Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Predictors for eltrombopag response in patients with hepatitis C virus-associated thrombocytopenia
Authors Elbedewy TA, Elsebaey MA, Elshweikh SA, Elashry H, Abd-Elsalam S
Received 1 September 2018
Accepted for publication 3 December 2018
Published 11 February 2019 Volume 2019:15 Pages 269—274
DOI https://doi.org/10.2147/TCRM.S186106
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Tamer A Elbedewy,1 Mohamed A Elsebaey,1 Samah A Elshweikh,1 Heba Elashry,2 Sherief Abd-Elsalam2
1Internal Medicine Department, Faculty of Medicine, Tanta University, Tanta, Egypt; 2Tropical Medicine Department, Faculty of Medicine, Tanta University, Tanta, Egypt
Background and aims: Thrombocytopenia is a common hematological abnormality observed in patients infected with hepatitis C virus (HCV). The use of eltrombopag has been approved for HCV-associated thrombocytopenia. This is the first study aiming to determine the predictive factors of response to eltrombopag therapy in patients with HCV-associated thrombocytopenia.
Patients and methods: This prospective study was carried out on 130 patients with chronic HCV-associated thrombocytopenia (<50,000×109/L) that precludes the initiation of HCV therapy. Eltrombopag was initiated at a dose of 25 mg once daily; the dose was adjusted with 25 mg increments every 2 weeks to achieve the target platelet count. The primary end point was to achieve stable target platelet count (50,000–100,000×109/L) required to initiate antiviral therapy.
Results: Treatment response was achieved in 111 (85.38%) patients. This prospective study showed that megakaryocyte hypoplasia or aplasia and splenectomy were independent risk factors for eltrombopag nonresponse in chronic HCV-associated thrombocytopenic patients.
Conclusion: Eltrombopag is safe and effective for patients with HCV-associated thrombocytopenia. Bone marrow examination should be considered before initiating treatment with eltrombopag in chronic HCV-associated thrombocytopenic patients, especially in patients with splenectomy.
Keywords: HCV, therapy, thrombocytopenia, predictors, splenectomy, direct-acting antivirals, DAAs
Introduction
Thrombocytopenia is a common hematological abnormality observed in patients with hepatitis C virus (HCV)-related chronic liver disease and is considered as an indicator of disease severity. The incidence of HCV-associated thrombocytopenia ranges from 0.16% to 45% as reported by multiple studies.1–3
The pathophysiology of HCV-associated thrombocytopenia is complex and multifactorial involving the following mechanisms: HCV-induced immune-mediated platelet destruction, direct bone marrow suppression, platelet sequestration related to hypersplenism, and impaired thrombopoietin production related to HCV-induced liver cirrhosis.4
Thrombocytopenia represents a major clinical challenge in the care of chronic HCV patients. It may impede the initiation of anti-HCV therapy as well as necessary diagnostic or therapeutic interventions because of concerns about increased risk of bleeding.1
The optimal management of thrombocytopenia among patients with HCV-related chronic liver disease is still a matter of discussion.5 While eradication of HCV infection is the most practical option for amelioration of HCV-induced thrombocytopenia,6–10 other therapeutic modalities such as steroids, platelet transfusion, splenic artery embolization, and splenectomy are available.11,12 Hypersplenism occurs in patients with chronic liver disease, for which splenectomy is the ultimate treatment. However, the operation may be serious in patients with impaired liver function. In recent years, partial splenic embolization has been used extensively in patients with cirrhosis and hypersplenism. It allows preservation of enough splenic tissue to protect against overwhelming sepsis. However, the routine use of these invasive procedures is limited by the occurrence of complications.13
The use of thrombopoietin-mimetic agents, in particular, eltrombopag, has been approved for HCV-associated thrombocytopenia. Eltrombopag interacts with the thrombopoietin receptor on megakaryocyte precursors and megakaryocytes, and induces their proliferation and differentiation to increase platelet production.14 Some data concerning the safety and efficacy of eltrombopag therapy in patients with HCV-associated thrombocytopenia have been reported,14–16 yet there are no studies published on the predictors of response to eltrombopag therapy. Therefore, we conducted this study to determine the predictive factors of response to eltrombopag therapy in patients with HCV-associated thrombocytopenia.
Patients and methods
This prospective study was conducted at the departments of internal medicine and tropical, Tanta University Hospitals, and Tanta Insurance Hospital in the period between December 2016 and April 2018. The study was carried out on 130 patients with chronic HCV-associated thrombocytopenia (platelet count <50,000×109/L) that precludes the initiation of HCV therapy.17 Patients with thrombocytopenia due to causes other than HCV infection were excluded from this study. The patients were divided into two groups (responders and nonresponders) according to their response to eltrombopag.
This study was conducted in accordance with the guidelines of the Declaration of Helsinki (1975) and its subsequent amendments (1983). Written informed consent was obtained from all patients prior to the study after full explanation of benefits and risks of the study. This study was approved by the Tanta University Faculty of Medicine research ethical committee.
Full medical history was obtained from the patients, and a thorough clinical examination was conducted for each patient.
Laboratory and other assessments
- Complete blood count including hemoglobin level, total leukocytic count, and platelet count (automatic blood cell counter model PCE-210N; Erma Inc) was evaluated.
- Liver function tests (alanine transaminase, aspartate transaminase, serum bilirubin, serum albumin, and international normalized ratio) were performed.
- HCV-RNA PCR assay was carried out by real-time PCR using StepOne instrument and software (Applied Biosystems).
- Bone marrow aspiration was performed for detection of megakaryocyte hypoplasia or aplasia.
- Abdominal ultrasonography was conducted (splenomegaly and huge splenomegaly were considered if the splenic span was >13 and 20 cm, respectively).18
- Child–Pugh score was calculated for the assessment of liver status in cirrhotic patients.19
Eltrombopag (Revolade®; Novartis Pharmaceuticals UK Ltd) was initiated at a dose of 25 mg once daily. The dose was adjusted with 25 mg increments every 2 weeks – when needed – to achieve the stable target platelet count (50,000–100,000×109/L) required to initiate antiviral therapy. Dose reduction by 25 mg was considered if platelet counts were in the range of 101,000–150,000×109/L. The maximum dose of eltrombopag was 100 mg once daily. Nonresponders were identified as those patients who had received eltrombopag 100 mg/day for 2 weeks and failed to meet the platelets threshold. Complete blood picture and liver function tests were requested once weekly until the target platelet count was maintained for 1 month. Thereafter, monitoring frequency was reduced to once a month.20
Statistics
SPSS (version 23; IBM Corporation, Armonk, NY, USA) was used for analysis of patients’ data. Sample size calculation performed according to previous studies rendered a sample size of 100 patients at a power of 90%. Quantitative and qualitative data were compared using unpaired t-test and chi-squared test, respectively. Univariate analysis was performed to identify the predictive factors of eltrombopag response. Variables that showed statistical significance in the univariate analysis were subsequently included in multivariate analyses. Probability (P) value <0.05 was considered significant.
Results
One hundred and thirty patients with chronic HCV-associated thrombocytopenia were enrolled in this study. Their mean age was 44.3±9.97. Eighty-two patients were males, and 48 were females. About 71% of patients were cirrhotic. The mean platelet count of the enrolled patients was 28.06±9.48 (×103)/mm3. All patients received interferon-free treatment. The baseline demographic and laboratory data are shown in Table 1.
Of the 130 patients enrolled in the study, 28 (21.54%) reported adverse events as shown in Table 2. Regarding the side effects associated with eltrombopag therapy, 28 patients (21.54%) reported adverse events mainly in the form of mild elevated liver enzymes (13 patients, 10%), headache (10 patients, 7.69%), easy fatigue (seven patients, 5.38%), and dry mouth (two patients, 1.54%).
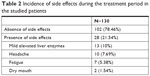  | Table 2 Incidence of side effects during the treatment period in the studied patients |
Eltrombopag response was achieved in 111 (85.38%) patients. The mean duration of treatment with eltrombopag for the responders was 3.89±1.64 weeks, ranging between 2 and 8 weeks, and the mean dose was 48.65±20.18 mg/day, ranging between 25 and 100 mg/day.
The univariate analysis performed to analyze the factors that might affect the response to eltrombopag revealed that megakaryocyte hypoplasia or aplasia, splenectomy, and huge splenomegaly were significantly associated with nonresponse to eltrombopag as shown in Table 3.
The multivariate analysis revealed that megakaryocyte hypoplasia or aplasia and splenectomy were significantly associated with nonresponse to eltrombopag as shown in Table 4.
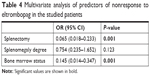  | Table 4 Multivariate analysis of predictors of nonresponse to eltrombopag in the studied patients |
Discussion
The optimal management of thrombocytopenia associated with HCV infection is still a matter of discussion.5 The use of eltrombopag therapy in patients with HCV-associated thrombocytopenia has been reported,11–13 yet there are no studies published on the predictors of response to eltrombopag therapy in this special category of patients.
One hundred and thirty patients with chronic HCV-associated thrombocytopenia with platelet count <50,000×109/L were included in this study. The aim was to assess the ability of eltrombopag to increase the platelet count above the threshold (50–100×109/L), and thus enable the initiation of antiviral therapy in patients. Eltrombopag response was achieved in 111 (85.38%) patients.
The results of our study were slightly better than those of the ENABLE-1 and ENABLE-2 studies. These two studies included 715 and 805 patients with chronic HCV infection, respectively, with a platelet count <75×109/L, and their purpose was to assess the ability of eltrombopag to increase the platelet count above the threshold (90–100×109/L) to enable the initiation of interferon treatment in patients.21
In addition, the response rate in our study was better than that of the ELEVATE study which included 292 patients with chronic liver disease and thrombocytopenia with a platelet count <50×109/L before performing elective invasive procedures. Eltrombopag response was achieved in 72% of patients.22
The discrepancy between our results and the results of the previous clinical studies might be attributed to the differences in number of included patients, their liver status, platelet count before treatment with eltrombopag, as well as platelets threshold required for initiation of HCV treatment or performing invasive procedures.
Regarding the side effects associated with eltrombopag therapy in the current study, 28 patients (21.54%) reported adverse events mainly in the form of mild elevated liver enzymes (13 patients, 10%), headache (10 patients, 7.69%), easy fatigue (seven patients, 5.38%), and dry mouth (two patients, 1.54%).
Eltrombopag is well tolerated.23 The most common side effects are nausea, vomiting, headache, dry mouth, and abdominal pain.24 However, serious adverse events such as thromboembolism might occur which necessitates termination of eltrombopag therapy.25
This is the first study to assess the predictive factors of response to eltrombopag therapy in patients with HCV-associated thrombocytopenia. This prospective study showed that bone marrow suppression (megakaryocyte hypoplasia or aplasia) and splenectomy were independent risk factors of nonresponse to eltrombopag treatment in chronic HCV-associated thrombocytopenic patients.
It is well known that splenectomy has been used to correct thrombocytopenia due to splenic sequestration in patients with hypersplenism, producing significant and persistent increases in platelet count.11,26 However, in our study, 13 patients with splenectomy were presented with thrombocytopenia, and even with eltrombopag, seven patients did not show increase in platelet count. This implies that the pathogenesis of thrombocytopenia in patients with HCV-associated liver disease is multifactorial rather than involving hypersplenism alone.27
The pathogenesis of thrombocytopenia in patients with chronic liver disease involves reduced thrombopoietin production, spleen sequestration of platelets, and myelosuppression of platelet production due to HCV-induced bone marrow suppression.28–30 The response to eltrombopag is achieved via its interaction with the thrombopoietin receptor on megakaryocyte precursors and megakaryocytes in bone marrow to induce their proliferation and differentiation in order to increase platelet production.31 This clearly explains the lower response to eltrombopag in patients with HCV-induced megakaryocyte hypoplasia or aplasia.
Conclusion
Eltrombopag is safe and effective for patients with HCV-associated thrombocytopenia. However, its efficacy is reduced in patients with HCV-induced bone marrow suppression. So, bone marrow examination should be considered before initiating treatment with eltrombopag in patients with chronic HCV-associated thrombocytopenia, especially in patients with splenectomy.
Disclosure
The authors report no conflicts of interest in this work.
References
Fouad YM. Chronic hepatitis C-associated thrombocytopenia: aetiology and management. Trop Gastroenterol. 2013;34:58–67. | ||
Mohamed SF. Prevalence of thrombocytopenia in Egyptian patients with chronic hepatitis C virus. J Egypt Soc Parasitol. 2013;43(3):617–628. | ||
Louie KS, Micallef JM, Pimenta JM, Forssen UM. Prevalence of thrombocytopenia among patients with chronic hepatitis C: a systematic review. J Viral Hepat. 2011;18(1):1–7. | ||
Weksler BB. Review article: the pathophysiology of thrombocytopenia in hepatitis C virus infection and chronic liver disease. Aliment Pharma. 2007;26(Suppl. D):13–19. | ||
Lebano R, Rosato V, Masarone M, Romano M, Persico M. The effect of antiviral therapy on hepatitis C virus-related thrombocytopenia: a case report. BMC Res Notes. 2014;7(1):59. | ||
Ahmed OA, Kaisar HH, Hawash N, et al. Efficacy of sofosbuvir plus ribavirin with or without Peginterferon- alfa in treatment of a cohort of Egyptian patients with hepatitis C virus infection. IDDT. 2017;17(2):95–100. | ||
Abd-Elsalam S, Sharaf-Eldin M, Soliman S, Elfert A, Badawi R, Ahmad YK. Efficacy and safety of sofosbuvir plus ribavirin for treatment of cirrhotic patients with genotype 4 hepatitis C virus in real-life clinical practice. Arch Virol. 2018;163(1):51–56. | ||
Ahmed OA, Kaisar HH, Badawi R, et al. Efficacy and safety of sofosbuvir-ledipasvir for treatment of a cohort of Egyptian patients with chronic hepatitis C genotype 4 infection. Infect Drug Resist. 2018;11:295–298. | ||
Ahmed OA, Elsebaey MA, Fouad MHA, et al. Outcomes and predictors of treatment response with sofosbuvir plus daclatasvir with or without ribavirin in Egyptian patients with genotype 4 hepatitis C virus infection. Infect Drug Resist. 2018;11:441–445. | ||
Ahmed OA, Safwat E, Khalifa MO, et al. Sofosbuvir plus daclatasvir in treatment of chronic hepatitis C genotype 4 infection in a cohort of Egyptian patients: an experiment the size of Egyptian village. Int J Hepatol. 2018;2018:1–5. | ||
Akahoshi T, Tomikawa M, Kawanaka H, et al. Laparoscopic splenectomy with interferon therapy in 100 hepatitis-C-virus-cirrhotic patients with hypersplenism and thrombocytopenia. J Gastroenterol Hepatol. 2012;27(2):286–290. | ||
Bárcena R, Gil-Grande L, Moreno J, et al. Partial splenic embolization for the treatment of hypersplenism in liver transplanted patients with hepatitis C virus recurrence before peg-interferon plus ribavirin. Transplantation. 2005;79(11):1634–1635. | ||
Amin MA, El-Gendy MM, Dawoud IE, Shoma A, Negm AM, Amer TA. Partial splenic embolization versus splenectomy for the management of hypersplenism in cirrhotic patients. World J Surg. 2009;33(8):1702–1710. | ||
Burness CB. Eltrombopag: a review of its use in the treatment of thrombocytopenia in patients with chronic hepatitis C. Drugs. 2014;74(16):1961–1971. | ||
Sharma V. Use of eltrombopag in thrombocytopenia of liver disease. World J Pharmacol. 2014;3(4):186–192. | ||
Mihăilă RG, Cipăian RC. Eltrombopag in chronic hepatitis C. World J Gastroenterol. 2014;20(35):12517–12521. | ||
El-Akel W, El-Sayed MH, El Kassas M, et al. National treatment programme of hepatitis C in Egypt: hepatitis C virus model of care. J Viral Hepat. 2017;24(4):262–267. | ||
Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21(9):1023–1032. | ||
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. | ||
GlaxoSmithKline. Revolade 25 mg film-coated tablets: summary of product characteristics. 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001110/WC500089964.pdf. Accessed September 15, 2014. | ||
Afdhal NH, Dusheiko GM, Giannini EG, et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146(2):442–452. | ||
Afdhal NH, Giannini EG, Tayyab G, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367(8):716–724. | ||
Tillmann HL, McHutchison JG. Use of thrombopoietic agents for the thrombocytopenia of liver disease. Semin Hematol. 2010;47(3):266–273. | ||
Mchutchison JG, Dusheiko G, Shiffman ML, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357(22):2227–2236. | ||
Giannini EG, Afdhal NH. Eltrombopag in patients with chronic liver disease. Expert Opin Pharmacother. 2013;14(5):669–678. | ||
McCormick PA, Murphy KM. Splenomegaly, hypersplenism and coagulation abnormalities in liver disease. Baillieres Best Pract Res Clin Gastroenterol. 2000;14(6):1009–1031. | ||
Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14(Suppl D):60D–66D. | ||
Giannini EG. Review article: thrombocytopenia in chronic liver disease and pharmacologic treatment options. Aliment Pharmacol Ther. 2006;23(8):1055–1065. | ||
Drews RE. Critical issues in hematology: anemia, thrombocytopenia, coagulopathy, and blood product transfusions in critically ill patients. Clin Chest Med. 2003;24(4):607–622. | ||
Abd-Elsalam S, Habba E, Elkhalawany W, et al. Correlation of platelets count with endoscopic findings in a cohort of Egyptian patients with liver cirrhosis. Medicine. 2016;95(23):e3853. | ||
Burness CB. Eltrombopag: a review of its use in the treatment of thrombocytopenia in patients with chronic hepatitis C. Drugs. 2014;74(16):1961–1971. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


