Back to Journals » Clinical Ophthalmology » Volume 16
Predictors of Early Onset Glaucoma
Authors Wooliscroft J, Akram R, Zuberi H , Tong B, Gu J, Hurd A , Kooner K
Received 10 February 2022
Accepted for publication 3 June 2022
Published 10 June 2022 Volume 2022:16 Pages 1925—1932
DOI https://doi.org/10.2147/OPTH.S360719
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jeffrey Wooliscroft,1 Rubeel Akram,1 Hafsa Zuberi,1 Betty Tong,1 Jane Gu,1 Aaron Hurd,1 Karanjit Kooner1,2
1Department of Ophthalmology, University of Texas Southwestern Medical Center, Dallas, TX, USA; 2Department of Ophthalmology, Veteran Affairs North Texas Health Care System Medical Center, Dallas, TX, USA
Correspondence: Karanjit Kooner, Department of Ophthalmology, University of Texas Southwestern Medical Center, Dallas, TX, USA, Tel +1 (214) 648-4733, Fax +1 (214) 648-2270, Email [email protected]
Purpose/Relevance: To determine the influence of hypertension (HTN), type 2 diabetes (DM2), migraine, and obstructive sleep apnea (OSA) on the onset of primary open-angle glaucoma (POAG) to enhance predictive accuracy.
Methods: In this cross-sectional study, data for 389 eligible patients with POAG were collected through medical records review and phone surveys. All data were assessed collectively using stepwise multiple regression analysis to determine the relative contribution to age at POAG diagnosis. We used the following groups, based on age at diagnosis, HTN for patients with or without DM2 (model 1), HTN for patients with DM2 (model 2), DM2 for patients with or without HTN (model 3), and DM2 for patients with HTN (model 4).
Results: In model 1, age at HTN diagnosis was associated with age at POAG diagnosis (β = 0.14; 95% CI, 0.01– 0.26, p = 0.04). In model 2, age at HTN diagnosis was not associated with age at POAG diagnosis (p > 0.05). In model 3, age at DM2 diagnosis was associated with age at POAG diagnosis (β = 0.37; 95% CI 0.16– 0.58, p = 0.001). In model 4, age at DM2 diagnosis was associated with age at POAG diagnosis (β = 0.40; 95% CI 0.00– 0.15, p = 0.003). Asian race/ethnicity was associated with early onset of POAG in model 3 (β = − 6.44; 95% CI − 12.34– 0.54, p = 0.033). OSA and migraine did not influence the onset of POAG.
Conclusion: Our study found that the diagnosis of DM2 and HTN at an earlier age is associated with the early onset of POAG.
Keywords: primary open-angle glaucoma, hypertension, type 2 diabetes, migraine, obstructive sleep apnea, onset of glaucoma
Introduction
Glaucoma is the leading cause of irreversible blindness in the world and represents a significant public health issue.1–3 Many risk factors for developing glaucoma have been described in literature: family history, elevated intraocular pressure (IOP), older age, black race, vascular diseases including hypertension (HTN), type 2 diabetes (DM2), migraine, and obstructive sleep apnea (OSA).4–7 Primary open-angle glaucoma (POAG) is the most common type of glaucoma, accounting for 80% to 90% of all glaucoma.1 Despite these well-known risk factors, the early stages of glaucoma have few symptoms, so patients frequently present late in the course of the disease.8 Patients in the latter group have increased vision loss and fewer treatment options, significantly impacting their quality of life.8,9
The prevalence of DM2, HTN, migraine, and OSA in glaucoma has been well studied, but their influence on the onset of POAG is not well understood. Therefore, the aim of our study was to explore the relationship between the age at diagnosis of HTN, DM2, history of migraine or OSA, and the age at POAG diagnosis.
We hypothesize that the history of early onset of HTN, DM2, migraine, and OSA contributes to the early onset of POAG.
Methods
This cross-sectional study was approved by the Institutional Review Board (IRB) of UT Southwestern Medical Center (UTSW). As there was no in-person patient-physician interaction, the IRB determined that no consent form was necessary. We adhered to the principles of the Declaration of Helsinki and the United States Health Insurance Portability and Accountability Act (HIPAA) of 1996.
We used the International Statistical Classification of Diseases (ICD) diagnostic codes for POAG to select all consecutive patients from a single practitioner (KSK) seen at the UTSW Eye Clinic, Dallas, TX, between June 2019 and December 2019. The clinical data used in the study were gathered via a review of the electronic medical records of 1116 patients stored at Epic (Epic, Verona, WI, USA). The patient information was stored and managed using a secure REDCap (Research Electronic Data Capture) server hosted at UTSW.
Patient Selection
Patients with POAG were identified with the following criteria: open anterior chamber angle by gonioscopy, characteristic glaucomatous optic nerve changes (cupping, notching, disc hemorrhage) with corresponding visual field defects, and with or without elevated IOP (≥21 mm Hg). Eligibility was defined as follows: ≥18 years old, history of glaucoma, no significant retinal or vascular pathology or non-glaucoma related visual field defects, vision better than 20/200, and an ability to speak and understand the English language. Exclusion criteria were as follows: secondary glaucoma, angle-closure glaucoma, normal tension glaucoma, juvenile open-angle glaucoma, <3 clinic visits, type 1 diabetes, deceased, non-English speaking, refusal to participate in the study, and dementia or mental disability.
Data Collection
Data collected via a medical records review included age, race, gender, vision, IOP, and body mass index (BMI). Additional data collected via phone survey included date of disease diagnosis (POAG, HTN, DM2, and history of migraine or OSA). Ages at the diagnosis for POAG, HTN, and DM2 were calculated from patient-provided survey information and date of birth.
Visual acuity was measured using Snellen eye charts and IOP was checked by a Goldmann Applanation Tonometer (Haag-Streit, Inc., Koeniz, Switzerland). Visual field examinations were performed using Humphrey Visual Field Analyzer 3 (Carl Zeiss, Jena, Germany).
Data Analysis
All analyses were carried out using IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, N.Y., USA). Student’s t-test was used to compare the mean age of POAG diagnosis between cohorts based on past medical history including a history of OSA vs without, and a history of migraine vs without. We used analysis of variance (ANOVA) to compare mean age at POAG diagnosis among four cohorts based on past medical history: HTN only vs DM2 only vs HTN vs DM2 vs neither HTN nor DM2. Four stepwise multiple regression models were used to assess relationships between age at POAG diagnosis and all of the following predictor variables: age at HTN or DM2 diagnosis and history of migraine or OSA. Additionally, age, gender, race/ethnicity (as indicator variables), and BMI were included in each model to control for confounding influences. Race/ethnicity were separated into four indicator variables: non-Hispanic white, non-Hispanic black, Hispanic, and Asian. Stepwise regression models were separated to maximize the data available for each of them. Each model included the predictor variables: age, gender, race/ethnicity, BMI, history of migraine, and history of OSA. In addition to these predictor variables, each regression model had one of the following predictor variables: age at HTN diagnosis for individuals with or without DM2 (model 1), age at HTN diagnosis for individuals with DM2 (model 2), age at DM2 diagnosis for individuals with or without HTN (model 3), and age at DM2 diagnosis for individuals with HTN (model 4).
Results
Clinical Characteristics
Demographic and study characteristics are described in Table 1. Exclusion criteria and model structure are described in Figure 1. Over the course of the study, 1116 eligible patients were identified for medical records review. Only 389 POAG patients remained after all the exclusions. The key characteristics were 57.3% female and 42.7% male, with a mean age of 71.39 years (±10.4). The predominant race/ethnicity were non-Hispanic white (48.1%) and non-Hispanic black (36.0%). The mean age at POAG diagnosis was 58.58 years (±14.3), and the mean ages at HTN and DM2 diagnoses were 52.95 years (±13.28) and 55.32 years (±11.6), respectively.
 |
Table 1 Demographic and Study Characteristics |
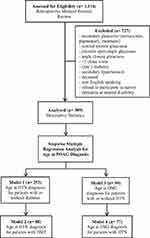 |
Figure 1 Exclusion criteria and model structure. Abbreviations: POAG, primary open-angle glaucoma; HTN, hypertension; DM2, type 2 diabetes. |
Migraine and Obstructive Sleep Apnea
There was no significant difference (t(385) = −0.428, p = 0.665) found in mean age at POAG diagnosis between patients with a history of migraine compared to those without a history of migraine (Table 2).
 |
Table 2 Mean Age at POAG Diagnosis Between Cohorts: History of Migraine and No History of Migraine |
There was no significant difference (t(385) = 1.053, p = 0.939) in mean age at POAG diagnosis between patients with a history of OSA compared to those without a history of OSA (Table 3).
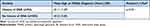 |
Table 3 Mean Age at POAG Diagnosis Between Cohorts: History of OSA and No History of OSA |
Hypertension and Type 2 Diabetes
A one-way ANOVA was conducted to compare mean age at POAG diagnosis based on past diagnoses: HTN only, DM2 only, HTN and DM2, and neither HTN nor DM2. There was not a significant effect of any independent variable on mean age at POAG diagnosis at the p < 0.05 level for the four conditions (F(3,385) = 0.58, p = 0.63) (Table 4).
 |
Table 4 Mean Age at POAG Diagnosis Between Cohorts: HTN Only, DM2 Only, HTN and DM2, and Neither HTN nor DM2 |
By design, age was a significant predictor in each model. Model 1 found that both age and age at HTN diagnosis for patients with or without DM2 explained a significant amount of variance in age at POAG diagnosis (F(2,250) = 52.65, p < 0.001, R2 = 0.30, R2adjusted = 0.39) (Table 5). Model 2 found that only age explained a significant amount of variance in age at POAG diagnosis (F(1,78) = 28.76, p < 0.001, R2 = 0.27, R2adjusted = 0.26) (Table 5). Model 3 found that age, age at DM2 diagnosis with or without HTN, and Asian race/ethnicity explained a significant amount of variance in age at POAG diagnosis (F (3,95) = 26.80, p < 0.001, R2 = 0.46, R2adjusted = 0.44) (Table 5). Model 4 found that both age and age at DM2 diagnosis for patients with HTN explained a significant amount of variance in age at POAG diagnosis (F(2,74) = 19.56, p < 0.001, R2 = 0.35, R2adjusted = 0.33) (Table 5).
 |
Table 5 Results of Stepwise Regression Analysis of Age at POAG Diagnosis for Models 1–4 |
Discussion
In this study, we addressed the relationship between age at DM2 and HTN diagnoses and age at POAG diagnosis using our single practitioner database at an academic center. We found an association between age at HTN diagnosis and age at POAG diagnosis in patients with or without DM2 (p = 0.04). We also found an association between age at DM2 diagnosis and age at POAG diagnosis in patients with both HTN and DM2, and those with or without HTN (p = 0.003; p = 0.001). Our results indicate that the earlier a patient is diagnosed with HTN or DM2, the earlier they are diagnosed with POAG.
While the association between age at HTN diagnosis and age at POAG diagnosis (1.3% change in R2 in model 1) was significant, it was noticeably weaker than age at DM2 diagnosis and age at POAG diagnosis (8.6% and 6.9% change in R2 in models 3 and 4, respectively). Unexpectedly, Asian race/ethnicity was incorporated into model 3. The model indicated that Asian race/ethnicity was associated with earlier development of POAG for patients with DM2 with or without HTN (2.7% change in R2). We did not find significant differences or trends for age at POAG diagnosis based on a history of migraine or OSA. We also did not find significant contributions in predicting age at POAG diagnosis in our models from gender, race/ethnicity in all other cases, or BMI. Finally, we did not find significant differences for the mean age at POAG diagnosis between individuals with HTN only, DM2 only, HTN and DM2, or neither HTN nor DM2.
In this study, we focused on the relationship between HTN, DM2, migraine, OSA, and POAG. HTN has previously been assessed for its possible contribution as a risk factor for POAG.7,10 Zhao et al found during a 2014 meta-analysis that the pooled relative risk for POAG for patients with HTN was 1.16, and there existed a weak dose–response relationship as systolic and diastolic blood pressure increased.7 Furthermore, Leeman noted in a 2019 systematic review that “both high [blood pressure (BP)] and low BP are associated with an increased risk of [POAG] and that there is mounting evidence that low nighttime BP or excessive dipping could adversely affect [POAG] progression”.11
DM2 has been previously assessed for its possible contribution as a risk factor for POAG.6 Zhao et al also found during a 2015 meta-analysis that the pooled relative risk for POAG for patients with DM2 was 1.48, and the risk of POAG increased by 5% each year since DM2 diagnosis.6 Although POAG risk was addressed for each disease, there was a lack of data on their effects on age at POAG diagnosis.
The history of migraine has been addressed previously as a possible POAG risk factor, though the relationship is still controversial.12 Xu et al found in a 2018 meta-analysis that there was a mild but significant increase in POAG risk associated with history of migraine (RR = 1.24).12 In addition, several other studies have indicated migraine as a probable risk factor for POAG.12–15 Despite this, our understanding of how migraine affects POAG development is poorly understood. In addition to migraine, OSA has limited data available as a POAG risk factor. A cohort study from 2013 found that, after a 5-year follow-up period, individuals with OSA had a hazard ratio of 1.67 for developing POAG relative to other patients.16 A 2016 review also indicated that the prevalence of POAG is higher in OSA patients relative to their peers.17 These data suggest that OSA may be a risk factor, but further research is merited in this area. Furthermore, OSA and its effects on age at POAG diagnosis have not been further analyzed.
Limitations and Strengths
This is a cross-sectional study, and as such, it shares all the weaknesses associated with this study type. Additionally, the sample size of this study was limited by the number of patients seen in the 6-month study period and by patient phone survey completion. These constraints resulted in a smaller sample size than the initial 1116 patients as not all patients were eligible or had complete phone survey data available during the time of data analysis. Furthermore, the small sample size of disease subgroups limits the ability to draw generalizability conclusions. Patients were also asked to confirm the date of their disease diagnosis from what had been documented in the electronic medical record. This creates an additional area of uncertainty as we incorporated the patient’s memory of their disease diagnosis. Due to study setting constraints, patients did not have their initial IOP assessed as they were frequently seen later in the course of their disease. This could provide further insight within patient groups as variations in IOP are well known to contribute to POAG risk. Finally, this study focused solely on type 2 diabetes and its relationship with POAG. Diabetic patients treated with insulin have been noted in prior studies to have a higher risk of POAG compared to patients not receiving insulin.6 Our study did not analyze the relationship between POAG and type 1 diabetes or the role of insulin.
Although type 1 diabetes was not addressed in this study, the study is strengthened by its differentiation between types of diabetes in the context of POAG patients and the subsequent focus on the effects of DM2. Studies assessing this relationship traditionally do not divide diabetes into separate categories, potentially confounding the results.6 Furthermore, although the sample size was significantly limited by the study method, the study is strengthened by a wide diversity in racial/ethnic composition. We also attempted to limit confounding factors in this study by including several demographic variables other than age at HTN and DM2 diagnoses.
Finally, all patients in this study belonged to one physician. We recognize that this introduces additional bias as treatment is dependent on physician training and practice opinion.
Relevance and Use
Glaucoma poses a significant public health concern despite a greater understanding of the potential risk factors over the last few decades.2,3 In order to reduce disease burden, general practitioners require a better understanding of when to refer patients to ophthalmologists for screening before disease develops and when to anticipate disease in patient subgroups. In this study, we further elaborated on the relationship between several well-understood risk factors while also looking further at the role these risk factors may play in the age individuals develop POAG. A better understanding of the relationship between early development of DM2 or HTN, history of migraine or OSA, and a potential younger age at POAG development could further reduce the public health burden of glaucoma.
Conclusion
In our sample, an earlier age of diagnosis of DM2 and HTN was associated with an earlier POAG diagnosis. The history of migraine and OSA was not predictive of earlier POAG diagnosis. Patients with early-onset DM2 or HTN may benefit from earlier POAG screening, especially if they have other significant risk factors (ie, family history).
Abbreviations
HTN, hypertension; DM2, type 2 diabetes; OSA, obstructive sleep apnea; BMI, body mass index; ANOVA, analysis of variance; POAG, primary open-angle glaucoma; IOP, intraocular pressure.
Data Sharing Statement
Data included in this study and further patient data can be obtained for research purposes only by contacting the primary author and providing appropriate IRB support. The primary author can be reached at: [email protected].
Acknowledgments
We are grateful to Drs. Matthew Petroll and Joan Reisch for their assistance and contributions to statistical work for this manuscript.
Funding
Supported in part by an unrestricted grant from the Research to Prevent Blindness, New York, NY; NIH grant P30 EY030413 and the support of the University of Texas Southwestern Medical Student Research Program, Dallas, TX.Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under the award number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
The authors have no conflicts of interest in this work.
References
1. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
2. Kolko M. Opsporing og forebyggelse af blindhed hos patienter med glaukom er en samfundsøkonomisk udfordring [Detection and prevention of blindness in patients with glaucoma is a socio-economical challenge]. Ugeskr Laeger. 2017;179(5)56. Danish.
3. Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82(11):887–888.
4. Czudowska MA, Ramdas WD, Wolfs RC, et al. Incidence of glaucomatous visual field loss: a ten-year follow-up from the Rotterdam Study. Ophthalmology. 2010;117(9):1705–1712. doi:10.1016/j.ophtha.2010.01.034
5. Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360(11):1113–1124. doi:10.1056/NEJMra0804630
6. Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015;122(1):72–78. doi:10.1016/j.ophtha.2014.07.051
7. Zhao D, Cho J, Kim MH, Guallar E. The association of blood pressure and primary open-angle glaucoma: a meta-analysis. Am J Ophthalmol. 2014;158(3):615–27.e9. doi:10.1016/j.ajo.2014.05.029
8. Kastner A, King AJ. Advanced glaucoma at diagnosis: current perspectives. Eye. 2020;34(1):116–128. doi:10.1038/s41433-019-0637-2
9. Ang GS, Eke T. Lifetime visual prognosis for patients with primary open-angle glaucoma. Eye. 2007;21(5):604–608. doi:10.1038/sj.eye.6702284
10. Choi J, Kook MS. Systemic and ocular hemodynamic risk factors in glaucoma. Biomed Res Int. 2015;2015:141905. doi:10.1155/2015/141905
11. Leeman M, Kestelyn P. Glaucoma and blood pressure. Hypertension. 2019;73(5):944–950. doi:10.1161/hypertensionaha.118.11507
12. Xu C, Li J, Li Z, Mao X. Migraine as a risk factor for primary open angle glaucoma: a systematic review and meta-analysis. Medicine. 2018;97(28):e11377. doi:10.1097/MD.0000000000011377
13. Cursiefen C, Wisse M, Cursiefen S, Jünemann A, Martus P, Korth M. Migraine and tension headache in high-pressure and normal-pressure glaucoma. Am J Ophthalmol. 2000;129(1):102–104. doi:10.1016/S0002-9394(99)00289-5
14. Huang JY, Su CC, Wang TH, Tsai I-J. Migraine and increased risk of developing open angle glaucoma: a population-based cohort study. BMC Ophthalmol. 2019;19(50). doi:10.1186/s12886-019-1062-9
15. Wang JJ, Mitchell P, Smith W. Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study. Ophthalmology. 1997;104(10):1714–1719. doi:10.1016/s0161-6420(97)30075-x
16. Lin CC, Hu CC, Ho JD, Chiu HW, Lin HC. Obstructive sleep apnea and increased risk of glaucoma: a population-based matched-cohort study. Ophthalmology. 2013;120(8):1559–1564. doi:10.1016/j.ophtha.2013.01.006
17. Chaitanya A, Pai VH, Mohapatra AK, Ve RS. Glaucoma and its association with obstructive sleep apnea: a narrative review. Oman J Ophthalmol. 2016;9(3):125–134. doi:10.4103/0974-620X.192261
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
