Back to Journals » Clinical Ophthalmology » Volume 13
Predictability of different calculators in the minimization of postoperative astigmatism after implantation of a toric intraocular lens
Authors Ribeiro FJ , Ferreira TB , Relha C, Esteves C, Gaspar S
Received 23 April 2019
Accepted for publication 1 August 2019
Published 29 August 2019 Volume 2019:13 Pages 1649—1656
DOI https://doi.org/10.2147/OPTH.S213132
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Filomena J Ribeiro,1,2 Tiago B Ferreira,1,3 Catarina Relha,1 Carina Esteves,1 Sylvia Gaspar1
1Ophthalmology Department, Luz Hospital, Lisbon, Portugal; 2Faculdade De Medicina Da Universidade De Lisboa, Lisbon, Portugal; 3NOVA Medical School, Lisbon, Portugal
Correspondence: Filomena J Ribeiro
Luz Hospital, Avenida Lusíada 100, Lisbon 1500-650, Portugal
Tel +351 21 710 4400
Email [email protected]
Purpose: To assess the efficacy of five calculators for toric intraocular lenses (IOL).
Methods: Retrospective comparative case series in cataract patients undergoing implantation of trifocal toric IOLs (PhysIOL FineVision POD FT). Inclusion criteria were age-related cataract and a corneal astigmatism between 0.90D and 4.50D. Refractive astigmatism predictability of five different toric calculators or calculation methods were compared. Furthermore, two groups were differentiated according to the type of astigmatism. The mean absolute error and the centroid errors in the predicted residual astigmatism from each calculator were evaluated.
Results: Fifty-one eyes of 43 patients were included in the study. For the standard toric calculator using anterior keratometry values only, the centroid prediction error was 0.39D±0.41@166º, which was reduced by the application of the PhysIOL toric calculator that includes the Abulafia-Koch regression formula and adjustment for the effective lens position (0.05D±0.34@167º), and also by the application of the Barrett toric calculator (0.07D±0.28@160º). Regarding the techniques that directly evaluate posterior corneal surface, the Holladay toric calculator, using total corneal power provided by a color-LED topographer, generated better results (0.10D±0.44@156º) than those using Scheimpflug camera data (0.23D±0.56@158º). Similar results were found for both types of astigmatism.
Conclusion: The PhysIOL and the Barrett toric calculators taking into account the posterior corneal astigmatism by mathematical models, yielded lower astigmatic prediction errors compared to a standard toric calculator based on anterior keratometry data only. When total corneal power measurements were used, prediction errors were lower with color-LED than with Scheimpflug based topography.
Keywords: astigmatism, toric IOL, toric IOL calculation
Introduction
Recent advances in microsurgery and the latest developments in intraocular lenses (IOLs) have allowed surgeons to achieve more accurate and predictable postoperative refractive results.1 In fact, nowadays, the main aim of cataract surgery is to obtain postoperative spectacle-independence – at least for distance – with the maximum visual quality. A recent study of 13,012 eyes of 6,506 cataract patients showed that 43.5% of eyes had a corneal astigmatism ≥1.00 diopters (D), which is enough to significantly affect postoperative vision.2 In agreement with this, a safe and predictable compensation for pre-existing corneal astigmatism has become one of the main challenges of eye surgeons over the last years. Since Shimizu et al developed the first model of a toric IOL,3 the efficacy of this method in the compensation of preoperative corneal astigmatism compared to incisional techniques has been demonstrated.4–6 Crystalline lens exchange using toric IOLs does not require significant changes in the classical phacoemulsification surgical technique. Also, it permits the compensation of high degrees of corneal astigmatism. However, several limitations still remain, specifically related to the risk of postoperative IOL rotation and intraoperative misalignment,7 the accurate measurement of preoperative astigmatism, and IOL power calculation to minimize postoperative refractive astigmatism.8–10
The purpose of this study was to evaluate the predictability of postoperative refraction through the assessment of the prediction error in residual astigmatism after implantation of a multifocal toric IOL (PhysIOL FineVision POD FT) using five calculation methods based on different measurement strategies.
Patients and methods
Patients
This was a retrospective, non-randomized, interventional case series. Inclusion criteria comprised diagnosis of age-related cataract and a corneal astigmatism equal or higher than 0.9D and up to 4.50D. Patients were excluded if they presented a relevant ophthalmic condition that might impair the surgical procedure or negatively affect its outcome, such as pseudoexfoliation syndrome, corneal pathologies or retinal pathologies (diabetic maculopathy, myopic maculopathy, age-related macular degeneration (AMD), etc.). Further exclusion criteria were previous ocular surgeries that could affect the capsular bag stability, irregular astigmatism, topographic abnormalities, use of contact lenses 2 weeks prior to the corneal topography assessment, systemic diseases with potential impact on visual outcomes, and an expected postoperative cylinder equal or higher than 0.50 D. All patients were informed regarding the study and signed an informed consent to undergo the clinical examinations in accordance with the tenets of the Declaration of Helsinki. The study protocol and informed consent from were approved by Hospital da Luz Clinical Investigation Ethics Committee.
Methods
Surgery
Topical anesthesia was administered in all cases. All surgeries were performed by two surgeons (F.R. and T.F.). With the patient seated to prevent cyclotorsion, the previously calculated IOL implantation axis was marked using a Robomarker device (Surgilum, Wilmington, NC, USA). A clear corneal temporal microincision of 2.2 mm was made with a disposable knife. After crystalline lens removal with microcoaxial phacoemulsification, the IOL was implanted through the main incision using the Accuject injector (Medicel AG, Altenrhein, Switzerland). The viscoelastic material was then meticulously removed, including behind the IOL, using an irrigation-aspiration system. Finally, the IOL was rotated to its final position by aligning the corneal marks with the reference marks in the IOL.
Intraocular lens
The FineVision POD FT is a single-piece trifocal, aspheric and diffractive IOL made of a hydrophilic acrylate. The diffractive pattern provides 2 effective additions for near and intermediate distance, +3.50 D in the first diffractive order and +1.75 D in the second diffractive order. The refractive index is 1.46, the total diameter of the IOL is 11.40 mm and the optical zone diameter is 6.0 mm. The IOL consists of double-C-loop haptics to reduce rotation of the lens and the cylindrical power at the IOL plane is available from +1.00 to +6.00 D. The manufacturer-labelled A-constant is 118.95.
Clinical protocol
Before surgery, all patients underwent a comprehensive preoperative ophthalmological examination that included manifest refraction, measurement of monocular uncorrected distance (UDVA) and best-corrected distance visual acuity (CDVA), biometry and keratometry (Lenstar LS900, Haag-Streit AG, Koenitz, Switzerland), slit-lamp examination, Scheimpflug imaging-based topography (Pentacam HR, Oculus, Wetzlar, Germany), color-LED topography (Cassini, I-optics, Den Haag, The Netherlands), Goldmann applanation tonometry, and dilated fundoscopy. The power calculation of the toric IOL to achieve emmetropia was performed using the SRK/T formula when the axial length (AL) was >22 mm and the Hoffer-Q formula when this value was ≤22 mm. The IOL cylindrical power was calculated with a standard toric calculator based on anterior keratometry values. The used toric calculator does not take into account the posterior curvature of the cornea, nor the individual anterior chamber depth or axial length. It was the standard toric calculator provided by PhysIOL until March 2017 before new regression formula (eg by Dr. Barrett or Dr. Abulafia and Dr. Koch) were integrated into the latest toric standard calculators.
Patients were evaluated at least 3 months postoperatively with a mean ± standard deviation (SD) follow-up time of 9.01 months ±7.05 (range 3–18 months). At the last follow-up visit, patients were examined using the preoperative protocol. Refraction was evaluated by the same examiner using the cross-cylinder method and pupils were dilated to assess the IOL axis and potential IOL tilt or decentration by means of slit-lamp examination and photography according to a previously published method.11 Lenstar keratometry, Scheimpflug and color-LED topography were repeated. For the 3 methods, 3 consecutive readings were performed to improve repeatability and an average of the magnitude and axis of the 3 readings was used for calculations. The Pentacam values used were the total corneal refractive power (TCRP) including the anterior and posterior surfaces within the central 4 mm. Also, the Cassini device was used to assess total corneal astigmatism (TCA). The calculation of the IOL cylinder power was repeated with five different methods: 1) the standard toric calculator, 2) the latest PhysIOL calculator that includes the Abulafia-Koch formula and the adjustment of effective lens position (ELP) according to the Holladay 1 formula with the Wang-Koch correction for eyes with AL over 25 mm (https://physioltoric.eu/),12 3) the Barrett toric calculator (http://ascrs.org/barrett-toric-calculator), 4) the Holladay’s IOL consultant toric calculator using the TCRP-values provided by the Scheimpflug camera, and 5) the Holladay calculator using the TCA values from the color-LED topography.
To take the ELP and the spherical equivalent power of the IOL into account, as the other calculators do, the Scheimpflug camera and the color-LED topography values were used in accordance with Holladay’s IOL consultant toric calculator. To avoid any effects of surgically induced astigmatism or IOL misalignment, the postoperative keratometry readings, total corneal astigmatism values, and measured IOL alignment axis were used.
Prediction error
The prediction error was calculated for each calculation method as the difference between postoperative manifest refraction (corrected for the corneal plane) and predicted residual astigmatism as described previously.13,14 Predicted residual astigmatism was calculated as:
Predicted residual astigmatism = Toric IOL cylindrical power (corneal plane) + Corneal astigmatism (derived from measured keratometry or total corneal astigmatism, if applicable).
The error in predicted residual astigmatism was calculated as:
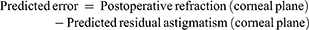
Vector analysis was performed in all calculations and mean absolute error (MAE) and centroid error in predicted residual astigmatism (PRA) were calculated.
Eyes were further divided into three groups: a “with-the-rule” (WTR) group if the keratometric steep meridian was oriented between 60 and 120 degrees; an “against-the-rule” (ATR) group if the steep meridian was oriented between 0 and 30 degrees, or 150 and 180 degrees; and an “oblique” (OB) group if the steep meridian was between 31 and 59 degrees, or 121 and 149 degrees.
Statistical analysis
Data analysis was performed using the software SPSS for Windows version 19.0 (IBM, Armonk, NY, USA). Normality of variables was evaluated by the Kolmogorov-Smirnov test. The distribution was normal for all variables. Comparisons of each method against the standard toric calculator were performed using a paired samples t-test. Centroid SDs were calculated in accordance with the method described by Holladay et al13. Statistical significance was set at p<0.05.
Results
The study involved a sample of 51 eyes of 43 patients with a mean age of 68.0 years ±8.0 (SD) (range 50–82 years). Nineteen patients were male (44.2%) and 24 were female (55.8%). Twenty-seven eyes had with-the-rule astigmatism (WTR) and 16 eyes against-the-rule astigmatism (ATR). Eight eyes showed oblique astigmatism. Due to the low number of eyes in this group, only eyes showing WTR or ATR group were included in the final analysis.
Biometric data and IOL power calculations
Mean AL in the overall sample was 23.40 mm ±1.54 (SD) (range 20.11–28.36 mm). Mean corneal astigmatism measured with the Lenstar device was 1.91 D ±0.76 (SD) (range 0.90–4.41 D). The mean IOL spherical power was 22.07 D ±3.91 (SD) (range 11.50–33.00 D) and the mean IOL cylinder power of the whole sample was 2.40 D ±1.11 (SD) (range 1.00–6.00 D).
Rotation/misalignment of the toric IOL
Mean rotation/misalignment of the toric IOL at the follow-up examination was 1.33° ±0.90 (SD) (range 0–4°).
MAE in the PRA compared to the original calculator
Comparison of the results of MAE in the PRA obtained by each calculator with the standard toric calculator is shown in Table 1. This calculator achieved a mean MAE in the PRA of 0.77 D ±0.40 (SD) (range 0.01–1.91 D). The PhysIOL calculator and the Barrett toric calculator provided significantly lower values (p<0.001). Specifically, the latest PhysIOL calculator showed a MAE in the PRA of 0.35 D ±0.29 (SD) (range 0.00–1.36 D) and the Barrett toric calculator achieved a value of 0.40 D ±0.29 (SD) (range 0–1.07 D). The color-LED topographer based TCA values also showed statistically significant differences compared to the standard toric calculator, with a mean value of MAE in the PRA of 0.49 D ±0.30 (SD) (range 0.00–1.07 D) (p=0.002). In contrast, the Scheimpflug camera based TCRP-values did not show any clinical or statistically significant differences compared to the original calculator, with a mean MAE of 0.72 D ±0.41 (SD) (range 0.02–1.87 D) (p=0.656).
 |
Table 1 Mean absolute error in predicted residual astigmatism for each calculator |
Absolute and centroid errors in PRA
Table 2 and Figure 1 show the centroid error for each calculator for the whole sample, and Table 3 shows those values stratified for type of astigmatism, WTR or ATR. In eyes with WTR astigmatism, the PhysIOL calculator revealed the best results with a mean MAE in PRA of 0.31 D±0.23 (SD) (range 0.00–1.36 D) and a centroid of 0.08 D±0.39 (SD) @161°. The Barrett toric calculator and the Holladay calculator using color-LED TCA values achieved the best results in the MAE in PRA and a low centroid error (0.09 D±0.32 (SD) @155º and 0.12 D±0.43 (SD) @145º, respectively). The Holladay calculator using Pentacam TCRP-values showed a lower reduction in the MAE in PRA and thus the highest centroid error value (0.39 D±0.61 (SD) @177º). In eyes with ATR astigmatism, all calculators yielded lower MAEs and centroid residual astigmatism prediction errors than in eyes with WTR astigmatism. Specifically, the Barrett toric calculator and the PhysIOL calculator revealed very similar results (0.01 D±0.25 (SD) @167º and 0.01 D±0.26 (SD) @175º). The Holladay calculator using color-LED TCA values also obtained a low prediction error (0.07 D±0.39 (SD) @160º) and again the TCRP-based calculation showed only a very slight reduction of the MAE in PRA compared to the standard toric calculator. The percentage of eyes within 0.25, 0.50, 0.75 and 1.00 D of absolute astigmatic prediction error for each calculation method are shown in Figure 2. Both the PhysIOL calculator and the Barrett toric calculator resulted in comparable outcomes superior to all other methods.
 |
Table 2 Centroid error in predicted residual astigmatism for each calculator |
 |
Table 3 Mean absolute and centroid errors in predicted residual astigmatism for eyes with with-the-rule (WTR) and against-the-rule (ATR) corneal astigmatism |
 |
Figure 1 Double-angle plots illustrating the centroid prediction errors in residual astigmatism for each calculation method. |
 |
Figure 2 Percentage of eyes within 0.25D–1.00D of absolute astigmatic prediction error with each calculation method. |
Discussion
The methodological error in predicting the toricity of the IOL is a main source of residual refractive errors after cataract surgery in eyes with corneal astigmatism. Conventional keratometry, that measures anterior corneal surface, can lead to a sub-optimal refractive outcome, specifically due to overcorrection in WTR and undercorrection in ATR astigmatism.15 These devices calculate the total corneal power assuming a fixed posterior versus anterior curvature ratio and use a standard keratometric index of 1.3375 to convert anterior surface measures in total corneal power and astigmatism.16 Calculations with older toric IOL calculators assume a fixed ratio (generally 1.46) between the cylindrical power at the corneal plane and at the IOL plane. This fixed ratio results in overcorrection in short eyes and undercorrection in long eyes.17 In order to overcome this limitation, Koch et al published their results as a population based regression analysis named Baylor nomogram that predicts posterior corneal astigmatism (PCA).10 Additionally, a regression formula to estimate TCA based on standard keratometry values, named the Abulafia-Koch formula, was developed in 2016.18 PCA can also be predicted by means of a theoretical model, as in the Barrett toric calculator, included in some commercially available calculators19 and also freely available online. The application of this calculator combined with the data obtained from an optical low-coherence reflectometry biometer resulted in significant lower values of residual refractive cylinder compared to standard calculators.14 Both mathematical models resulted in an improvement of outcomes, but still with a wide distribution of errors.20
It is also possible to directly evaluate anterior and posterior corneal surfaces, and thus calculate the TCP through the use of technology based on the Scheimpflug principle or color-LED topography. Both technologies have demonstrated to be more accurate in TCA measurements compared to corneal astigmatism measurements that are based only on the anterior corneal surface.21 The color-LED topographer has shown high rates of repeatability both in normal and post cataract surgery corneas.22,23 Regarding astigmatism assessment, our research group demonstrated a better performance with TCA obtained by the color-LED topography device when compared to the Lenstar and the Orbscan devices.24 When used to predict postoperative astigmatism, Park et al found a better performance of the vector summation using anterior and posterior corneal surface powers with the Pentacam device compared to other methods that included the application of the Baylor nomogram or the Barrett toric calculator with the IOLMaster (Carl Zeiss Meditec AG, Jena, Germany) readings.25 Also, Savini et al used the Pentacam HR to calculate total corneal astigmatism and found optimized outcomes in comparison to keratometric astigmatism.17 These authors concluded that the efficacy of this method was comparable to the application of the Barrett toric calculator or the Abulafia-Koch formula.26 To the best of our knowledge, the TCA obtained from the color-LED topographer has not been previously evaluated to predict postoperative astigmatism after the implantation of toric IOLs. Our results suggest that this color-LED technology improves the performance of the Scheimpflug camera, although these outcomes are still slightly less accurate than the mathematical models in the Abulafia-Koch formula or the Barrett toric calculator. These findings are consistent with results from our recently published study.27
In the future, and in order to improve the accuracy and personalize the measurements, the direct evaluation of the posterior corneal surface will be mandatory. The anterior segment optical coherence tomography (AS-OCT) could be an option. This technology has been used to evaluate the alignment of toric IOLs comparing the corneal astigmatic axis to the IOL cylinder axis shown in real time on the same screen in the topographic map to avoid any errors from head tilting.28 Also, the swept-source technology implemented in the newest biometers is able to predict the postoperative tilt of the IOL and probably will measure the posterior corneal surface with higher accuracy than the methods currently available.29 In fact, Hoffman et al have demonstrated the superiority of swept source Fourier domain AS-OCT in combination with autokeratometry over the Scheimpflug technology in the predictive quality after implantation of toric IOLs.30 Intraoperative aberrometry (IA) also seems to be an alternative method to accurately calculate IOL power. Although there is limited research with this technology, it appears that it slightly increases the predictability of postoperative refractive astigmatism compared with standard methods.31 On the contrary, Davison and Potvin did not find a clear benefit of IA in the calculation of different types of IOLs, including toric and multifocals, in healthy patients.32
In summary, the latest PhysIOL toric calculator and the Barrett toric calculator delivered the best predictive quality of postoperative refractive astigmatism in our sample, showing lower values of the centroid errors and the lowest MAE with PRA. All calculators yielded lower MAE and centroid residual astigmatism prediction errors in ATR astigmatism compared to eyes with WTR astigmatism. Regarding direct measurement of the posterior corneal surface, the color-LED topographer showed a better performance than the Scheimpflug camera.
Acknowledgments
This research has received financial support from a grant from PhysIOL. PhysIOL had no involvement in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. This study was presented at the 36th Congress of the ESCRS as a free paper presentation with interim findings. The paper’s abstract is available at: https://www.escrs.org/vienna2018/programme/free-papers-details.asp?id=30079&day=0.
Disclosure
The authors have no commercial or proprietary interests in any of the materials mentioned in this article. The authors have no financial interests as consultants, reviewers or evaluators.
References
1. Kretz FT, Muller M, Gerl M, Gerl RH, Auffarth GU. Binocular function to increase visual outcome in patients implanted with a diffractive trifocal intraocular lens. BMC Ophthalmol. 2015;15:110. doi:10.1186/s12886-015-0089-9
2. Ferreira TB, Hoffer KJ, Ribeiro F, Ribeiro P, O’Neill JG. Ocular biometric measurements in cataract surgery candidates in Portugal. PLoS One. 2017;12(10):e0184837. doi:10.1371/journal.pone.0184837
3. Shimizu K, Misawa A, Suzuki Y. Toric intraocular lenses: correcting astigmatism while controlling axis shift. J Cataract Refract Surg. 1994;20(5):523–526.
4. Kessel L, Andresen J, Tendal B, Erngaard D, Flesner P, Hjortdal J. Toric intraocular lenses in the correction of astigmatism during cataract surgery: a systematic review and meta-analysis. Ophthalmology. 2016;123(2):275–286. doi:10.1016/j.ophtha.2015.10.002
5. Razmjoo H, Ghoreishi M, Milasi AM, et al. Toric intraocular lens for astigmatism correction in cataract patients. Adv Biomed Res. 2017;6:123. doi:10.4103/2277-9175.216777
6. Nanavaty MA, Bedi KK, Ali S, Holmes M, Rajak S. Toric intraocular lenses versus peripheral corneal relaxing incisions for astigmatism between 0.75 and 2.5 diopters during cataract surgery. Am J Ophthalmol. 2017;180:165–177. doi:10.1016/j.ajo.2017.06.007
7. Potvin R, Kramer BA, Hardten DR, Berdahl JP. Toric intraocular lens orientation and residual refractive astigmatism: an analysis. Clin Ophthalmol. 2016;10:1829–1836. doi:10.2147/OPTH.S114118
8. Hirnschall N, Hoffmann PC, Draschl P, Maedel S, Findl O. Evaluation of factors influencing the remaining astigmatism after toric intraocular lens implantation. J Refract Surg. 2014;30(6):394–400. doi:10.3928/1081597X-20140429-01
9. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936–944. doi:10.1016/j.jcrs.2014.08.036
10. Koch DD, Jenkins RB, Weikert MP, Yeu E, Wang L. Correcting astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J Cataract Refract Surg. 2013;39(12):1803–1809. doi:10.1016/j.jcrs.2013.06.027
11. Shah GD, Praveen MR, Vasavada AR, et al. Software-based assessment of postoperative rotation of toric intraocular lens. J Cataract Refract Surg. 2009;35(3):413–418. doi:10.1016/j.jcrs.2008.10.057
12. Wang L, Shirayama M, Ma XJ, Kohnen T, Koch DD. Optimizing intraocular lens power calculations in eyes with axial lengths above 25.0 mm. J Cataract Refract Surg. 2011;37(11):2018–2027. doi:10.1016/j.jcrs.2011.05.042
13. Holladay JT, Moran JR, Kezirian GM. Analysis of aggregate surgically induced refractive change, prediction error, and intraocular astigmatism. J Cataract Refract Surg. 2001;27(1):61–79.
14. Gundersen KG, Potvin R. Clinical outcomes with toric intraocular lenses planned using an optical low coherence reflectometry ocular biometer with a new toric calculator. Clin Ophthalmol. 2016;10:2141–2147. doi:10.2147/OPTH.S120414
15. Savini G, Naeser K. An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest Ophthalmol Vis Sci. 2015;56(2):827–835. doi:10.1167/iovs.14-15903
16. Fam HB, Lim KL. Meridional analysis for calculating the expected spherocylindrical refraction in eyes with toric intraocular lenses. J Cataract Refract Surg. 2007;33(12):2072–2076. doi:10.1016/j.jcrs.2007.07.034
17. Savini G, Hoffer KJ, Carbonelli M, Barboni P. Scheimpflug analysis of corneal power changes after myopic excimer laser surgery. J Cataract Refract Surg. 2013;39(4):605–610. doi:10.1016/j.jcrs.2012.12.031
18. Abulafia A, Koch DD, Wang L, et al. New regression formula for toric intraocular lens calculations. J Cataract Refract Surg. 2016;42(5):663–671. doi:10.1016/j.jcrs.2016.02.038
19. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699–707. doi:10.3928/1081597X-20150928-03
20. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340–347. doi:10.1016/j.jcrs.2016.12.031
21. Klijn S, Reus NJ, van der Sommen CM, Sicam VA. Accuracy of total corneal astigmatism measurements with a scheimpflug imager and a color light-emitting diode corneal topographer. Am J Ophthalmol. 2016;167:72–78. doi:10.1016/j.ajo.2016.04.011
22. Hidalgo IR, Rozema JJ, Dhubhghaill SN, Zakaria N, Koppen C, Tassignon MJ. Repeatability and inter-device agreement for three different methods of keratometry: Placido, Scheimpflug, and color LED corneal topography. J Refract Surg. 2015;31(3):176–181. doi:10.3928/1081597X-20150224-01
23. Ventura BV, Al-Mohtaseb Z, Wang L, Koch DD, Weikert MP. Repeatability and comparability of corneal power and corneal astigmatism obtained from a point-source color light-emitting diode topographer, a Placido-based corneal topographer, and a low-coherence reflectometer. J Cataract Refract Surg. 2015;41(10):2242–2250. doi:10.1016/j.jcrs.2015.11.003
24. Ferreira TB, Ribeiro FJ. A novel color-LED corneal topographer to assess astigmatism in pseudophakic eyes. Clin Ophthalmol. 2016;10:1521–1529. doi:10.2147/OPTH.S113027
25. Park DY, Lim DH, Hwang S, Hyun J, Chung TY. Comparison of astigmatism prediction error taken with the Pentacam measurements, Baylor nomogram, and Barrett formula for toric intraocular lens implantation. BMC Ophthalmol. 2017;17(1):156. doi:10.1186/s12886-017-0550-z
26. Savini G, Naeser K, Schiano-Lomoriello D, Ducoli P. Optimized keratometry and total corneal astigmatism for toric intraocular lens calculation. J Cataract Refract Surg. 2017;43(9):1140–1148. doi:10.1016/j.jcrs.2017.06.040
27. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794–800. doi:10.3928/1081597X-20171004-03
28. Lucisano A, Ferrise M, Balestrieri M, Busin M, Scorcia V. Evaluation of postoperative toric intraocular lens alignment with anterior segment optical coherence tomography. J Cataract Refract Surg. 2017;43(8):1007–1009. doi:10.1016/j.jcrs.2017.05.025
29. Hirnschall N, Buehren T, Bajramovic F, Trost M, Teuber T, Findl O. Prediction of postoperative intraocular lens tilt using swept-source optical coherence tomography. J Cataract Refract Surg. 2017;43(6):732–736. doi:10.1016/j.jcrs.2017.01.026
30. Hoffmann PC, Abraham M, Hirnschall N, Findl O. Prediction of residual astigmatism after cataract surgery using swept source fourier domain optical coherence tomography. Curr Eye Res. 2014;39(12):1178–1186. doi:10.3109/02713683.2014.898376
31. Woodcock MG, Lehmann R, Cionni RJ, Breen M, Scott MC. Intraoperative aberrometry versus standard preoperative biometry and a toric IOL calculator for bilateral toric IOL implantation with a femtosecond laser: one-month results. J Cataract Refract Surg. 2016;42(6):817–825. doi:10.1016/j.jcrs.2016.02.048
32. Davison JA, Potvin R. Preoperative measurement vs intraoperative aberrometry for the selection of intraocular lens sphere power in normal eyes. Clin Ophthalmol. 2017;11:923–929. doi:10.2147/OPTH.S135659
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
