion therapies (70%, p = 0.037).
In pwMS discontinuing their treatment, a higher decrease in importance was reported for ease of application (d = 0.26, p = 0.030), overall tolerance (d = 0.33, p = 0.006), low frequency of administration (d = 0.33, p = 0.011), few psychological side effects (d = 0.28, p = 0.022), interference with work (d = 0.38, p=0.002), and a well-known long-term safety profile (d = 0.32, p = 0.012).
Discussion
In the observational ANTHEMS study, we found that pwMS starting on a new DMT expressed specific needs and demands regarding their treatment with a high and stable focus on relapse prevention and a good overall safety over the course of up to 36 months. Individual preferences may have been part of the initial shared decision process, but new emerging therapy options and shifts in preferences may also directly affect the decision to continue or discontinue treatment.
Participants
The study population represents a largely homogeneous cohort of rather early, active RRMS, being characterised by an average age of 42 years and 6 years since diagnosis, low disability, and relapse activity in the previous year. For most of these pwMS, therefore, a broad range of on-label DMT alternatives were applicable, highlighting the importance of understanding factors involved in treatment choice. At the start of the inclusion period (2015–2018), available treatments included beta interferons and glatiramer acetate for injection, teriflunomide, dimethyl fumarate and fingolimod as oral DMTs, as well as the highly effective monoclonal antibodies natalizumab and alemtuzumab for infusion. Oral cladribine was approved in August 2017. Daclizumab injections were available from July 2016 to March 2018, being restricted from July 2017 due to safety concerns.
The proportion of participants starting infusion therapies was considerably higher at 22.2% than in a German registry with 17,553 participants who were treated by office-based neurologists during the same period (10%).31 In contrast, injectable therapies were less frequently used in the present cohort than in the registry (26.1% vs 46%). During the study period, therapeutic strategies were shifting from an escalation paradigm (ie, generally starting with a moderately effective DMT and switching to higher efficacy DMTs only in the case of high and persistent disease activity) towards first-line use of high-efficacy DMTs for active disease, considering also MRI findings and other patient characteristics.11,20 Since the study protocol did not guide the choice of DMT, a stronger embracement of this development by the participating academic MS centres is suggested.
Initial Treatment Preference and DMT Selection
At baseline, the highest priority was stated for efficacy and tolerability, and lower preference for safety and convenience. Similar preferences were reported in earlier studies.32,33 In the domain of efficacy, suppression of relapses was ranked much higher than prevention of MRI activity. Of note, subclinical disease activity on MRI, especially while on DMT, is a predictor of relapses, disability, and shorter time to SPSS, and it is a central means to monitor individual treatment effectiveness.34–37 Not closely related to short-time changes in disability, the importance of MRI activity is often underestimated by physicians and pwMS.38,39 Improvement of MS symptoms, as has been observed with HET, was not ranked as an important aspect of selecting a DMT.40
Other leading considerations were the availability of an oral formulation, absence of physical side effects, and long-term safety. Different findings were reported in a recent discrete choice experiment, where safety and convenience (route and frequency of administration) were ranked twice as important as the effects on relapse rate and disease progression.41 Another study reported high valuation of symptomatic improvement by DMT.42
Decision tree analysis suggests that treatment preferences were not closely reflected in the actual selection of DMT. This mismatch was particularly apparent in the aspect of efficacy. On the one hand, pwMS starting highly effective infusion therapies assigned relatively lower preference to efficacy than those who did not. At the same time, a rather large proportion of patients favouring high efficacy started moderately effective injection therapies. Unrelated to patient preference, age and level of disease activity appeared to be important factors in selecting infusion therapies. Further contextual factors are likely to have a major impact on DMT choice.22
By comparison, safety-related preferences were more closely aligned with the DMT selection. Available for more than 25 years, injection therapies are considered particularly safe while only moderately effective. PwMS stating high preferences for safety during pregnancy or for long-term safety were likely to be treated with injectable DMTs. These safety needs appeared to override other factors: Even in active RRMS, these pwMS were less likely to receive infusion therapies, and despite at least equal efficacy, higher convenience, and fewer tolerability issues, they were less likely to receive a more recent oral therapy. In the mentioned discrete choice experiment, pwMS on injectable DMTs also gave highest priority to safety, unlike patients on oral or infusion therapies.41 MS neurologists and nurses may assign equal or higher priority to safety than pwMS.43,44
The findings indicate that the perceived balance of treatment risks and benefits by pwMS remains central to treatment decision. Shared decision-making is negatively impacted by knowledge gaps of pwMS, but effective means to support the provision of effective and accurate information remain unclear.32,45,46 Overestimation of benefits and underestimation of risks by pwMS may increase the risk of treatment dissatisfaction and lower adherence.32 In the present study, study physicians and nurses provided information on MS-related topics in a semi-structured manner; results will be presented elsewhere.
Adherence and Time on Treatment
Participants estimated their prospective treatment adherence at baseline at 8.7/10. Self-assessed adherence increased steadily in all subgroups, reaching 9.9/10 at 36 months. Near-perfect adherence is not usually found in real-world scenarios. A recent study evaluating insurance claims of 3347 pwMS in the US estimated adherence during one year at 72–76%, based on a medication possession ratio of at least 80%, regardless of the route of administration.47 While self-assessed adherence in our study may seem overly optimistic, we interpret the finding as a sign of growing confidence in DMT and perceived self-efficacy of the participants.
DMT persistence was 69.0% over the entire study period, with a shorter median time to discontinuation for injectable therapies. A comparison to previous studies is difficult given variable definitions and methods of assessment.48 With injectable DMTs, two-year persistence was calculated at 32.3% in a study of pharmacy claims for 50,057 German patients.49 Although participants reported stable adherence, a higher on-treatment relapse rate was found in pwMS discontinuing or switching their DMT, suggesting that breakthrough disease activity was a prominent reason for non-persistence. Due to the limited granularity of the visit schedule, we did, however, not identify a close temporal relation of relapses or EDSS progression events with discontinuation at an individual level. Similarly, no deterioration in patient-reported treatment satisfaction or quality of life was captured before discontinuation. In contrast, in an earlier study of first-line injectable therapies, pwMS identified side effects, lack of perceived effectiveness and psychological support as the main reasons for discontinuation.50,51
Quality of Life and Treatment Satisfaction
Overall, participants reported an improvement in physical and mental well-being during the first two years of the study, with a later return to baseline levels. A non-specific”honey-moon” period of transient improvement in SF-36 sum scores has been described during the first two years after a range of diverse medical interventions, eg bariatric surgery.52 However, psychological well-being appeared to benefit from positive treatment experiences, particularly in pwMS who attributed high importance to safety and stable MRI results. Moreover, pwMS reporting increased physical well-being de-prioritized the absence of physical DMT side effects as a therapeutic preference. Translation of these positive effects into everyday life appeared to be geared more towards the personal sphere than the workplace.
On a cross-sectional level, global treatment satisfaction was generally high. For injection therapies, similar results were reported in an earlier study.33 Over time, we observed an increase in global treatment satisfaction in all subgroups, showing a non-significant trend towards higher scores in pwMS receiving oral therapies. In this latter group, the amount of improvement can be considered as clinically meaningful.53 Since no difference was found between pwMS who stayed on their DMT vs those who discontinued, increased satisfaction is not likely explained by attrition bias.
Satisfaction with efficacy remained stable over time and did not differ between DMT groups. An effect of relapses or EDSS progression was not detected. Thus, efficacy did not appear to be the main driver of treatment satisfaction during the observation period. Instead, differential effects were found for treatment convenience. In all groups, convenience ratings deteriorated on average after the first year, but did not predict discontinuation. In pwMS using oral DMTs, the preference for oral application increased in parallel with increasing satisfaction, convenience, and efficacy, as well as global treatment experience. Overall, QoL and treatment satisfaction results may point to self-amplifying mechanisms of positive attribution.
Changes in Preferences Over Time
Attitudes and preferences of pwMS towards their disease and therapy are prone to change, especially in the first two years of therapy.54 Initial preferences for prevention of relapses and long-term safety retained a very high ranking throughout our study. Other aspects lost importance with increasing treatment duration. Convenience, eg, relating to a low frequency of DMT administration or of appointments, was stated as an important priority in selecting a therapy, but was less emphasized over time. Since convenience-related treatment satisfaction decreased, we consider habituation and adjustment of expectations to the actual treatment experience as likely explanations. Along the same lines, an initial focus of pwMS on externally available safety information may have shifted towards personal experience, consistent with the overall increase in satisfaction with treatment.
Furthermore, pwMS considered prevention of relapse and preservation of their personal lifestyle to be central to their treatment experience, whereas preventing disability progression, symptom improvement, and tolerability became even less important, especially for pwMS who discontinued their DMT. Thus, relapses appeared to be central to pwMS’ disease perception, whereas newer concepts such as progression independent of relapse active (PIRA) or smouldering MS were not yet considered.55,56
Considering a trade-off between safety and efficacy preferences, we found shifts within these categories but less between them. These results extend and strengthen findings from larger cross-sectional studies that identified satisfaction with treatment efficacy and side effects as major determinants of HRQoL, making them key components of treatment monitoring and holistic disease management.50,51 In this regard, longitudinal research approaches with a representative sample size, executed in a real-world setting, will provide more relevant information, whereas smaller studies on that topic provided less generalizable results.15,33,41,42,51
Limitations
Our study was conducted in highly specialized treatment centres that may not be fully representative of routine MS care. Combined with the study procedures, this may have implicitly led to increased adherence. The self-reported level of adherence was assessed using a non-standardized instrument. Recording of time to discontinuation of medication was tied to visits, so no day-by-day resolution was possible. However, this level of accuracy appears sufficient in the context of DMTs for MS. In a small number of affected patients, outcomes were influenced by the withdrawal of daclizumab from the market due to safety concerns. Due to the observational character, our study was not designed to provide enough cases to compare single DMTs. Some selection bias may have manifested over time as pwMS staying on DMT contributed more data than pwMS discounting their DMT. However, our analyses indicated that these two groups did not differ systematically. Satisfaction with treatment-associated side effects was not assessed due to the applied version of the TSQM.
Conclusion
In this prospective observational study, we observed important differences between the stated therapeutic preferences of pwMS and the actual selection of DMT. Nonetheless, self-reported adherence, treatment satisfaction, and HRQoL remained high or improved throughout the study. Disease activity rather than patient-reported outcomes appeared to be the main driver of DMT discontinuation (switch) in pwMS, with a lower persistence on injectable therapy. Several changes in leading treatment preferences were observed during the first years of treatment experience. To promote treatment satisfaction and adherence, treatment decisions should be aligned with the preferences of pwMS, also considering their evolution during the actual treatment experience.
Acknowledgments
Partial funding for this investigator-initiated research was provided by Biogen, Novartis Pharma, and Sanofi-Genzyme. The sponsors did not participate in the design, conduct, or evaluation of the study or in the preparation of the manuscript. Those seeking information regarding or permission to use the TSQM are directed to IQVIA at www.iqvia.com/TSQM or [email protected].
Disclosure
OH served on scientific advisory boards and/or received speaker honoraria and/or support for attending in scientific meetings from Alexion, Bayer Healthcare, Biogen, Bristol Myers Squibb/Celgene, Janssen, Merck, Novartis, Roche, Sandoz, Sanofi, and Teva as well as research support from Biogen, Novartis, and Sanofi. FP served on steering committees for Novartis and MedImmune/Viela Bio; received speaker honoraria and travel grants from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, Shire, Roche, Actelion, Celgene; received consultancy honoraria from Sanofi Genzyme, Biogen Idec, MedImmune, Shire, Alexion, Hoffmann-La Roche, Celgene, Horizon Therapeutics, Merck, Neurogenesis Ltd.; received research support from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Alexion, and Merck Serono; from German Research Council (DFG Exc 257), Werth Stiftung of the City of Cologne, German Ministry of Education and Research (BMBF Competence Network MultipleSclerosis), Arthur Arnstein Stiftung Berlin, EU FP7Framework Program (combims.eu), Guthy Jackson Charitable Foundation, National Multiple Sclerosis Society of the USA. He is an academic editor for PLoS ONE and an associate editor for Neurology Neuroimmunology and Neuroinflammation. RK provided technical services, data management, and analyses for Alexianer St. Josef Potsdam GmbH in connection with this study and received compensation for consulting services from AMS, Bristol Myers Squibb, Bayer HealthCare, Biogen, Merck Deutschland, Novartis, Sanofi, and Teva. TZ reports scientific advisory board and/or consulting for Almirall, Biogen, BMS, Sandoz, Roche, Novartis, Celgene, and Merck; compensation for serving on speakers bureaus for Roche, Novartis, Merck, Sanofi, Celgene, and Biogen; research support from Biogen, Novartis, Merck, Sanofi, and Teva. The authors report no other conflicts of interest in this work.
References
1. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26:1816–1821. doi:10.1177/1352458520970841
2. Hauser SL, Cree BAC. Treatment of Multiple Sclerosis: a Review. Am J Med. 2020;133:1380–1390 e1382. doi:10.1016/j.amjmed.2020.05.049
3. Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147–3161. doi:10.1093/brain/awac016
4. Vukusic S, Confavreux C. Natural history of multiple sclerosis: risk factors and prognostic indicators. Curr Opin Neurol. 2007;20:269–274. doi:10.1097/WCO.0b013e32812583ad
5. Yang JH, Rempe T, Whitmire N, et al. Therapeutic Advances in Multiple Sclerosis. Front Neurol. 2022;13:824926. doi:10.3389/fneur.2022.824926
6. Tedeholm H, Piehl F, Lycke J, et al. Effectiveness of first generation disease-modifying therapy to prevent conversion to secondary progressive multiple sclerosis. Mult Scler Relat Disord. 2022;68:104220. doi:10.1016/j.msard.2022.104220
7. McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and Treatment of Multiple Sclerosis A Review. JAMA-J Am Med Assoc. 2021;325:765–779. doi:10.1001/jama.2020.26858
8. Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol. 2019;15:287–300. doi:10.1038/s41582-019-0170-8
9. Van Wijmeersch B, Hartung HP, Vermersch P, et al. Using personalized prognosis in the treatment of relapsing multiple sclerosis: a practical guide. Front Immunol. 2022;13:ARTN 991291. doi:10.3389/fimmu.2022.991291
10. Hoffmann O, Gold R, Meuth SG, et al. Prognostic relevance of MRI in early relapsing multiple sclerosis: ready to guide treatment decision making? Ther Adv Neurol Disord. 2024;17:17562864241229325. doi:10.1177/1756286424122932
11. Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9(Suppl 1):S5–S48. doi:10.1016/j.msard.2016.07.003
12. Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the Age-Dependent Efficacy of Multiple Sclerosis Treatments. Front Neurol. 2017;8:ARTN 577. doi:10.3389/fneur.2017.00577
13. Wiendl H, Gold R, Berger T, et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Diso. 2021;14:Artn 17562864211039648. doi:10.1177/17562864211039648
14. Burks J, Marshall TS, Ye XL. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoeconomic Outc. 2017;9:251–260. doi:10.2147/Ceor.S130334
15. Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18:69–77. doi:10.1111/j.1468-1331.2010.03110.x
16. Bischoff C, Schreiber H, Bergmann A. Background information on multiple sclerosis patients stopping ongoing immunomodulatory therapy: a multicenter study in a community-based environment. J Neurol. 2012;259:2347–2353. doi:10.1007/s00415-012-6499-1
17. Klotz L, Havla J, Schwab N, et al. Risks and risk management in modern multiple sclerosis immunotherapeutic treatment. Ther Adv Neurol Disord. 2019;12:1756286419836571. doi:10.1177/1756286419836571
18. Klauer T, Zettl UK. Compliance, adherence, and the treatment of multiple sclerosis. J Neurol. 2008;255(Suppl 6):87–92. doi:10.1007/s00415-008-6016-8
19. Visser LA, Louapre C, Uyl-de Groot CA, Redekop WK. Patient needs and preferences in relapsing-remitting multiple sclerosis: a systematic review. Mult Scler Relat Disord. 2020;39:101929. doi:10.1016/j.msard.2020.101929
20. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol. 2018;25:215–237. doi:10.1111/ene.13536
21. Rae-Grant A. Author response: practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;92:110–111. doi:10.1212/WNL.0000000000006735
22. Eskyte I, Manzano A, Pepper G, et al. Understanding treatment decisions from the perspective of people with relapsing remitting multiple Sclerosis: a critical interpretive synthesis. Mult Scler Relat Dis. 2019;27:370–377. doi:10.1016/j.msard.2018.11.016
23. Muller S, Ziemssen T, Diehm C, et al. How to Implement Adherence-Promoting Programs in Clinical Practice? A Discrete Choice Experiment on Physicians’ Preferences. Patient Prefer Adherence. 2020;14:267–276. doi:10.2147/PPA.S222725
24. Lugaresi A, Ziemssen T, Oreja-Guevara C, et al. Improving patient-physician dialog: commentary on the results of the MS Choices survey. Patient Prefer Adherence. 2012;6:143–152. doi:10.2147/PPA.S27932
25. Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7: 36:20090427. doi:10.1186/1477-7525-7-36
26. Ware JE, Sherbourne CD. The Mos 36-Item Short-Form Health Survey (Sf-36).1. Conceptual-Framework and Item Selection. Med Care. 1992;30:473–483. doi:10.1097/00005650-199206000-00002
27. Greiner W, Claes C, Busschbach JJ, von der Schulenburg JM. Validating the EQ-5D with time trade off for the German population. Eur J Health Econ. 2005;6:124–130. doi:10.1007/s10198-004-0264-z
28. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–1452. doi:10.1212/wnl.33.11.1444
29. Ziemssen T, Kern R, Voigt I, Haase R. Data Collection in Multiple Sclerosis: the MSDS Approach. Front Neurol. 2020;11:445. doi:10.3389/fneur.2020.00445
30. Kass GV. An Exploratory Technique for Investigating Large Quantities of Categorical Data. J Royal Statistical Soc. 1980;29:119–127. doi:10.2307/2986296
31. Braune S, Rossnagel F, Dikow H, et al. Impact of drug diversity on treatment effectiveness in relapsing-remitting multiple sclerosis (RRMS) in Germany between 2010 and 2018: real-world data from the German NeuroTransData multiple sclerosis registry. BMJ Open. 2021;11:e042480. doi:10.1136/bmjopen-2020-042480
32. Reen GK, Silber E, Langdon DW. Multiple sclerosis patients’ understanding and preferences for risks and benefits of disease-modifying drugs: a systematic review. J Neurol Sci. 2017;375:107–122. doi:10.1016/j.jns.2016.12.038
33. Haase R, Kullmann JS, Ziemssen T. Therapy satisfaction and adherence in patients with relapsing-remitting multiple sclerosis: the THEPA-MS survey. Ther Adv Neurol Disord. 2016;9:250–263. doi:10.1177/1756285616634247
34. Brex PA, Ciccarelli O, O’Riordan JI, et al. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346:158–164. doi:10.1056/NEJMoa011341
35. Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131:808–817. doi:10.1093/brain/awm329
36. Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236–242. doi:10.1002/ana.20883
37. Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20:653–670. doi:10.1016/S1474-4422(21)00095-8
38. Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol. 2002;15:239–245. doi:10.1097/00019052-200206000-00003
39. Filippi M, Paty DW, Kappos L, et al. Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: a follow-up study. Neurology. 1995;45:255–260. doi:10.1212/wnl.45.2.255
40. Bielekova B, Tintore M. Sustained reduction of MS disability: new player in comparing disease-modifying treatments. Neurology. 2016;87:1966–1967. doi:10.1212/WNL.0000000000003314
41. Bauer B, Brockmeier B, Devonshire V, et al. An international discrete choice experiment assessing patients’ preferences for disease-modifying therapy attributes in multiple sclerosis. Neurodegener Dis Manag. 2020;10:369–382. doi:10.2217/nmt-2020-0034
42. Wilson LS, Loucks A, Gipson G, et al. Patient preferences for attributes of multiple sclerosis disease-modifying therapies: development and results of a ratings-based conjoint analysis. Int J MS Care. 2015;17:74–82. doi:10.7224/1537-2073.2013-053
43. Tintoré M, Alexander M, Costello K, et al. The state of multiple sclerosis: current insight into the patient/health care provider relationship, treatment challenges, and satisfaction. Patient Prefer Adher. 2017;11:33–45. doi:10.2147/Ppa.S115090
44. Kremer IEH, Evers S, Jongen PJ, Hiligsmann M. Comparison of preferences of healthcare professionals and MS patients for attributes of disease-modifying drugs: a best-worst scaling. Health Expect. 2018;21:171–180. doi:10.1111/hex.12599
45. de Seze J, Borgel F, Brudon F. Patient perceptions of multiple sclerosis and its treatment. Patient Prefer Adher. 2012;6:263–273. doi:10.2147/Ppa.S27038
46. Kopke S, Solari A, Rahn A, et al. Information provision for people with multiple sclerosis. Cochrane Database Syst Rev. 2018;10:CD008757. doi:10.1002/14651858.CD008757.pub3
47. Burkhard A, Toliver J, Rascati K. Association between multiple sclerosis disease severity and adherence to disease-modifying therapies. J Manag Care Spec Ph. 2021;27:915–923. doi:10.18553/jmcp.2021.27.7.915
48. Ben-Zacharia AB, Walker B, Ross AP, et al. Factors Associated With Disease-Modifying Therapy Adherence and Persistence in Multiple Sclerosis: a Scoping Literature Review. Int J MS Care. 2023;25:188–195. doi:10.7224/1537-2073.2021-139
49. Hansen K, Schussel K, Kieble M, et al. Adherence to Disease Modifying Drugs among Patients with Multiple Sclerosis in Germany: a Retrospective Cohort Study. PLoS One. 2015;10:e0133279. doi:10.1371/journal.pone.0133279
50. Schriefer D, Haase R, Kullmann JS, Ziemssen T. Health-Related Quality of Life and the Relationship to Treatment Satisfaction in Patients with Multiple Sclerosis: insights from a Large Observational Study. Patient Prefer Adherence. 2020;14:869–880. doi:10.2147/PPA.S248272
51. Donze C, Malapel L, Kwiatkowski A, et al. Treatment discontinuation in multiple sclerosis: the French Web-based survey ALLIANCE. Mult Scler J Exp Transl Clin. 2015;1:2055217315600720. doi:10.1177/2055217315600720
52. Andersen JR, Aasprang A, Karlsen TI, et al. Health-related quality of life after bariatric surgery: a systematic review of prospective long-term studies. Surg Obes Relat Dis. 2015;11:466–473. doi:10.1016/j.soard.2014.10.027
53. Greene N, Quere S, Bury DP, et al. Establishing clinically meaningful within-individual improvement thresholds for eight patient-reported outcome measures in people with relapsing-remitting multiple sclerosis. J Patient Rep Outcomes. 2023;7:61. doi:10.1186/s41687-023-00594-8
54. Heesen C, Haase R, Melzig S, et al. Perceptions on the value of bodily functions in multiple sclerosis. Acta Neurol Scand. 2018;137:356–362. doi:10.1111/ane.12881
55. Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the ‘real MS’. Ther Adv Neurol Disord. 2022;15:17562864211066751. doi:10.1177/17562864211066751
56. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. 2020;77:1132–1140. doi:10.1001/jamaneurol.2020.1568
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

