Back to Journals » Clinical Interventions in Aging » Volume 18
Prediction of Outcomes Through Cystatin C and cTnI in Elderly Type 2 Myocardial Infarction Patients
Received 6 May 2023
Accepted for publication 26 July 2023
Published 25 August 2023 Volume 2023:18 Pages 1415—1422
DOI https://doi.org/10.2147/CIA.S416372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nandu Goswami
Jinling Ma, Suyan Bian, Meng Gao
Department of Geriatric Cardiology, the Second Medical Center and National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China
Correspondence: Jinling Ma, Department of Geriatric Cardiology, the Second Medical Center and National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, No. 28, Fuxing Road, Haidian District, Beijing, 100853, People’s Republic of China, Email [email protected]
Background: Chronic kidney disease (CKD) and coronary artery disease (CAD) are strongly associated. Cystatin C (Cys C) is a more sensitive marker of early renal insufficiency. This study aimed to evaluate the prognostic implications of combined of Cys C and cardiac troponin I (cTnI) on 90-day outcomes in elderly patients with type 2 myocardial infarction (MI).
Methods: The data of consecutive type 2 MI patients aged 80 years and older who received Cys C and cTnI measurements within 24 h of admission were retrospectively reviewed. The endpoint was a 90-day all-cause and cardiac mortality.
Results: A total of 4326 patients were included. During the 90-day follow-up period, a higher all-cause and cardiac mortality was observed in patients with Cys C ≥ 1.49mg/L than in patients with Cys C < 1.49 mg/L (P < 0.001). After the multivariate logistic regression adjustments, the higher CysC and cTnI levels remained independent predictors of the 90-day all-cause mortality and cardiac mortality. Moreover, the Kaplan–Meier all-cause and cardiac mortality event-free survival curves showed that the patients with the presence of elevated levels of both Cys C and cTnI had a significantly increased risk than those with Cys C or cTnI alone.
Conclusion: Elevated Cys C level is an independent risk factor for all-cause and cardiac mortality in the elderly type 2 MI population. The predictive ability of the combined use of Cys C and cTnI in elderly type 2 MI patients is stronger than that of Cys C or cTnI alone.
Keywords: myocardial infarction, cystatin C, elderly
Introduction
Chronic kidney disease (CKD) and coronary artery disease (CAD) are strongly associated.1 CKD is a well-known risk factor for the development and progression of CAD,2,3 and adversely affects outcomes in patients with CAD.4–9 Low estimated glomerular filtration rate (eGFR) is associated with an increasing risk of all-cause mortality in patients with CAD.10–12 CKD is an important independent risk factor for cardiovascular events.13,14 Even a minor reduction in eGFR is associated with increased cardiovascular morbidity and mortality.15,16 CKD confers a high risk for poor cardiovascular events with 50% of all patients of CKD stage 4/5 experiencing cardiovascular disease. Moreover, cardiovascular mortality accounts for 40% to 50% of all deaths in patients of advanced CKD stages 4 and 5.17
Cystatin C (Cys C) is a more sensitive marker of early renal insufficiency.18 A significant association exists between elevated Cys C and the risk of cardiovascular events or mortality in heterogeneous populations with normal renal function.19 In addition, Cys C has been shown to be a strong predictor of major adverse cardiovascular events and all-cause mortality,20–22 and the association between high levels of Cys C and any cause-related mortality is age-independent.18 Age is an established risk factor for both CKD and type 2 myocardial infarction (MI), especially in elderly patients. Type 2 MI is a heterogeneous entity with varying etiologies and triggers.23 Patients with type 2 MI were markedly older with more frequent comorbidities.24,25 The levels of cardiac troponin I (cTnI) were significantly associated with the severity of CAD in patients with CKD.26
Type 2 MI is associated with CKD, and aging contributes to its incidence and poor prognosis. Accordingly, it is of clinical significance to understand the clinical features and risk factors for 90-day all-cause and cardiac mortality in type 2 MI patients. This study aimed to evaluate the prognostic implications of the combined use of Cys C and cTnI on 90-day outcomes in patients aged over 80 years old with type 2 MI.
Methods
Study Population
The present work was an observational and retrospective cohort study. Among the consecutive patients aged 80 years and older with a diagnosis of type 2 MI at Chinese PLA General Hospital between December 2010 and December 2021, only those patients whose serum Cys C and cTnI levels were available based on clinical indication within 24 h of admission were included. The clinical and demographic data were collected from related electronic medical records. The age-adjusted Charlson comorbidity index (ACCI) score27 was calculated to assess the severity of comorbidity. The echocardiographic variables were obtained during the first days of hospitalization by transthoracic echocardiography.
cTnI and Cys C Testing
cTnI was measured with electrochemiluminescence immunoassay on a Dimension Vista 500 Intelligent Laboratory System. An increased level of cTnI was defined as ≥0.07 ng/mL.
The serum Cys C levels were measured using a nephelometry assay. An increased level of Cys C was defined as ≥1.49mg/L.
Diagnosis of Type 2 MI
Type 2 MI was defined in accordance with the Fourth Universal Definition of MI as the presence of myocardial necrosis detected by elevated cTnI, which is a result of the imbalance between myocardial oxygen supply and demand in the presence or absence of a fixed atherosclerotic coronary disease.28 Two independent cardiologists reviewed all available medical records and decided the final adjudication of type 2 MI, with discordant diagnoses resolved by a third cardiologist. Patients with end-stage kidney failure who were receiving long-term dialysis were excluded. Patients with Takotsubo cardiomyopathy, type 3, 4, or 5 MI, or whose complete data were not available from the electronic medical record were also excluded from the analysis.
Clinical Outcomes
The primary endpoint in this study was the 90-day all-cause mortality, and the secondary endpoint was cardiac mortality in type 2 MI elderly patients. The follow-up data were obtained from medical records or by telephone interview. Cardiac death included deaths caused by myocardial infarction, heart failure, or significant arrhythmias. Sudden unexpected death without another explanation was also considered cardiac death.
Statistical Analysis
Normally distributed continuous variables were expressed as the mean ± standard deviation. Categorical variables were expressed as frequencies and percentages. Categorical variables were compared using χ2 tests. All variables with P < 0.05 in the univariate analysis were considered in the multivariate analysis. Multivariate logistic regression analysis was used to predict the prevalence of the 90-day all-cause and cardiac mortality with adjustments for age, ACCI, and co-morbidities. The results were presented as adjusted odds ratios (OR) and their 95% confidence interval (CI). The survival curves were estimated by the Kaplan–Meier analysis and compared using the Log rank test. The event-time curve was separated into three curves according to the Cys C and cTnI. All data were processed using SPSS 20 (IBM SPSS Statistics, Armonk, New York, USA). P< 0.05 was considered statistically significant.
Results
Baseline Clinical Characteristics
A total of 4326 were included in this study. The mean age of the subjects was 88.51 ± 5.26 years, and men comprised 71.3% of the group. According to the serum Cys C level on admission, the population was divided into two groups: Cys C ≥ 1.49mg/L (n = 2385, 55.1%) and Cys C < 1.49 mg/L (n = 1941, 44.9%).
Overall All-Cause and Cardiac Mortality
During the 90-day follow-up period, a higher all-cause mortality was observed in patients with Cys C ≥ 1.49mg/L than in patients with Cys C < 1.49 mg/L (P <0.001 Table 1). Moreover, the cardiac mortality was higher in patients with Cys C ≥ 1.49mg/L than in patients with Cys C < 1.49 mg/L (P <0.001 Table 1).
 |
Table 1 Clinical Outcomes According to Cys C During the 90-Day Follow-Up Period |
Factors Associated with 90-Day All-Cause and Cardiac Mortality of Type 2 MI
The baseline characteristics of the study population according to the occurrence of 90-day all-cause mortality are presented in Table 2. In comparison with the survivors, non-survivors were older and had significantly higher Cys C, cTnI levels ≥ 1.4 ng/mL, prevalence of congestive heart failure NYHA III/IV, hemoglobin < 80 g/L, as well as higher ACCI score (Table 2). However, survivors and non-survivors showed no differences in terms of gender, BMI, history of hypertension, previous MI, atrial fibrillation, dyslipidemia, DM, peripheral vascular disease, or left ventricular ejection fraction (LVEF). Furthermore, the Cys C and cTnI levels were significantly higher in the individuals that experienced cardiac death than in those who did not (Table 3). After multivariate logistic regression adjustments, the higher CysC and cTnI levels remained independent predictors of the 90-day all-cause and cardiac mortality (Table 4).
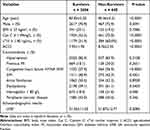 |
Table 2 Characteristics of Type 2 MI Based on the Occurrence of 90-Day Survival |
 |
Table 3 Characteristics of Type 2 MI Based on the Occurrence of 90-Day Cardiac Survival |
 |
Table 4 Risk Factors Associated with 90-Day Mortality in a Multivariable Logistic Regression Analysis |
The Kaplan–Meier all-cause mortality event-free survival curves showed that the patients with the presence of elevated levels of both Cys C and cTnI had a significantly increased risk than Cys C or cTnI alone (P < 0.001) (Figure 1). Moreover, the Kaplan–Meier cardiac death event-free survival curves showed that the patients with the presence of elevated levels of both Cys C and cTnI had a significantly higher incidence of cardiac death than Cys C or cTnI alone (P < 0.001) (Figure 2).
 |
Figure 1 Kaplan–Meier all-cause mortality event-free survival curves according to the Cys C and cTnI levels. Abbreviations: Cys C, Cystatin C; cTnI, cardiac troponin I. |
 |
Figure 2 Kaplan–Meier cardiac mortality event-free survival curves according to the Cys C and cTnI levels. Abbreviations: Cys C, Cystatin C; cTnI, cardiac troponin I. |
Discussion
The results of the present real-world study demonstrate that elevated Cys C levels were associated with increased risk of cardiac death and all-cause mortality in elderly patients with type 2 MI during the 90-day follow-up period. The combined use of Cys C and cTnI also provided incremental prognostic information that enhanced the prediction of increased risk of cardiac death and all-cause mortality. The predictive ability of the combined use of Cys C and cTnI in elderly type 2 MI patients is stronger than that of Cys C alone. Simultaneous assessment of the two biomarkers would be more useful in risk stratification than using either biomarker separately.
CAD and CKD are risk factors for each other. Elderly individuals are more likely to be complicated with CAD and CKD.29 Renal dysfunction has become a cardiovascular risk factor of equal importance as diabetes mellitus.30 The results of another study strengthened the role of kidney disease as a risk multiplier for cardiovascular and all-cause mortality and highlighted the need to prevent its onset and progression.13 Moreover, patients with renal impairment more frequently presented with multivessel disease and calcified lesions.5 Elevated cTnI concentrations were 3-fold more common in patients with renal impairment than in those with normal renal function.31 In patients with impaired renal function, blood levels of hsTnI were significantly associated with the severity of stable CAD.26 Bidirectional interactions exist between heart disease and kidney disease.32 The potential mechanisms to explain the relationship may be complex and multifactorial. Several mechanistic pathways may contribute to the observed worsening of outcomes, including sharing risk factors (such as hypertension, diabetes and hyperlipidemia) and some kidney-related risk factors.33 Another possible mechanism is related to endothelial dysfunction, cardiac remodeling, vascular calcification,34 neuro-hormonal activation, inflammatory cascade activation,35 renal venous hypertension and elevated intra-abdominal pressures.36 Furthermore, the poorer prognosis is possibly explained by more prevalent comorbidity, age, more frequent high-risk presentations of acute coronary syndrome, lower rates of complete revascularization, and underutilization of guideline-recommended therapies. A combination of these factors might explain the influence on adverse clinical outcomes.
In the present study, the combined use of Cys C and cTnI further improves the predictive accuracy. Accurate screening and assessment of the risk factors for the 90-day outcomes play a significant role in type 2MI patients, especially for very old patients. CKD overlapped with several conditions and showed a significant association with decreased clearance of cardiac troponins.37 There may be an elevated cTnI level due to CKD. We identified 1.40 ng/mL (2×0.07) as the optimal cut-off cTnI value. This value was in accordance to the basis of our experience. This result appears to be different from other reports. Such a situation can be attributed to differences in the prevalence of comorbidities, especially age. Moreover, the patients with concomitant renal impairment and type 2 MI are less likely to receive treatment in clinical practice, which may hamper the implementation to optimal treatment with early coronary intervention, revascularization or drugs known to favorably impact cardiovascular and renal outcome. In addition, elderly patients are underrepresented in many clinical trials, resulting in sparsity of evidence-based therapies. Further studies are needed to explore the mechanism linking Cys C among type 2 MI patients to improve the care of these patients.
This study has several limitations that should be considered. First, this work was a non-randomized, single-center retrospective observational study, which may have certain selection bias and confounding factors. Unmeasured confounders and selection bias might have affected the results. Second, the determination of CKD stage was based on a single Cys C measurement and measured only once. The other markers of kidney damage, such as albuminuria or eGFR, were not available in our study. Third, the evaluation of Cys C and cTnI levels was only measured once at admission without serial measurements. The circulating Cys C and cTnI levels may significantly change over time, particularly in patients with unstable hemodynamics and varying medical therapy. Nevertheless, these dynamic changes were not taken into account. The effects should be further investigated. Fourth, no data were available on medications taken. Consequently, the influence of drugs on Cys C and cTnI levels were not analyzed. This will lead to the credibility of the results. The decision to select medications was up to the discretion of the cardiologist, which led to a bias in the analysis. Fifth, the indices of left ventricular diastolic function were not comprehensively measured. Sixth, only elderly patients were included in this study. Accordingly, the present findings may not be extrapolated to the general population or other patients with specific diseases. Finally, it is likely there were still many important biomarkers for risk were not included in the analyses, such as N-terminal pro-B-type natriuretic peptide, cytokine levels, frailty, the use of glucocorticoid, or thyroid dysfunctions which could have affected Cys C level, making it challenging to establish the optimal cut-off value for prognosis prediction. Despite these limitations, the main strength of our study was the large cohort of patients aged over 80 years. Our findings should be confirmed through a long-term observational study, with medicines included.
Conclusion
In conclusion, the present study found that elevated Cys C level is an independent risk factor for all-cause and cardiac mortality in the elderly type 2 MI population. The combined effects of Cys C and cTnI on the 90-day outcomes appear to be worse than that of Cys C or cTnI alone. Further multi-center prospective studies are needed to provide more clinical evidence.
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki, and ethical approval was obtained from Chinese PLA General Hospital (No. S2021–096-01). This study was retrospective and did not require written informed consent. Patient data were kept confidential in this study.
Funding
Research reported in this article was supported by Public Service Platform Project of Industrial Technology Foundation, China under grant number 2020-0103-3-1-2. The funding organization had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Engelbertz C, Reinecke H, Breithardt G, et al. Two-year outcome and risk factors for mortality in patients with coronary artery disease and renal failure: the prospective, observational CAD-REF Registry. Int J Cardiol. 2017;243:65–72. doi:10.1016/j.ijcard.2017.05.022
2. Holzmann MJ, Siddiqui AJ. Outcome of percutaneous coronary intervention during non-ST-segment-elevation myocardial infarction in elderly patients with chronic kidney disease. J Am Heart Assoc. 2020;9(12):e015084. doi:10.1161/JAHA.119.015084
3. Ram E, Beckerman P, Segev A, et al. The predictive value of creatinine clearance for mortality in patients undergoing revascularization. J Cardiothorac Surg. 2021;16(1):120. doi:10.1186/s13019-021-01502-1
4. Watanabe Y, Mitomo S, Naganuma T, et al. Impact of chronic kidney disease in patients with diabetes mellitus after percutaneous coronary intervention for left main distal bifurcation (from the Milan and new-Tokyo (MITO) registry). Am J Cardiol. 2021;138:33–39. doi:10.1016/j.amjcard.2020.10.021
5. Stähli BE, Gebhard C, Gick M, et al. Outcomes after percutaneous coronary intervention for chronic total occlusion according to baseline renal function. Clin Res Cardiol. 2018;107(3):259–267. doi:10.1007/s00392-017-1179-x
6. Lima EG, Charytan DM, Hueb W, et al. Long-term outcomes of patients with stable coronary disease and chronic kidney dysfunction: 10-year follow-up of the medicine, angioplasty, or surgery study II trial. Nephrol Dial Transplant. 2020;35(8):1369–1376. doi:10.1093/ndt/gfy379
7. Thomas B, Matsushita K, Abate KH, et al. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28(7):2167–2179. doi:10.1681/ASN.2016050562
8. Jamthikar AD, Puvvula A, Gupta D, et al. Cardiovascular disease and stroke risk assessment in patients with chronic kidney disease using integration of estimated glomerular filtration rate, ultrasonic image phenotypes, and artificial intelligence: a narrative review. Int Angiol. 2021;40(2):150–164. doi:10.23736/S0392-9590.20.04538-1
9. Peng Y, Du X, Li X, et al. Association of renal insufficiency with treatments and outcomes in patients with acute coronary syndrome in China. Int J Cardiol. 2021;323:7–12. doi:10.1016/j.ijcard.2020.08.022
10. Amdur RL, Feldman HI, Dominic EA, et al. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings from the CRIC study. Am J Kidney Dis. 2019;73(3):344–353. doi:10.1053/j.ajkd.2018.09.012
11. Lees JS, Welsh CE, Celis-Morales CA, et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med. 2019;25(11):1753–1760. doi:10.1038/s41591-019-0627-8
12. De Luca L, Cappadona F, Temporelli PL, et al. Impact of eGFR rate on 1-year all-cause mortality in patients with stable coronary artery disease. Eur J Intern Med. 2022;101:98–105. doi:10.1016/j.ejim.2022.04.021
13. Piscitelli P, D’Errico MM, Mirijello A, et al. Low GFR amplifies the association between coronary three-vessel disease and all-cause mortality. Nutr Metab Cardiovasc Dis. 2022;32(2):402–409. doi:10.1016/j.numecd.2021.09.036
14. Lee HF, Cheng YW, Peng JR, et al. Impact of chronic kidney disease on long-term outcomes for coronary in-stent restenosis after drug-coated balloon angioplasty. J Cardiol. 2021;78(6):564–570.
15. Bikbov B, Purcell CA, Levey AS; GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–733. doi:10.1016/S0140-6736(20)30045-3
16. Benzing T, Salant D. Insights into glomerular filtration and albuminuria. N Engl J Med. 2021;384(15):1437–1446. doi:10.1056/NEJMra1808786
17. Schuett K, Marx N, Lehrke M. The cardio-kidney patient: epidemiology, clinical characteristics and therapy. Circ Res. 2023;132(8):902–914. doi:10.1161/CIRCRESAHA.122.321748
18. Kim TJ, Kang MK, Jeong HG, et al. Cystatin C is a useful predictor of early neurological deterioration following ischaemic stroke in elderly patients with normal renal function. Eur Stroke J. 2017;2(1):23–30. doi:10.1177/2396987316677197
19. Einwoegerer CF, Domingueti CP. Association between increased levels of cystatin C and the development of cardiovascular events or mortality: a systematic review and meta-analysis. Arq Bras Cardiol. 2018;111(6):796–807. doi:10.5935/abc.20180171
20. Lou B, Luo Y, Zhang H, et al. Association between cystatin C and cardiac function in acute myocardial infarction patients: a real-world analysis. Dis Markers. 2022;2022:7267937. doi:10.1155/2022/7267937
21. Correa S, Morrow DA, Braunwald E, et al. Cystatin C for risk stratification in patients after an acute coronary syndrome. J Am Heart Assoc. 2018;7(20):e009077. doi:10.1161/JAHA.118.009077
22. Jin S, Xu J, Shen G, Gu P. Predictive value of circulating cystatin C level in patients with acute coronary syndrome: a meta-analysis. Scand J Clin Lab Invest. 2021;81(1):1–7. doi:10.1080/00365513.2020.1846212
23. Sandoval Y, Jaffe AS. Type 2 myocardial infarction: JACC review topic of the week. J Am Coll Cardiol. 2019;73(14):1846–1860. doi:10.1016/j.jacc.2019.02.018
24. Singh A, Gupta A, DeFilippis EM, et al. Cardiovascular mortality after type 1 and type 2 myocardial infarction in young adults. J Am Coll Cardiol. 2020;75(9):1003–1013. doi:10.1016/j.jacc.2019.12.052
25. McCarthy CP, Kolte D, Kennedy KF, Vaduganathan M, Wasfy JH, Januzzi JL. Patient characteristics and clinical outcomes of type 1 versus type 2 myocardial infarction. J Am Coll Cardiol. 2021;77(7):848–857. doi:10.1016/j.jacc.2020.12.034
26. Brunner FJ, Kröger F, Blaum C, et al. Association of high-sensitivity troponin T and I with the severity of stable coronary artery disease in patients with chronic kidney disease. Atherosclerosis. 2020;313:81–87. doi:10.1016/j.atherosclerosis.2020.09.024
27. Zhang XM, Wu XJ, Cao J, et al. Effect of the age-adjusted Charlson comorbidity index on all-cause mortality and readmission in older surgical patients: a national multicenter, prospective cohort study. Front Med. 2022;9:896451. doi:10.3389/fmed.2022.896451
28. Nestelberger T, Boeddinghaus J, Badertscher P, et al. Effect of definition on incidence and prognosis of type 2 myocardial infarction. J Am Coll Cardiol. 2017;70(13):1558–1568. doi:10.1016/j.jacc.2017.07.774
29. Zhang Y, Zhai G, Wang J, Zhou Y. Risk factors of cardiac death for elderly patients with severe chronic kidney disease after percutaneous coronary intervention. Clin Appl Thromb Hemost. 2022;28:10760296221081848. doi:10.1177/10760296221081848
30. Panayiotou AG, Spaak J, Kalani M. Kidney function is associated with short-term, mid-term and long-term clinical outcome after coronary angiography and intervention. Acta Cardiol. 2018;73(4):362–369. doi:10.1080/00015385.2017.1395546
31. Gallacher PJ, Miller-Hodges E, Shah ASV, et al. High-sensitivity cardiac troponin and the diagnosis of myocardial infarction in patients with kidney impairment. Kidney Int. 2022;102(1):149–159. doi:10.1016/j.kint.2022.02.019
32. Kang J, Park JJ, Cho YJ, et al. Predictors and prognostic value of worsening renal function during admission in HFpEF versus HFrEF: data from the KorAHF (Korean Acute Heart Failure) registry. J Am Heart Assoc. 2018;7(6):e007910. doi:10.1161/JAHA.117.007910
33. Duran M, Elcik D, Inanc MT, et al. Relationship between mild renal dysfunction and coronary artery disease in young patients with stable angina pectoris. Biomark Med. 2020;14(6):433–440. doi:10.2217/bmm-2019-0319
34. Ataklte F, Song RJ, Upadhyay A, Musa Yola I, Vasan RS, Xanthakis V. Association of mildly reduced kidney function with cardiovascular disease: the Framingham heart study. J Am Heart Assoc. 2021;10(16):e020301. doi:10.1161/JAHA.120.020301
35. Batra G, Ghukasyan Lakic T, Lindbäck J, et al. Interleukin 6 and cardiovascular outcomes in patients with chronic kidney disease and chronic coronary syndrome. JAMA Cardiol. 2021;6(12):1440–1445. doi:10.1001/jamacardio.2021.3079
36. Toroghi HM, Lo KB, Ziccardi MR, et al. Renal implications of pulmonary arterial capacitance in acute heart failure with preserved ejection fraction. Rev Cardiovasc Med. 2019;20(4):267–272.
37. Mohamed MO, Contractor T, Abramov D, et al. Sex-based differences in prevalence and outcomes of common acute conditions associated with type 2 myocardial infarction. Am J Cardiol. 2021;147:8–15. doi:10.1016/j.amjcard.2021.02.011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
