Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
Predictors of Survival Time Among HIV-Infected Adults After Initiating Anti-Retroviral Therapy in Kombolcha Town: A 5-Year Retrospective Cohort Study
Authors Siraj M, Gedamu S , Tegegne B
Received 4 February 2022
Accepted for publication 8 April 2022
Published 16 April 2022 Volume 2022:14 Pages 181—194
DOI https://doi.org/10.2147/HIV.S359495
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Muhammed Siraj,1 Sisay Gedamu,2 Belachew Tegegne2
1Department of Nursing, Tropical College of Medicine, Dessie, Ethiopia; 2Department of Nursing, School of Nursing and Midwifery, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Correspondence: Belachew Tegegne, Department of Nursing, School of Nursing and Midwifery, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia, Email [email protected]
Background: Antiretroviral therapy (ART) enhances the survival of HIV-infected patients by reducing viral load and increasing CD4. As CD4 count increases, patients are more protected against opportunistic infections. In developing countries including Ethiopia, there were limited studies about the survival benefit of ART particularly no study in Kombolcha Town. Thus, this study was aimed to address the gap.
Methods: A hospital-based retrospective cohort study was employed in Kombolcha town among 510 patients’ records from January 2015 to December 2019. A systematic random sampling technique was used to select patient records. The collected data were checked, coded, and entered into Epidata version 4.6 and exported to Statistical Package for Social Sciences version 26 for data cleaning and analysis. Kaplan–Meier was used to estimate the probability of category of each predictor and a log rank test was used to compare survival curves. Bivariate and multivariate Cox-regression were employed using a 95% CI and variables with p-value < 0.05 were declared as predictors of poor survival time.
Results: In this cohort, out of 510 HIV-infected patients, 39 (7.65%) were died, and 471 (92.35%) were censored. Fair drug adherence (AHR=6.88, 95% CI: 4.31– 24.04), Poor drug adherence (AHR=9.58, 95% CI: 8.72– 30.97), CD4 count < 50 cell/μL (AHR=9.38, 95% CI: 1.48– 59.31), CD4 count 50– 99 cell/μL (AHR=9.67, 95% CI: 1.80– 51.73), bedridden (AHR=9.5, 95% CI: 4.49– 18.66), opportunistic infections (AHR=4.58, 95% CI: 1.20– 5.65), weight < 60kg (AHR=2.48, 95% CI:1.59, 10.38), WHO stage III (AHR=3.56, 95% CI: 1.71– 17.89), WHO stage IV (AHR=4.42, 95% CI:1.75– 25.93) were predictors of poor survival time.
Conclusion: The Kaplan–Meier result showed that the estimated median survival time of patients after ART initiation in Kombolcha town was higher (32 months) as compared to other studies. Poor drug adherence, WHO stage III & IV, Lower baseline CD4 count, presence of opportunistic infections, weight < 60kg, and being bedridden were predictors of poor survival time. Thus, early initiation of ART ought to be encouraged among HIV-infected patients and good patient counseling on the level of adherence should be strengthened.
Keywords: survival time, predictors, Kombolcha, HIV/AIDS
Introduction
Antiretroviral therapy (ART) is defined as the use of a combination of three or more antiretroviral drugs for the treatment of HIV infection, and it involves lifelong treatment.1 WHO (2015) recommended that all HIV-positive patients start ART irrespective of clinical staging and CD4 count.
United Nations program on HIV/AIDS (USAID) and partners proposed three new and ambitious targets, three 90ʹs. These targets are diagnosing 90% of people living with HIV, providing ART to 90% of for those diagnosed with HIV, and achieving viral suppression for 90% of patients receiving treatment.2 To improve HIV care and treatment to achieve the goal of 90–90–90 following strategies are crucial. Working with religious leaders; implementing new programs (self-HIV testing, house-to-house HIV testing, community ART distribution, and teach-test-treat –link strategy); decentralizing ART services in health posts and private clinics; integrating HIV care services with mental illness and other non-communicable diseases; filling gaps in legislation issues related with HIV status disclosure and traditional helping practices.3
Globally, around 36,990 new HIV infections have occurred.4 In Ethiopia, 67% of people living with HIV know their status, 88% of them are on treatment, and 86% of people are on treatment have viral suppression. By 2020, 79% of people living with HIV know their status, of which 96–99% are on ART and 86% of viral suppression.5
ART enhances the survival of HIV-positive patients by reducing viral load and increasing CD4. As CD4 count increases, patients are more protected against opportunistic infections. In developing countries including Ethiopia, there were limited studies about the survival benefit of ART particularly no study in Kombolcha Town. Thus, this study was aimed to address the gap.
Materials and Methods
Study Design and Study Area Description
Five (5) years retrospective cohort study was conducted from 1 January 2015 to 30 December 2019 in Kombolcha town health centers. Kombolcha town is located in North Eastern part of Ethiopia at a distance of 379 kilometer from the capital city, Addis Ababa. The town had a total population of 162,533 (77,041 were males and 85,492 were females (CSA, 2013)). In Kombolcha town there were 4 public health centers, 12 health posts and 16 private clinics. Three public health centers were provided ART services for 3348 HIV-infected patients.
Study Participants and Eligibility Criteria
All HIV-infected patients who were enrolled in ART clinic from January 2015 to December 2019 in Kombolcha town health centers were study participants. All HIV-infected patients aged greater than 18 years and who started ART in three health centers were included in the study whereas age <18 years, patients who started ART outside the study health centers and had incomplete medical records were excluded from the study.
Sample Size Determination and Sampling Technique
The sample size was calculated using double population proportion formula by using Epi info version 7.1 through considering different factors. Adherence was chosen as the main exposure because it gave the maximum sample size. Statistical assumptions were considered (ie power = 80%, confidence interval = 95%, Ratio (number in exposed: unexposed) =1:1, estimated proportion of mortality in Ethiopia taken as 9.9% for non-exposed group). Hence, 512 patients living with HIV/AIDS and started ART were included in this study. Study participants were selected from the registration book by using systematic random sampling technique. The sample interval was calculated as 3348 divided by 512. The sample was allocated proportionally to three health centers (Kombolcha 03, Kombolcha 02, and Kombolcha 05). Profiles of all patients on ART between 1 January 2015, and 30 December 2019 was evaluated from available standard national medical registers, which have been adopted by the Ethiopian Ministry of Health.
Data Collection and Quality Management
The pre-prepared data retrieval (data abstraction tool) form was developed from records of the Federal Ministry of Health of Ethiopia, ART entry and follow-up forms, patient records, laboratory requests and computer data. The information related to socio-demographic, medical, and behavioral related factors was retrieved from ART case notes using pretested information extraction tool during the data collection period. To ensure data quality, data was collected by ART staff nurses and the tool was pretested in 5% at Dessie health center. The training was given to data collectors and supervisors regarding the data abstraction technique. Data quality was also controlled through continuous supervision and ongoing formative checkup for completeness and consistency of responses.
Outcome Variable
The outcome variable is the time from initiation of ART to an event, categorized to death and censored (alive, transferred out, and lost-to-follow-up).
Independent Variables
Socio-demographic characteristics (Age, Sex, Marital status, Educational status, Occupation).
Base Line Factors (Weight, Height, WHO clinical staging, CD4 count, Hemoglobin level, functional status, past medication).
ART treatment (ART eligibility criteria, OI prophylaxis, Regimens given at follow up time, drug regimen change).
Patient Follow Up Factors (Date of HIV confirmed, Eligible date, duration ART, Drug side effect, TB screened and prophylaxis, TB treatment and co-trimoxazole preventive therapy, Recent ARV adherence, Recent CD4 count, Recent Hemoglobin).
Operational Definitions
Drop out: if a patient discontinued ART for at least three months as recorded by the ART physician.6
Lost: if a patient discontinued ART for 1 to 3 months as recorded by the ART physician.6
Good Adherence: if the percentage of missed dose is >95% (<2 doses of 30 doses or <3 doses of 60 doses) as documented by ART physicians.6
Fair Adherence: if the percentage of missed dose is 85–95% (3–5 doses of 30 doses or 3–9 doses of 60 doses) as documented by ART physicians.6
Poor Adherence: if the percentage of missed dose <85% (>6 doses of 30 doses or >9 doses of 60 doses) as documented by physicians.6
Survival time: The number of months from initiation of ART until the death of the patient.7
Event case: all HIV patients who will die after ART medication was initiated.
Censored: A patient does not develop event (transferred out, lost to follow up and alive).
Baseline: is the measurement taken nearest to the date of ART initiation.
Prophylaxis: aims to avoid either the first occurrence of infections (primary prophylaxis) or their recurrence (secondary prophylaxis or maintenance).1
Data Management and Analysis
The collected data was entered into Epidata version 4.6 and exported to SPSS version 26.0 for data cleaning and analysis. The patients’ cohort characteristics were described in terms of the mean value for continuous data and percentage for categorical data. Death was confirmed by reviewing the death registration in the hospital, or registration by ART adherence supporter through calling using the registered phone number and individuals alive. Finally, the outcome of each subject was dichotomized into censored or death. Kaplan–Meier was used to estimate the probability of category of each predictor and a Log rank test was used to compare survival curves. Bivariate (crude hazard ratio) and multivariate (adjusted hazard ratio) Cox-regression were employed using a 95% confidence interval (CI). From bivariable Cox-regression, variables with p-value<0.25 were candidate for Cox-regression. Then, from Cox-regression variables with p-value <0.05 were declared as predictors of poor survival time.
Results
Socio-Demographic Characteristics
The majority, 289 (56.7%) of study participants were females and the mean (±SD) age of respondents was 39.9 (±12.168) years. Over half, 262 (51.4%) of participants were married and 359 (70.4%) of them had lived in urban (Table 1).
 |
Table 1 Socio-Demographic Characteristics of HIV-Infected Adults After Initiating ART in Kombolcha Town from January 2015 to December 2019 (n=510) |
Baseline Clinical Characteristics
The majority, 214 (42%) of participants had CD4 count ≥350 and 194 (38%) of them had classified under WHO stage 1. Most, 409 (80.2%) of them had hemoglobin level ≥10g/dl with working 421 (82.5%) functional status at the baseline. Additionally, 213 (41.8%) had opportunistic infections (Table 2).
 |
Table 2 Baseline Clinical Characteristics of HIV-Infected Adults After Initiating ART in Kombolcha Town from January 2015 to December 2019 (n=510) |
Treatment and Patient Follow Up Characteristics
Three-fourths, 387 (75.9%) of participants had taken CPT (Co-trimoxazole preventive therapy) and 386 (75.7%) of them had taken the drug regimen AZT-3TC-EFV. Most, 424 (83.1%) and one-fourths, 130 (25.5%) of participants respectively had good drug adherence and drug side effect (Table 3).
 |
Table 3 Treatment and Patient Follow Up Characteristics of HIV-Infected Adults After Initiating ART in Kombolcha Town from January 2015 to December 2019 (n=510) |
Pattern of Death
As life table showed, the pattern of death with time intervals among HIV-infected adults on ART revealed that the proportion of surviving decreases as the duration of ART increases (Table 4).
 |
Table 4 Life Table Revealing Pattern of Death with Time Intervals of HIV-Infected Adults After Initiating ART in Kombolcha Town from January 2015 to December 2019 |
Survival Status HIV-Infected Patients After Initiation of ART
The study participants were followed for 60 months and the follow-up outcome was categorized into died and censored (patients who transfer out, lost to follow-up, and survived beyond the study period). In this cohort, out of 510 HIV-infected patients on ART, 471 (92.35%) were censored and 39 (7.65%) have died. From 471 censored patients, 430 (84.31%) were alive, 26 (5.1%) transferred out and 15 (2.94%) lost to follow up (Figure 1). The median survival time was 32 months. The cumulative probability of survival at 20, 40, and 60 months after ART initiation was 93%, 87%, and 83%, respectively (Figure 2). Starting co-trimoxazole therapy (CPT) early increases the survival function of HIV infected patients (Figure 3). Having good adherence enhances the survival rate of HIV-infected patients (Figure 4). Early initiation of ART is crucial to enhance the survival rate of HIV-infected patients. Patients who started ART in WHO stage I had better survival outcomes as compared to WHO stage IV (Figure 5).
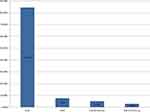 |
Figure 1 Survival status (outcome of follow-up) of HIV-infected adults after initiating ART in Kombolcha town from January 2015 to December 2019. |
 |
Figure 2 The probability of survival time of HIV-infected adults after initiating ART in Kombolcha town from January 2015 to December 2019. |
 |
Figure 3 Survival functions of HIV-infected adults after initiating ART in Kombolcha town by baseline CPT from January 2015 to December 2019. |
 |
Figure 4 Survival functions of HIV-infected adults by baseline ART adherence in Kombolcha town from January 2015 to December 2019. |
 |
Figure 5 Survival function of HIV-infected adults by baseline WHO clinical staging in Kombolcha town from January 2015 to December 2019. |
Kaplan–Meier Analysis on Socio-Demographic Characteristics
Kaplan–Meier’s analysis revealed that participants categorized in age 18–29 years had better survival than age ≥60 years (56.96 months, 95% CI: 54.8–59.12 Vs 49.22 months, 95% CI: 44.29–54.14). Participants who had lived in rural areas showed less survival time than urban residents (53.41 months, 95% CI: 50.85–55.96 Vs 56.48, 95% CI: 55.14–57.82). Likewise, participants who had not attended any education were less survival time than those who had attended primary education (53.5 months, 95% CI: 51.22–55.77 Vs 57.15 months, 95% CI: 55.54–58.77) (Table 5).
 |
Table 5 Kaplan–Meier Analysis on Socio-Demographic Characteristics of HIV-Infected Adults After Initiating ART in Kombolcha Town from January 2015 to December 2019 |
Kaplan–Meier Analysis on Baseline Characteristics
Patients having weight ≥60 kg had better survival time than <60 kg (58.02 months, 95% CI: 56.13–59.88 Vs 55.3 months, 95% CI: 53.84–56.75). Patients who had CD4 Count ≥350 were better surviving as compared to having CD4 count <50 (58.46 months, 95% CI: 57.26–59.67 Vs 40.08 months, 95% CI: 33.0–47.15). Patients who had good medication adherence were lived better than those who had poor adherence (59.45 months, 95% CI: 58.92–60.0 Vs 32.47 months, 95% CI: 27.6 −37.34). Patients who had opportunistic infections were less survive than their counterparts (54.96 months, 95% CI: 53.25–56.67 Vs 57.31, 95% CI: 55.73–58.9) (Table 6).
 |
Table 6 Kaplan–Meier Analysis on Baseline Characteristics of HIV-Infected Adults After Initiating ART in Kombolcha Town from January 2015 to December 2019 |
Predictors of Mortality After Initiation of ART
From multivariable Cox regression model, fair and poor drug adherence, CD4 count<50andCD4 count 50–99, functional status of Bedridden, presence of OIs, Weight < 60kg, WHO staging III, and WHO staging IV were independent predictors of mortality. The hazard of patients’ mortality was 6.88 times higher among adults with fair drug adherence than patients who had good adherence to ART (AHR= 6.88, 95% CI: 4.31–24.04). Likewise, the hazard of adult mortality was 9.58 times higher among adults with poor drug adherence than adults who had good adherence to ART (AHR=9.58, 95% CI: 8.72–30.97). The hazard of mortality was 9.67 times higher among adults with CD4 count < 50 than adults who had CD4 count ≥350 (AHR= 9.67, 95% CI: 1.48–59.31). Similarly, the hazard of mortality was 9.38 times higher among adults with CD4 count 50–99 than adults who had CD4 count ≥350 (AHR= 9.67, 95% CI: 1.80–51.73). The hazard of mortality was 9.5 times higher among bedridden patients than patients who had working functional status (AHR=9.5, 95% CI: 4.49–18.66). The risk of mortality was 4.58 times higher among patients having opportunistic infections than their counterparts (AHR=4.58, 95% CI: 1.20–5.65). The hazard of mortality was 2.48 times higher among patients with body weight < 60kg than their counterparts (AHR= 2.48, 95% CI: 1.59–10.38). The hazard of mortality was 3.56 times higher among patients with WHO clinical stage III than patients having WHO clinical stage I (AHR=3.56, 95% CI: 1.71–17.89). Likewise, the hazard of mortality was 4.42 times higher among patients with WHO clinical stage IV than patients having WHO clinical stage I (AHR=4.42, 95% CI: 1.75–25.93) (Table 7).
 |
Table 7 Predictors of Survival Time in Cox-Regression Among HIV-Infected Adults After Initiating ART in Kombolcha Town from January 2015 to December 2019 (n=510) |
Discussion
This cohort study was identified predictors of survival time among HIV-infected adults after initiation of ART. Accordingly, fair and poor ART adherence, CD4 count<50andCD4 count 50–99, functional status of bedridden, presence of OIs, Weight < 60kg, and WHO clinical staging III and IV were independent predictors of poor survival time.
In this cohort, 7.65% (95% CI: 5.21–10.42) patients have died. This is in line with other studies in Aksum hospital (8.85%)8 Oromia region (10.3%),9 Eastern Ethiopia (5.9%),10 Tigray central zone (7.5%),11 Jinka hospital (10%),12 Gofa zone hospital (7.5%)13 and Latin America (7.3%).14 However, this finding is lower than studies in Hiwot Fana teaching hospital (11.9%),15 Kambata tambaro zone (12.63%),16 Malawi (12.6%),17 Durban, South Africa (16%)18 and India (13%, 16.1%).19,20 The difference might be due to differences in socioeconomic characteristics and duration of follow-up.
The mortality hazard ratio of those with baseline weight <60kg compared with those with baseline weight >60kg is 2.48 (95% CI: 1.59–10.38). This is supported by other studies, Gambia,21 Aksum8 (AHR = 2.3, 95% CI:1.24, 4.55), Oromia,9 India.19 The hazard of patients’ mortality was 6.88 times higher among adults with fair drug adherence than patients who had good drug adherence (AHR = 6.88, 95% CI: 4.31–24.04). Likewise, the hazard of adult mortality was 9.58 times higher among adults with poor drug adherence than adults who had good adherence to ART (AHR=9.58, 95% CI: 8.72–30.97). This is consistent with other studies in Nekemet,22 Kambata Tambaro Zone16 (AHR = 3.8, 95% CI: 1.88, 7.96) Goffa zone,13 Canada.23
The hazard of mortality was 9.67 times higher among adults with CD4 count <50 than adults who had CD4 count ≥350 (AHR = 9.67, 95% CI: 1.48–59.31). Similarly, the hazard of mortality was 9.38 times higher among adults with CD4 count 50–99 than adults who had CD4 count ≥350 (AHR = 9.67, 95% CI: 1.80–51.73). This is congruent with other studies; India,19 Cameron,24 Durban, South Africa,18 Canada,23 Latin America,14 Singapore,25 Ethiopia.26
The hazard of mortality was 9.5 times higher among bedridden patients than patients who had working functional status (AHR = 9.5, 95% CI: 4.49–18.66). This finding is supported by similar studies in India,19 eastern Ethiopia,10 Tigray central zone,11 Jinka Hospital,12 kamata tambaro zone,16 Goffa zone public hospitals.13 The risk of mortality was 4.58 times higher among patients having opportunistic infections than their counter parts (AHR = 4.58, 95% CI: 1.20–5.65). This is supported by South Africa,27 kambata tambaro zone,16 Goffa zone public hospitals.13
The hazard of mortality was 3.56 times higher among patients with WHO clinical stage III than patients having WHO clinical stage I (AHR=3.56, 95% CI: 1.71–17.89). Likewise, the hazard of mortality was 4.42 times higher among patients with WHO clinical stage IV than patients having WHO clinical stage I (AHR=4.42, 95% CI: 1.75–25.93). This is supported by studies in Latin America,14 Oromia region,9 eastern Ethiopia,10 Cameron,24 India,20 Jinka hospital,12 Zewuditu hospital,28 teaching hospitals in Ethiopia,26 Hiwot Fana hospital,15 and Malawi.17
Conclusion
The Kaplan–Meier result showed that the estimated median survival time of patients after ART initiation in Kombolcha town was higher (32 months) as compared to other studies. Poor drug adherence, WHO stage III & IV, Lower baseline CD4 count, presence of opportunistic infections, weight < 60kg, and being bedridden were predictors of poor survival time. Thus, early initiation of ART ought to be encouraged among HIV-infected patients and good patient counseling on the level of adherence should be strengthened.
Abbreviations
AIDS, acquired immune deficiency syndrome; AHR, adjusted hazard ratio; ART, antiretroviral therapy; CHR, crude hazard ratio; CI, confidence interval; HIV, human immunodeficiency virus; MOH, Ministry Of Health.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Consideration
Ethical clearance was obtained from the Tropical College of medicine ethical review committee (Reference: ERC/2129/2021). Letter of permission was obtained from Kombolcha town administration health office and consent was secured from each health centered. As the study was conducted through a review of medical records, obtain informed consent from patients’ was difficult. Data were extracted anonymously ensuring patient data confidentiality, and all data collection was conducted in compliance with the declaration of Helsinki.
Consent for Publication
Not applicable. No individual personal details, images, or videos are being used in this study.
Acknowledgments
We would like to thank data collectors, supervisors, and the study participants for their valuable contribution to the study.
Author Information
MS: Muhammed Siraj (BSc, MSc in adult health nursing), department of nursing, tropical college of medicine, Dessie, Ethiopia. BT: Belachew Tegegne (BSc, MSc in adult health nursing), department of nursing, school of nursing and midwifery, college of medicine and health sciences, Wollo University, Dessie, Ethiopia ([email protected]). SG: Sisay Gedamu (BSc, MSc, Assistant professor in adult health nursing), department of nursing, school of nursing and midwifery, college of medicine and health sciences, Wollo University, Dessie, Ethiopia.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No organization funded this study.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. World Health Organization. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy, July 2017. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2017.
2. UNAIDS. UNAIDS 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, Switzerland: UNAIDS; 2014.
3. Gesesew H, Ward P, Woldemichael K, Mwanri L. Improving the UNAIDS 90- 90-90Treatment targets: solutions suggested from a qualitative study of HIV patients, community advocates, health workers and program managers in Jimma, Southwest Ethiopia. Int J Environ Res Public Health. 2020;17:378. doi:10.3390/ijerph17010378
4. Deribew A, Biadgilign S, Deribe K, et al. The burden of HIV/AIDS in Ethiopia from 1990 to 2016: evidence from the global burden of diseases 2016 study. Ethiop J Health Sci. 2019;29(1):859–868. doi:10.4314/ejhs.v29i1.7
5. Girum T, Wasie A, Worku A. Trend of HIV/AIDS for the last 26 years and predicting achievement of the 90–90-90 HIV prevention targets by 2020 in Ethiopia: a time series analysis. BMC Infect Dis. 2018;18(1):1–10. doi:10.1186/s12879-018-3214-6
6. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection 2016 Recommendations for a Public Health Approach Second Edition. WHO; 2016.
7. Zachariah RFM, Massquoi M. Risk factors for high early mortality in Patients on antiretroviral treatment in a rural district of Malawi. 2013.
8. Tadesse K, Haile F, Hiruy N. Predictors of mortality among patients enrolled on antiretroviral therapy in Aksum hospital, northern Ethiopia: a retrospective cohort study. PLoS One. 2014;9(1):e87392. doi:10.1371/journal.pone.0087392
9. Alemu AW, Sebastián MS. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob Health Action. 2010;3(1):5398. doi:10.3402/gha.v3i0.5398
10. Biadgilign S, Reda AA, Digaffe T. Predictors of mortality among HIV infected patients taking antiretroviral treatment in Ethiopia: a retrospective cohort study. AIDS Res Ther. 2012;9(1):1–7. doi:10.1186/1742-6405-9-15
11. Belay H, Alemseged F, Angesom T, Hintsa S, Abay M. Effect of late HIV diagnosis on HIV-related mortality among adults in general hospitals of Central Zone Tigray, northern Ethiopia: a retrospective cohort study. Hiv/Aids. 2017;9:187.
12. Tachbele E, Ameni G. Survival and predictors of mortality among human immunodeficiency virus patients on anti-retroviral treatment at Jinka Hospital, South Omo, Ethiopia: a six years retrospective cohort study. Epidemiol Health. 2016;38. doi:10.4178/epih.e2016049
13. Kebede A, Tessema F, Bekele G, Kura Z, Merga H. Epidemiology of survival pattern and its predictors among HIV positive patients on highly active antiretroviral therapy in Southern Ethiopia public health facilities: a retrospective cohort study. AIDS Res Ther. 2020;17(1):1–8. doi:10.1186/s12981-020-00307-x
14. Carriquiry G, Fink V, Koethe JR, et al. Mortality and loss to follow‐up among HIV‐infected persons on long‐term antiretroviral therapy in Latin America and the Caribbean. J Int AIDS Soc. 2015;18(1):20016. doi:10.7448/IAS.18.1.20016
15. Harar E, Jigjiga E, Hallard M, Gros F. Predictors of mortality among adult patients enrolled on antiretroviral therapy in Hiwotfana specialized University Hospital, Eastern Ethiopia: retrospective Cohort study. La Nouvelle Presse Medicale. 2018;3:1753–1754.
16. Abuto W, Abera A, Gobena T, Dingeta T, Markos M. Survival and predictors of mortality among HIV positive adult patients on highly active antiretroviral therapy in public hospitals of Kambata Tambaro Zone, Southern Ethiopia: a retrospective cohort study. HIV/AIDS. 2021;13:271.
17. Zachariah R, Fitzgerald M, Massaquoi M, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. Aids. 2006;20(18):2355–2360. doi:10.1097/QAD.0b013e32801086b0
18. Ojikutu BO, Zheng H, Walensky RP, et al. Predictors of mortality in patients initiating antiretroviral therapy in Durban, South Africa: original article. South African Med J. 2008;98(3):204–208.
19. Chakravarty J, Tiwary NK, Prasad SR, et al. Determinants of survival in adult HIV patients on antiretroviral therapy in Eastern Uttar Pradesh: a prospective study. Indian J Med Res. 2014;140(4):491.
20. Bachani D, Garg R, Rewari BB, et al. Two-year treatment outcomes of patients enrolled in India’s national first-line antiretrovital therapy programme. Natl Med J India. 2010;23(1):7.
21. Van der Sande MA, van der Loeff MFS, Aveika AA, et al. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. JAIDS. 2004;37(2):1288–1294. doi:10.1097/01.qai.0000122708.59121.03
22. Hambisa MT, Ali A, Dessie Y. Determinants of mortality among HIV positives after initiating antiretroviral therapy in Western Ethiopia: a hospital-based retrospective cohort study. Int Scholar Res Notices. 2013;2013. doi:10.1155/2013/491601
23. Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350× 109 cells/L. Ann Intern Med. 2003;139(10):810–816. doi:10.7326/0003-4819-139-10-200311180-00008
24. Sieleunou I, Souleymanou M, Schönenberger AM, Menten J, Boelaert M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far‐North Province, Cameroon. Trop Med Int Health. 2009;14(1):36–43. doi:10.1111/j.1365-3156.2008.02183.x
25. Chow K, Ang L, Verghesse I, Chew S, Leo Y. Measurable predictive factors for progression to AIDS among HIV-infected patients in Singapore. Ann Acad Med Singapore. 2005;34(1):84–89.
26. Fekade D, Weldegebreal T, Teklu AM, et al. Predictors of survival among adult Ethiopian patients in the national ART program at Seven University Teaching Hospitals: a prospective cohort study. Ethiop J Health Sci. 2017;27(1):63–71. doi:10.4314/ejhs.v27i1.7S
27. Mee P, Collinson MA, Madhavan S, et al. Determinants of the risk of dying of HIV/AIDS in a rural South African community over the period of the decentralised roll-out of antiretroviral therapy: a longitudinal study. Glob Health Action. 2014;7(1):24826. doi:10.3402/gha.v7.24826
28. Mengesha S, Belayihun B, Kumie A. Predictors of survival in HIV-infected patient after Initiation of HAART in Zewditu Memorial Hospital, Addis Ababa, Ethiopia. Int Scholar Res Notices. 2014;2014:1–6. doi:10.1155/2014/250913
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
