Back to Journals » Infection and Drug Resistance » Volume 14
Predictive Value of Urinalysis and Recent Antibiotic Exposure to Distinguish Between Bacteriuria, Candiduria, and No-Growth Urine
Authors Chotiprasitsakul D, Kijnithikul A, Uamkhayan A, Santanirand P
Received 6 October 2021
Accepted for publication 8 December 2021
Published 30 December 2021 Volume 2021:14 Pages 5699—5709
DOI https://doi.org/10.2147/IDR.S343021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Darunee Chotiprasitsakul,1 Akara Kijnithikul,1 Anuchat Uamkhayan,2 Pitak Santanirand2
1Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Darunee Chotiprasitsakul
Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Bangkok, 10400, Thailand
Tel +66 2 201 1581
Fax +66 2 201 1647
Email [email protected]
Purpose: Urinary tract infections are diagnosed by clinical symptoms and detection of causative uropathogen. Antibiotics are usually not indicated in candiduria and no-growth urine. We aimed to develop a predictive score to distinguish bacteriuria, candiduria, and no-growth urine, and to describe the distribution of microorganisms in urine.
Patients and Methods: A single-center, retrospective cohort study was conducted between January 2017 and November 2017. Patients with concomitant urinalysis and urine culture were randomly sorted for a clinical prediction model. Multivariable regression analysis was performed to determine factors associated with bacteriuria, candiduria, and no-growth urine. A scoring system was constructed by rounding the regression coefficient for each predictor to integers. Accuracy of the score was measured by the concordance index (c-index).
Results: There were 8091 positive urine cultures: bacteria 85.6%, Candida 13.7%. Randomly selected cases were sorted into derivation and validation cohorts (448 cases and 272 cases, respectively). Numerous yeast on urinalysis predicted candiduria with complete accuracy; therefore, it was excluded from a score construction. We developed a NABY score based on: positive nitrite, 1 point; Antibiotic exposure within 30 days, – 2 points; numerous Bacteria in urine, 2 points; few Yeast in urine, – 2 points; moderate Yeast in urine, – 5 points. The c-index was 0.85 (derivation) and 0.82 (validation). A score ≥ 0 predicted 76% and 54% of bacteriuria in the derivation and validation cohorts, respectively. A score ≤− 3 predicted 96% of candiduria in both cohorts.
Conclusion: Numerous yeast on urinalysis and the NABY score may help identify patients with a low risk of bacteriuria in whom empiric antibiotics for UTIs can be avoided.
Keywords: urinary tract infection, Candida, antibiotics, microscopy, urine culture, NABY score
Introduction
Urinary tract infection (UTI) is a common infectious disease in both outpatient and inpatient settings. Diagnosis of UTI is based on the presence of clinical symptoms or signs consistent with UTI and detection of bacteria in urine culture; ≥105 colony-forming units (CFU)/mL for most symptomatic UTI,1 and ≥103 CFU/mL for catheter-associated (CA) UTI.2 Clinical symptoms of UTI include dysuria, urinary frequency or urgency, suprapubic pain, or flank pain.3,4 However, older patients, debilitated patients, or patients with an indwelling bladder catheter may present with nonspecific symptoms without the localized symptoms of UTIs. Furthermore, older patients are more likely to have atypical symptoms, such as anorexia, confusion, impaired functional status, and absence of fever.5,6 Patients with cognitive deficits might have impaired ability to communicate and may have existing chronic genitourinary symptoms (eg, incontinence, urgency, and frequency), which makes the diagnosis of UTIs more difficult.7 Patients with a long-term indwelling urethral catheter cannot discern frequency or dysuria; therefore, the diagnosis of UTI relies on urine workup and exclusion of other causes.7 The most common causative pathogens for uncomplicated UTI was Escherichia coli, followed by Klebsiella pneumoniae, Staphylococcus saprophyticus, Enterococcus faecalis, group B Streptococcus (GBS), Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus and Candida spp. For complicated UTI, E. coli was the most common etiologic agent, followed by Enterococcus spp., K. pneumoniae, Candida spp., S. aureus, P. mirabilis, P. aeruginosa and GBS.8
Although urine culture is the gold standard to support the diagnosis of UTI, the time required for organism growth is 24–48 h.9 Urinalysis is a useful tool for initial evaluation. Leukocytes in the urine imply inflammation and tissue invasion of the urinary tract.10 Pyuria is present in most cases of acute simple cystitis in women. The absence of pyuria suggests an alternative diagnosis and does not warrant antibiotic treatment.11 In contrast, pyuria is not diagnostic of CA-bacteriuria or CA-UTI in catheterized patients.12 Pyuria cannot be used to differentiate CA-asymptomatic bacteriuria (CA-ASB) from CA-UTI, as it is usually present in both conditions.2 Furthermore, catheterized patients can have pyuria without bacteriuria, as the catheter itself may cause bladder inflammation.12 Commercially available urine dipsticks that test for leukocyte esterase and nitrite are convenient for screening for UTI. Leukocyte esterase is an enzyme released by neutrophils and reflects pyuria. A review of studies reported that the sensitivity and specificity of leukocyte esterase for culture-confirmed UTI were 72–97% and 41–86%, respectively.13 Nitrite indicates the presence of bacteria, which convert urinary nitrate to nitrite. Many gram-negative and some gram-positive bacteria are able to make this conversion.13 The sensitivity and specificity of nitrite for culture-confirmed UTI were 19–48% and 92–100%, respectively.13
In a study by Van Nostrand et al, at least moderate numbers of bacteria on microscopy showed significant association with UTI. However, the use of this finding as the sole predictor of UTI was not sufficient. The sensitivity and specificity of this parameter were 46.4% and 89%, respectively.14
Candiduria is a common finding in hospitalized patients. Sobel et al reported that at least 10–15% of hospitalized patients had candiduria.15 Candiduria is more common in intensive care units (ICUs), diagnosed in 22–40% of the patients.15 Risk factors associated with candiduria included advanced age, female sex, antibiotic use, urinary drainage devices, previous surgical procedures, and diabetes mellitus.16 Yeast observed by microscopy may be the first clue for candiduria.17 The majority of cases were asymptomatic UTIs.18 Asymptomatic candiduria rarely needs treatment.19
Urinalysis and urine culture are commonly ordered tests in hospitals. Urinalysis and/or urine culture were obtained in 70% of 3748 admitted patients in a prospective multicenter trial.20 Unnecessary urine testing and false-positive results have led to overtreatment with antibiotics. Fever was the sole indication for obtaining a urine culture in 74% of patients.21 Positive urinalysis results were associated with antibiotic prescription in asymptomatic patients.22 Although there were substantial evidence and strong recommendations against treatment for ASB, inappropriate antibiotic use is very common for this condition. A retrospective cohort study of 2733 patients with ASB found that 83% were treated with antibiotics for a median of 7 days.23 Positive urinalysis and urine cultures with a bacteria ≥105 CFU per high-power field were the crucial factors for overtreatment of ASB.23 Lammers et al estimated that 23–50% of antibiotic-days for asymptomatic bacteriuria were unnecessary.24 Antibiotic exposure is a major component for antibacterial resistance.25 The higher resistance rate of uropathogen was reported in antibiotic groups, which have been frequently used for treating UTI.26 As the gaps in knowledge, attitude, and practice remain a challenging problem, early exclusion of bacteriuria could be useful to avoid unnecessary use of empirical antibiotics in the case of candiduria or no-growth urine.
The primary objective of this study was to develop a predictive score for distinguishing between bacteriuria, candiduria, and no-growth urine. The secondary objective was to assess the species distribution of microorganisms in positive urine cultures and the proportion of patients undergoing empirical treatment for UTI in a real-world setting.
Materials and Methods
Study Population
A single-center, retrospective cohort study was conducted at Ramathibodi Hospital, a 1300-bed, tertiary-care university hospital with kidney and stem cell transplant units in Bangkok, Thailand. The hospital served at least 5000 outpatient visits per day. Data were collected from January 2017 through November 2017. All urine sample data were retrieved from the laboratory database. Patients 18 years of age and older with concomitant microscopic urinalysis and urine culture on the same day were randomly selected for the analysis. The exclusion criteria were unavailable medical records, insufficient quantity of urine sample (<5 mL), and urine samples from the same patient within 30 days.
Urinalysis and Urine Culture
Fresh urine specimens were collected in preservative-free, antiseptic-free containers. Uncentrifuged urine samples were put into test tubes for processing upon arrival at the clinical microscopy laboratory. Physical and chemical analysis was performed with the cobas u 601 urine analyzer (Roche Diagnostics International AG, Rotkreuz, Switzerland). The cobas u 601 analyzer is a fully automated reflectance photometry urine test strip analyzer, as well as physical cell measurements of clarity and specific gravity.27 Urine sediment analysis was performed by the Sysmex UF-1000i urine particle analyzer (Sysmex, Kobe, Japan). The UF-1000i system is a fully automated flow cytometer for identification and quantification of microscopic materials suspended in the urine. It also has a dedicated chamber for bacteria and separate bacterial analysis channel to discriminate bacteria from debris. Detection is based on flow cytometry combined with impedance method.28 The abnormal sediments, including cells, casts, bacteria, and yeast were confirmed by microscopic examination of centrifuged urine by trained medical technicians. Visualized sediments were classified according to established guides.29 The turn-around time for the results was within 2 h of arrival in the laboratory.
Quantitative urine culture was performed using 1 μL of urine, spread in a pinwheel streak onto 5% human blood agar plates and MacConkey agar (BBL; BD, Sparks, MD, USA) prepared by the microbiology laboratory. The plates were incubated aerobically at 35°C for 24 h. The final urine culture result was reported 48 h after incubation.
Data Collection
The patients were classified by microorganism into bacteriuria or candiduria groups (or no-growth group). Demographic information, preexisting medical conditions, location at time of urine sample collection (emergency department, outpatient clinic, or hospital ward), recent antibiotic exposure (within 30 days prior to urine examination), presence of an indwelling urinary catheter, as well as the results of urinalysis and urine culture were collected from medical records. Information on empirical antibiotics initiated on the day of urine collection and documented indication for treatment were collected.
Statistical Analysis
The distribution of microorganisms (bacterial or Candida species) in urine culture was calculated from all urine cultures of adults submitted to the microbiology laboratory during the study period. A prediction model was developed and validated, as previously described.30 Sample size of the derivation cohort was estimated from at least 10 events (bacteriuria) per candidate predictor.31 Equal numbers, and equal quantity of bacteriuria, candiduria, and no-growth urine were randomly selected. Categorical and ordinal variables were presented as frequencies and percentages and were compared using the chi-square test and Kruskal–Wallis H-test, where appropriate. Continuous variables were presented as medians and interquartile ranges (IQRs) and were compared using Student’s t-test and the Wilcoxon rank-sum test, where appropriate. Logistic regression was used to estimate the odds ratios (ORs) and associated 95% confidence intervals (CIs) of factors associated with bacteriuria, candiduria, and no-growth urine. Variables with a P-value <0.10 in the univariate analysis were included in the logistic regression model for the multivariate analysis. All test P-values of <0.05 were considered to be statistically significant. Backward elimination was performed for variable selection. The Hosmer–Lemeshow goodness of fit test was used for the calibration of the predictive model. The receiver operating characteristic (ROC) curves and the concordance statistic (c-index) from 1000 bootstrap replications were used to evaluate discriminative ability. The scoring system was developed by rounding the regression coefficient to the nearest integers.32 External validation in randomly selected patients visiting the emergency room (other than in the derivation cohort) from the same dataset was evaluated. Stata software, version 16.0 (StataCorp LLC, College Station, TX, USA) was used for analyses.
Ethical Approval
This study was approved by the ethics institutional review board of Ramathibodi Hospital, with a waiver of informed consent due to the retrospective nature of the study (approval number: MURA2021/507). De-identification of data was used to prevent personal identifiers. All data was kept confidential. This study was conducted in accordance with the Declaration of Helsinki.
Results
Microorganism Distribution in Cultured Urine Samples
A total of 20,433 urine samples were collected between January 2017 and November 2017. There were 16,568 urine samples from adults. Of these, 8091 samples (48.8%) grew microorganisms: bacteria in 6929 samples (85.6%) and Candida in 1106 samples (13.7%). The most common bacterium was Escherichia coli (44.7%), followed by Enterococcus spp. (12.0%), Klebsiella spp. (11.3%), and Pseudomonas aeruginosa (9.4%). The most common fungus was C. albicans (53.6%), followed by C. tropicalis (23.3%) and C. glabrata (15.7%) (Figure 1).
 |
Figure 1 Distribution of microorganisms in urine culture. Abbreviation: CFU/mL, colony-forming unit per milliliter. |
Comparison of Bacteriuria, Candiduria, and No-Growth Urine in the Derivation Cohort
We randomly sorted 192 bacteriuria, 192 candiduria, and 64 no-growth urine samples into the derivation cohort. The median age of the bacteriuria, candiduria, and no-growth urine groups were 67 years, 69 years, and 67 years, respectively. There was no significant difference in the comorbidities among the three groups. The most common pre-existing condition was diabetes mellitus (29.0%). Several differences between the three groups were evident. Recent antibiotic exposure in 30 days in the bacteriuria, candiduria, and no-growth urine groups were 30.2%, 84.9%, and 56.3%, respectively (P < 0.001). The presence of an indwelling urinary catheter in the bacteriuria, candiduria, and no-growth urine groups were 35.9%, 62.5%, and 34.4%, respectively (P < 0.001) (Table 1).
 |
Table 1 Demographic Data and Other Characteristics of Patients with Candiduria, Bacteriuria, and No-Growth in the Derivation Cohort |
Positive nitrite was found more in the bacteriuria group (19.3%) than in the candiduria (5.7%) and no-growth urine groups (3.1%). The no-growth urine group was less likely to have moderately to markedly positive leukocyte esterase, positive blood, 3+ or 4+ protein, or white blood cells ≥5 cells/high power field. Bacteriuria specimens had a higher likelihood of numerous bacteria on microscopic examination. Candiduria specimens were more likely to have few, moderate, or numerous yeast. None of the bacteriuria specimens had numerous yeast cells. Of the 64 no-growth urine samples, only two patients had at least a moderate amount of bacteria, and none had at least a moderate amount of yeast (Table 2).
 |
Table 2 Urinalysis Profile of Patient with Candiduria, Bacteriuria, and No-Growth Urine in the Derivation Cohort |
Score for Distinguishing Bacteriuria, Candiduria, and No-Growth Urine
The univariate analysis of factors associated with bacteriuria, candiduria, and no-growth urine is shown in Table 3. In the multivariate analysis, factors significantly associated with bacteriuria were recent antibiotic exposure (OR 0.19; 95% CI 0.11–0.31, P < 0.001), positive nitrite (OR 3.39; 95% CI 1.24–9.28, P = 0.02), numerous bacteria (OR 7.35; 95% CI 2.44–22.20, P < 0.001), few yeast (OR 0.13; 95% CI 0.05–0.34, P < 0.001), and moderate yeast (OR 0.01; 95% CI 0.001–0.10, P < 0.001). Numerous yeast was perfectly predictive of not having bacteriuria, and that parameter was omitted from score construction (Table 4).
 |
Table 3 Univariate Analysis of Factors Associated Between Bacteriuria, Candiduria, and No-Growth Urine with P-value <0.10 in the Derivation Cohort |
 |
Table 4 Multivariate Analysis of Factors Associated Between Bacteriuria, Candiduria, and No-Growth Urine with P-value <0.05 in the Derivation Cohort |
We developed a score using the variables selected in the multivariate model. The synthetic score on a scale of −7 to 3 for the risk of bacteriuria was obtained from the weight of coefficient (β) in the model (Table 5). We called it the NABY score (positive Nitrite, recent Antibiotic exposure, amount of Bacteria in urine, amount of Yeast in urine). The best cutoff value for bacteriuria was established at a score ≥0 points. The Hosmer–Lemeshow goodness-of-fit test had a P-value of 0.51. The c-index in the derivation and validation cohorts was 0.85 (95% CI 0.81–0.88), and 0.82 (95% CI 0.78–0.86), respectively.
 |
Table 5 Scoring System to Calculate Point Values for the Risk Score of Bacteriuria vs Candiduria |
Risk Groups Within Derivation and Validation Cohorts
A total of 272 patients (108 bacteriuria, 112 candiduria, 52 no-growth urine) visiting the emergency room were randomly sorted into the validation cohort. Nineteen patients in the derivation cohort, and six patients in the validation cohort with numerous yeast cells in their urine were excluded from the risk score. The risk groups classified by the NABY score are shown in Figure 2. A NABY score of ≥0 correctly classified 76% and 54% of bacteriuria in the derivation and validation cohorts, respectively. A NABY score of ≤−3 correctly classified 96% of candiduria in both cohorts.
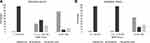 |
Figure 2 Percentage of patients with true bacteriuria, true candiduria, and true no-growth urine by NABY score category; (A) derivation cohort, (B) validation cohort. |
Empirical antibiotics for UTI on the day of urine examination was documented in 28.6% (55/192) of the bacteriuria, 30.2% (58/192) of the candiduria, and 10.9% (7/64) of the no-growth groups in the derivation cohort. These were 43.5% (47/108) of the bacteriuria, 46.4% (52/112) of the candiduria, and 23.1% (12/52) of the no-growth groups in the validation cohort.
Discussion
Almost 85% of the patients with candiduria in our study had recent antibiotic exposure within 30 days. This is consistent with a prospective multicenter surveillance study which found 90% of 861 hospitalized patients had received antibiotics within 1 month prior to the onset of funguria.33 Antibiotics suppress the susceptible endogenous bacterial flora and facilitate epithelial surface fungal colonization.34 The recent use of antibiotics is a risk factor for candiduria.16 Initial recognition of the possibilities of candiduria in patients receiving antibiotics for previous or concomitant bacterial infections in the last 30 days could be helpful in determining these patients whom UTI is subsequently suspected.
The suboptimal performance observed in our study of individual parameters in urinalysis for predicting bacteriuria agrees with most previous reports. This study found a higher proportion of leukocyte esterase among candiduria (58.9%) compared with bacteriuria (49.5%) and no-growth urine (26.6%) specimens. A similar finding was observed for white blood cells in urine. High false-positive rates of leukocyte esterase in the testing limit its effectiveness for the diagnosis of bacterial UTI.35 Nitrite was more likely to be positive in the bacteriuria group (19.3%), and it remained significant after controlling for the other variables in our study. The positive nitrite could be useful for ruling in patients who have bacteriuria, as it had high specificity (85–98%).35 However, its low sensitivity (45–60%) limits the use of negative nitrite for ruling out bacteriuria, as the majority of bacteriuria specimens were negative for nitrite.35
At least moderate numbers of bacteria on microscopy showed a significant association with UTI. However, this finding had a low sensitivity of 46.4%.14 We found that a moderate amount of bacteria was observed more in the candiduria group, while numerous bacteria were detected more in the bacteriuria group. Only numerous bacteria on microscopy was significantly associated with bacteriuria. Although the finding of yeast cells on microscopy may initially suggest candiduria,17 the degree of association has never been studied. Our results showed that the more yeast detected by microscopy, the more the likelihood of candiduria. Numerous yeast cells in particular perfectly predicted candiduria.
E. coli was the most common bacterium (38.3%). Candida was found in 13.7% of all positive urine cultures, similar to a previous report of 10–15% in hospitalized patients.15 Half of the urine cultures during the study period grew no organisms, comparable with a previous report.36 This might reflect the overuse of urine testing. Both bacteriuria and candiduria had a high proportion of positive urinalyses. Positive urinalysis could induce cognitive biases in favor of a bacterial UTI, even in patients with unfulfilled criteria of the recommended guideline.37 The perceived risk for bacterial UTI is higher in debilitated patients and patients with a urinary catheter given the difficulty of nonspecific symptoms and communication.7,16
No single parameter has good predictive ability to detect bacterial UTI. Score development using multiple components could improve the predictive ability. Clues in the form of a score might reduce the overdiagnosis of bacterial UTI. The c-index of our derivation cohort and validation cohort showed good diagnostic accuracy. However, our optimal cutoff score of ≥0 identified 76% and 54% of true bacteriuria in the derivation cohort and validation cohort, respectively. The NABY score is more useful for exclusion of bacteriuria, as most patients with a score ≤−3 points had candiduria in both cohorts. This score was greatly driven by the amount of yeast in the urine. Approximately 80% of no-growth urine had negative chemistry and sediment in the urine. The no-growth urine could be predicted by negative urinalysis, as in usual practice.
The NABY score provides an easy-to-use tool to identify patients with a low risk of bacteriuria, in whom empiric antibiotics can be avoided. This is done by focusing on recent antibiotic exposure in the last 30 days, nitrite, and the amount of bacteria and yeast in urinalysis. The security of having laboratory parameters to identify patients at low risk is likely to be helpful in avoiding empirical antibiotic treatment in the face of concern for bacterial UTI.
The step approach should start from pre-test probability of UTI. Common nonspecific cues such as delirium, weakness, falls, or any febrile illness must be carefully considered for alternate explanations, as these are guidelines-discordant cues for UTI.37 Urine tests should be ordered exclusively in patients with new urinary symptoms or no other plausible cause to explain clinical presentation in debilitated patients and catheterized patients.38 Urinalysis can be interpreted using NABY score to estimate the likelihood of bacteriuria and candiduria. If numerous yeast is reported or NABY score ≤−3, urine culture and antibiotics for bacterial UTI can be avoided. However, a routine activity across most healthcare settings is sending a urine test for many nonspecific reasons.38 The barriers against conforming to recommended guidelines might be improperly constructed clinicians’ mental models rather than lack of knowledge.37 Our study found pyuria had low predictive value for bacteriuria, similar to previous studies and guidelines.2,10,12,16 The influence of prior belief in pyuria and UTI perpetuate the guidelines-discordant cues,37 perhaps because of fear of missing an infection. There were 1.6% and 2.9% more empirical antibiotics for UTI in candiduria group than bacteriuria group in the derivation and validation cohorts, respectively. The no-growth urine group had empiric UTI treatment 10.9% in derivation cohort, and 23.1% in validation cohort. Although empirical treatment would be deemed appropriate for some bacteriuria which were true UTI, all candiduria and no-growth urine would not warrant the antibiotics for UTI. Early prediction to exclude bacteriuria might reduce overtreatment of presumed UTI. The NABY score can be integrated as a part of intervention to reduce unnecessary urine culture and antibiotic use, while working toward a sustainable practice change through effective system-level solutions.
Our study has several limitations. First, we could not include the entire data set in our analysis. Nevertheless, our sample size and random selection demonstrated the generalizability in the derivation and validation cohorts. Second, this study was conducted in a single center. The prevalence of candiduria varies depending on the hospital setting. The majority of candiduria occur in hospitalized patients, especially those in ICUs and those with an indwelling bladder catheter.15 Therefore, the NABY score is more advantageous in the inpatient setting than the outpatient setting. Third, the review of cases occurred retrospectively. There may be clinical data that informed decisions to initiate antibiotic that were unavailable in the medical records, along with missing records of the source of infection and indication for antibiotic. Consequently, our number of antibiotic prescriptions for UTI was likely to be underestimated. The results should be further validated in another cohort or prospective study.
We do not intend to put forward a proposition on the treatment of asymptomatic bacteriuria or candiduria. We continue to agree that treatment should be established based on clinical presentation, predisposing conditions, and guidelines, independently of this score.
Conclusion
Our study reiterates the proportion of bacteriuria and candiduria. Candiduria may be more frequent, depending on the healthcare setting and risk factors. Numerous yeast cells on urinalysis accurately predicted candiduria. Numerous yeast cells and the NABY score can be applied to identify patients with low risk of bacteriuria, in whom urine culture and empiric antibiotics for UTIs can be avoided.
Data Sharing Statement
Data can be made available through contact with the corresponding author.
Acknowledgments
We thank Rommanee Khositnithikul, PhD from Clinical Pathology Program, Faculty of Medicine Ramathibodi Hospital, Mahidol University for providing the information on urinalysis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schmiemann G, Kniehl E, Gebhardt K, Matejczyk MM, Hummers-Pradier E. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int. 2010;107:361–367. doi:10.3238/arztebl.2010.0361
2. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663. doi:10.1086/650482
3. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287:2701–2710. doi:10.1001/jama.287.20.2701
4. Sabih A, Leslie SW. Complicated urinary tract infections. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 [update August 12, 2021]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK436013/.
5. High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:149–171. doi:10.1086/595683
6. Juthani-Mehta M, Quagliarello VJ. Infectious diseases in the nursing home setting: challenges and opportunities for clinical investigation. Clin Infect Dis. 2010;51:931–936. doi:10.1086/656411
7. Rowe TA, Juthani-Mehta M. Urinary tract infection in older adults. Aging Health. 2013;9:519–528.
8. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–284. doi:10.1038/nrmicro3432
9. Price TK, Dune T, Hilt EE, et al. The clinical urine culture: enhanced techniques improve detection of clinically relevant microorganisms. J Clin Microbiol. 2016;54:1216–1222. doi:10.1128/JCM.00044-16
10. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75:53–58. doi:10.1016/0002-9343(83)90073-6
11. Stamm WE, Running K, McKevitt M, Counts GW, Turck M, Holmes KK. Treatment of the acute urethral syndrome. N Engl J Med. 1981;304:956–958. doi:10.1056/NEJM198104163041608
12. Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160:673–677. doi:10.1001/archinte.160.5.673
13. Simerville JA, Maxted WC, Pahira JJ. Urinalysis: a comprehensive review. Am Fam Physician. 2005;71:1153–1162.
14. Van Nostrand JD, Junkins AD, Bartholdi RK. Poor predictive ability of urinalysis and microscopic examination to detect urinary tract infection. Am J Clin Pathol. 2000;113:709–713. doi:10.1309/428N-60XK-UQ3Q-BXLC
15. Sobel JD, Fisher JF, Kauffman CA, Newman CA. Candida urinary tract infections—epidemiology. Clin Infect Dis. 2011;52(Suppl 6):S433–6. doi:10.1093/cid/cir109
16. Kauffman CA. Candiduria. Clin Infect Dis. 2005;41(Suppl 6):S371–6. doi:10.1086/430918
17. Kauffman CA, Fisher JF, Sobel JD, Newman CA. Candida urinary tract infections–diagnosis. Clin Infect Dis. 2011;52(Suppl 6):S452–6. doi:10.1093/cid/cir111
18. Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. doi:10.1097/00003246-199905000-00020
19. Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161–189. doi:10.1086/380796
20. Laan BJ, van Horrik T, Nanayakkara PWB, Geerlings SE. How many urinalysis and urine cultures are necessary? Eur J Intern Med. 2021;83:58–61. doi:10.1016/j.ejim.2020.08.013
21. Hartley S, Valley S, Kuhn L, et al. Inappropriate testing for urinary tract infection in hospitalized patients: an opportunity for improvement. Infect Control Hosp Epidemiol. 2013;34:1204–1207. doi:10.1086/673449
22. Yin P, Kiss A, Leis JA. Urinalysis orders among patients admitted to the general medicine service. JAMA Intern Med. 2015;175:1711–1713. doi:10.1001/jamainternmed.2015.4036
23. Petty LA, Vaughn VM, Flanders SA, et al. Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med. 2019;179:1519–1527. doi:10.1001/jamainternmed.2019.2871
24. Lammers RL, Gibson S, Kovacs D, Sears W, Strachan G. Comparison of test characteristics of urine dipstick and urinalysis at various test cutoff points. Ann Emerg Med. 2001;38:505–512. doi:10.1067/mem.2001.119427
25. Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122–9. doi:10.1038/nm1145
26. Gajdacs M, Abrok M, Lazar A, Burian K. Urinary tract infections in elderly patients: a 10-year study on their epidemiology and antibiotic resistance based on the WHO Access, Watch, Reserve (AWaRe) Classification. Antibiotics (Basel). 2021;10(9):1098. doi:10.3390/antibiotics10091098
27. Cobbaert CM, Arslan F, Caballe Martin I, et al. Automated urinalysis combining physicochemical analysis, on-board centrifugation, and digital imaging in one system: a multicenter performance evaluation of the cobas 6500 urine work area. Pract Lab Med. 2019;17:e00139. doi:10.1016/j.plabm.2019.e00139
28. van der Zwet WC, Hessels J, Canbolat F, Deckers MM. Evaluation of the Sysmex UF-1000i(R) urine flow cytometer in the diagnostic work-up of suspected urinary tract infection in a Dutch general hospital. Clin Chem Lab Med. 2010;48:1765–1771. doi:10.1515/CCLM.2010.342
29. Roxe DM. Urinalysis. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations.
30. Han K, Song K, Choi BW. How to develop, validate, and compare clinical prediction models involving radiological parameters: study design and statistical methods. Korean J Radiol. 2016;17:339–350. doi:10.3348/kjr.2016.17.3.339
31. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi:10.1016/S0895-4356(96)00236-3
32. Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML. Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol. 2016;79:22–28. doi:10.1016/j.jclinepi.2016.03.031
33. Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. The National Institute for Allergy and Infectious Diseases (NIAID) Mycoses Study Group. Clin Infect Dis. 2000;30:14–18. doi:10.1086/313583
34. Singla N, Gulati N, Kaistha N, Chander J. Candida colonization in urine samples of ICU patients: determination of etiology, antifungal susceptibility testing and evaluation of associated risk factors. Mycopathologia. 2012;174:149–155.
35. Deville WL, Yzermans JC, van Duijn NP, et al. The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. BMC Urol. 2004;4:4. doi:10.1186/1471-2490-4-4
36. Silver SA, Baillie L, Simor AE. Positive urine cultures: a major cause of inappropriate antimicrobial use in hospitals? Can J Infect Dis Med Microbiol. 2009;20:107–111. doi:10.1155/2009/702545
37. Trautner BW, Bhimani RD, Amspoker AB, et al. Development and validation of an algorithm to recalibrate mental models and reduce diagnostic errors associated with catheter-associated bacteriuria. BMC Med Inform Decis Mak. 2013;13:48. doi:10.1186/1472-6947-13-48
38. Leis JA, Soong C. De-adoption of routine urine culture testing-A call to action. JAMA Intern Med. 2019;179:1466–1468. doi:10.1001/jamainternmed.2019.4515
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
